Abstract
Three properties define hematopoietic stem cells (HSCs): their capacity for quiescence and long survival, their ability to self-renew, and their ability to give rise to a multilineage clone of differentiating and maturing blood cells. Although it is likely that different signals regulate these events, this has been difficult to dissect on a molecular level, since HSC division, their fate decisions, and the earliest differentiation events cannot be directly visualized. Our studies of c-Mpl, the cellular receptor for the cytokine thrombopoietin, suggest that c-Mpl does not control HSC numbers, as had been previously argued, but rather facilitates the early expansion of differentiating clones. These experiments provide a strategy to distinguish the actions of HSCs from earliest progenitor cells in vivo and demonstrate that a selective growth advantage at a level distal to HSC can result in a profound effect on multilineage hematopoiesis.
Introduction
Thrombopoietin is the primary regulator of megakaryocyte and platelet production1 and much experimental evidence suggests that it also regulates the survival and/or proliferation of hematopoietic stem cells (HSCs).2–8 For example, murine marrow cells significantly enriched for HSCs can survive in thrombopoietin alone in single-cell culture, and increased proliferation is noted when either stem cell factor (SCF) or interleukin-3 (IL-3) is added to the culture medium.3 Flow-cytometric cell-sorting studies demonstrate that the marrow cells capable of reconstituting hematopoiesis express c-Mpl.5 In addition, c-mpl−/− animals have decreased numbers of granulocytic and erythroid as well as megakaryocytic progenitor cells, and in competitive repopulation assays, it appears that c-mpl−/− mice have one seventh to one tenth of the number of HSCs as wild-type (WT) controls.2,4,5,7,8
Perhaps the most compelling data that support a role for c-Mpl in maintaining HSC number is the rare human disorder congenital amegakaryocytic thrombocytopenia (CAMT) that results from c-MPL gene mutation.9–12 Patients with this disorder have thrombocytopenia that progresses to aplastic anemia, suggesting an important nonredundant effect of thrombopoietin/c-MPL on HSCs.
Although these data indeed demonstrate that multilineage hematopoiesis is impacted, the conclusion that HSC survival or replication is impaired may be less secure, since each study quantitates HSCs by measuring their contribution to hematopoiesis. Competitive repopulation assays are considered a gold standard with which to determine murine HSC frequency.13 However, the assay actually relies on several discrete functional characteristics of HSCs: their ability to home from the circulation to marrow after transplantation, to engraft within the marrow microenvironment, to survive and self-renew, and to initiate a differentiation program. In addition, outcomes depend on the ability of the resulting progeny (progenitor) cells to proliferate and mature. Should any of these properties be influenced by c-Mpl, the experimental results would unintentionally reflect this abnormality and not only HSC frequency. Similarly, the finding that patients with CAMT have thrombocytopenia that progresses to aplastic anemia could imply either a deficient number of HSCs (because of impaired HSC replication or survival) or the deficient differentiation of an early multilineage progenitor cell.
In this report, we use alternative experimental strategies to distinguish these possibilities. The approaches are feasible because thrombopoietin uniquely signals via the binding and activation of c-Mpl and because both thrombopoietin−/− (tpo−/−)14 and c-mpl−/−2 mice have been generated and, with the exception of thrombocytopenia, are healthy. In all studies that enumerate HSCs, we transplant marrow cells into irradiated tpo−/− mice so that c-mpl−/− cells can compete as effectively as wild-type cells.
Materials and methods
These studies were performed with Institutional Animal Care and Use Committee (IACUC) approval at the University of Washington.
Mice
The c-Mpl null (c-mpl−/−) and thrombopoietin null (tpo−/−) mice were gifts from Warren Alexander (Walter and Eliza Hall Institute of Medical Research, Parkville, Australia) and Fred de Sauvage (Genentech, San Francisco, CA), respectively. Wild-type (WT) mice with and without genetic markers to track cell origin (ie, PeP3b [B6 SJL/Ly5.1], C57BL/6J [B6.Ly5.2], and heterozygous ROSA26 [C57BL/6J-Gtrosa26. Ly5.2] mice) were purchased from Jackson Laboratory (Bar Harbor, ME). All mice were bred and maintained at the University of Washington under specific pathogen-free conditions. ROSA26 homozygous offspring of heterozygous matings were identified by polymerase chain reaction (PCR) typing of tail-tip genomic DNA. PeP3b mice express the CD45.1 antigen on their hematopoietic cells, whereas cells in ROSA26, C57BL/6J, c-mpl−/−, and tpo−/− mice express the CD45.2 isoform, a phenotype that is readily identifiable by flow cytometry. In addition, ROSA26 mice express a transgene encoding β-galactosidase. CFU-GM–derived colonies from ROSA26 mice were distinguished from other CFU-GM–derived colonies in agar cultures by staining for β-galactosidase activity. Fluorescein di-β-D galactopyranoside (FDG) staining and flow cytometry was used to identify β-galactosidase expression in granulocytes. More detailed methods are in Abkowitz et al15 and Chen et al.16
Parabiosis studies
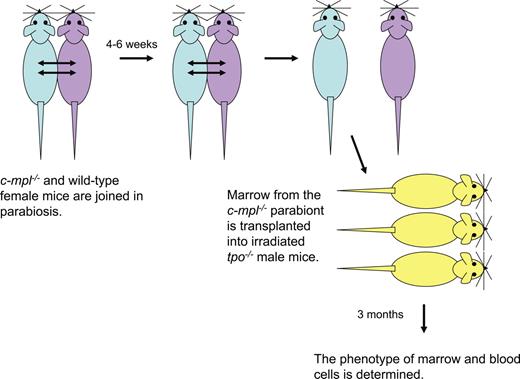
Female c-mpl−/− mice were attached to female PeP3b (or to female ROSA26) mice using methods previously described.15 As diagrammed in Figure 1, after 4 to 6 weeks of parabiosis, marrow cells from the c-mpl−/− parabionts were infused into 2 or 3 lethally irradiated male tpo−/− recipients. In each study, large numbers of marrow cells (15 × 106 to 20 × 106 per recipient) were transplanted to ensure that the phenotype of the reconstituted mice reflected the phenotype of donor HSCs and to avoid stochastic clonal contributions to hematopoiesis. Three months after transplantation, the phenotype of marrow and blood granulocytes and marrow CFU-GMs was determined and analyzed using the methods of Abkowitz et al.15 To ensure that there was no endogenous recovery of tpo−/− marrow cells, Southern-blot analyses were performed using a Y-chromosome probe.
Experimental design of parabiosis studies. c-mpl−/− and WT female 10- to 14-week-old mice were linked in parabiosis by suturing tissue from shoulder to hip. After 10 days, approximately 50% of the blood granulocytes and lymphocytes in each parabiont are of partner origin, demonstrating that the circulations are joined (data not shown). After 4 to 6 weeks, the parabionts are separated and the percentages of partner phenotype granulocytes in marrow and blood are assessed by flow cytometry for CD45.1 (PeP3b) and CD45.2 (c-mpl−/−; Experiment 1) or β-galactosidase activity (ROSA26; Experiment 2). The transfer of HSCs from the WT parabiont to the c-mpl−/− parabiont was quantitated by the transplantation of marrow cells into irradiated male tpo−/− recipients. The contribution WT HSCs to recipient hematopoiesis was assessed 3 months later. In each experiment, we proved that there was no recovery of endogenous tpo−/− marrow cells using Southern-blot analyses and probing for the Y-chromosome (data not shown). Methodologic details can be found in Abkowitz et al15 and Chen et al.16
Experimental design of parabiosis studies. c-mpl−/− and WT female 10- to 14-week-old mice were linked in parabiosis by suturing tissue from shoulder to hip. After 10 days, approximately 50% of the blood granulocytes and lymphocytes in each parabiont are of partner origin, demonstrating that the circulations are joined (data not shown). After 4 to 6 weeks, the parabionts are separated and the percentages of partner phenotype granulocytes in marrow and blood are assessed by flow cytometry for CD45.1 (PeP3b) and CD45.2 (c-mpl−/−; Experiment 1) or β-galactosidase activity (ROSA26; Experiment 2). The transfer of HSCs from the WT parabiont to the c-mpl−/− parabiont was quantitated by the transplantation of marrow cells into irradiated male tpo−/− recipients. The contribution WT HSCs to recipient hematopoiesis was assessed 3 months later. In each experiment, we proved that there was no recovery of endogenous tpo−/− marrow cells using Southern-blot analyses and probing for the Y-chromosome (data not shown). Methodologic details can be found in Abkowitz et al15 and Chen et al.16
Competitive transplantation analyses
To distinguish the quantity of HSCs from their differentiation and proliferation potentials, we performed competitive repopulation assays using different ratios of donor cells and either lethally irradiated (1100 cGy) tpo−/− or lethally irradiated wild-type (ROSA26) recipients. Multiple independent cell markers were used to carefully dissect cell origin (eg, CD45.1 vs CD45.2, presence or absence of β-galactosidase activity, presence or absence of a Y-chromosome). Specifically, marrow mononuclear cells from c-mpl−/− and PeP3b mice were isolated and mixed at ratios of 1:1, 2:1, 4:1, or 8:1 prior to transplantation (5 × 106:5 × 106, 10 × 106:5 × 106, 20 × 106:5 × 106, or 20 × 106:2.5 × 106 cells/irradiated recipient mouse, respectively). Control experiments were performed with equivalent ratios of C57BL/6J and PeP3b donor marrow cells. Three months after transplantation, the percentages of blood and marrow granulocytes of c-mpl−/− (or C57BL/6J) versus PeP3b phenotype were determined as outlined in parabiosis studies. In some studies, the phenotypes of HSCs were also assayed by the secondary transplantation of marrow cells into irradiated tpo−/− mice.
AMD3100 mobilization studies
AMD3100 (a specific SDF1 antagonist, provided by AnorMED, Langley, BC, Canada) was used to mobilize HSCs and create open niches for the competitive engraftment of transplanted cells, using the strategy of Chen et al.16 c-mpl−/− mice were given 5 mg/kg of AMD3100, subcutaneously, 2 hours prior to the infusion of 40 × 106 wild-type (ROSA26) marrow cells. Control animals received donor cells but no AMD3100. The numbers of engrafting ROSA26 donor cells were determined after 3 months by assaying the percentages of blood and marrow granulocytes and marrow CFU-GMs with β-galactosidase activity as described in Chen et al16 and in “Parabiosis studies.”
Results
We first studied the relationship of c-Mpl and HSC number by observing the trafficking of HSCs from WT to c-mpl−/− mice joined in parabiosis. After 4 to 6 weeks, the animals were separated and the phenotype of HSCs in the c-mpl−/− parabionts was assayed by transplanting marrow cells into irradiated tpo−/− recipients. The experimental strategy is shown in Figure 1 and is premised on the studies of Abkowitz et al,15 Chen et al,16 and Wagers et al.17 If the c-mpl−/− parabiont lacked HSCs, we reasoned that there would be open (unoccupied) niches in which circulating WT HSCs could engraft. Surprisingly, however, less than 1% of HSCs exited the WT mouse's marrow, transited blood, and engrafted in the c-mpl−/− partner mouse's marrow (Table 1). This small amount of HSC trafficking is similar to that seen between normal WT mice pairs after 4 to 12 weeks of parabiosis15 ; suggests that few open sites were available in the c-mpl−/− mouse; and argues that the number of HSCs in c-mpl−/− mice are normal or that both the number of HSCs and the number of supportive niches are comparably decreased.
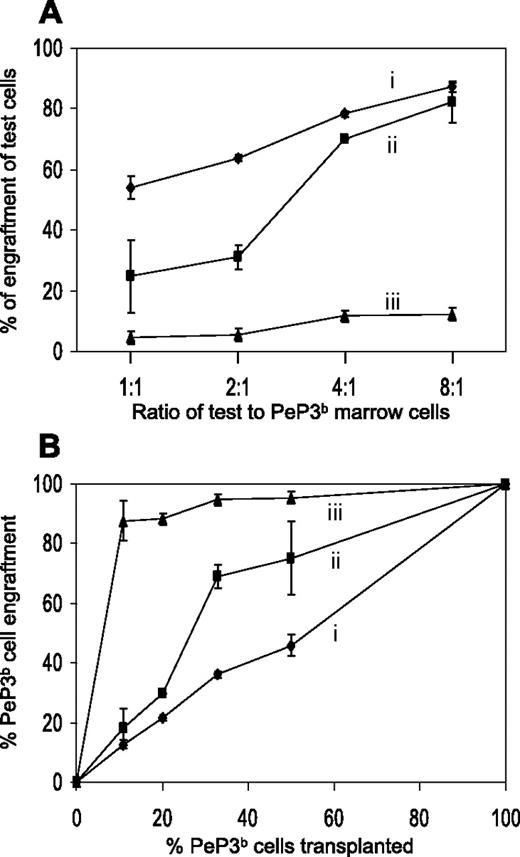
Next, competitive transplantation studies were performed in which various ratios of c-mpl−/− and WT marrow cells were transplanted into irradiated tpo−/− (or WT) recipients. The phenotypes of blood granulocytes, marrow granulocytes, and marrow progenitor cells were assessed 3 months later. As expected, when phenotypically distinct WT marrow cells were transplanted into irradiated tpo−/− recipients, the ratio of the reconstituting cell phenotypes was identical to the ratio of the infused cell phenotypes (Figure 2A, experiment A), and, as reported previously by others,2,4,7,8 when c-mpl−/− and WT cells were transplanted into WT recipients at a 1:1 ratio, the relative contribution of c-mpl−/− cells was low (5%; Figure 2A, experiment C), suggesting that the frequency of HSCs in c-mpl−/− marrow was one tenth of the frequency of HSCs in normal (WT) marrow. Even at a ratio of 8:1, c-mpl−/− cells contributed minimally, implying a major (exponential) selective advantage for WT (vs c-mpl−/−) cells in the WT thrombopoietin-containing environment, again consistent with previous data.4,7 Most importantly, however, are the studies labeled B in Figure 2A, where c-mpl−/− and WT marrow cells were transplanted into irradiated tpo−/− mice at ratios of 4:1 and 8:1. These studies demonstrate that in the absence of thrombopoietin, c-mpl−/− and WT marrow cells compete equivalently and hence prove that frequency of HSCs in c-mpl−/− marrow is normal.
HSC behavior in competitive transplantation experiments. (A) Various ratios of test and PeP3b (WT) marrow cells (1:1, 2:1, 4:1, 8:1) were transplanted into irradiated recipient ROSA26 (WT) or tpo−/− mice and their contributions to blood granulocytes, marrow granulocytes, and marrow progenitor cells were assessed 3 months later. The expected percentages of test cell engraftment (50%, 66%, 80%, and 88%, respectively) were only seen when C57BL/6J and PeP3b cells were transplanted into tpo−/− (i) (or into WT; data not shown) recipients. c-mpl−/− HSCs are poor competitors when transplanted into WT (thrombopoietin-producing) recipients (iii) yet compete appropriately at marrow cell dose ratios of 4:1 and 8:1 when transplanted into tpo−/− recipients (ii). See “Results” for details. (B) PeP3b cells outcompete c-mpl−/− cells in a WT host. Data from panel A are plotted as percentage PeP3b cells transplanted versus percentage PeP3b cells engrafting. Error bars indicate the mean ± standard deviation (SD) of experiments using 3 to 5 mice.
HSC behavior in competitive transplantation experiments. (A) Various ratios of test and PeP3b (WT) marrow cells (1:1, 2:1, 4:1, 8:1) were transplanted into irradiated recipient ROSA26 (WT) or tpo−/− mice and their contributions to blood granulocytes, marrow granulocytes, and marrow progenitor cells were assessed 3 months later. The expected percentages of test cell engraftment (50%, 66%, 80%, and 88%, respectively) were only seen when C57BL/6J and PeP3b cells were transplanted into tpo−/− (i) (or into WT; data not shown) recipients. c-mpl−/− HSCs are poor competitors when transplanted into WT (thrombopoietin-producing) recipients (iii) yet compete appropriately at marrow cell dose ratios of 4:1 and 8:1 when transplanted into tpo−/− recipients (ii). See “Results” for details. (B) PeP3b cells outcompete c-mpl−/− cells in a WT host. Data from panel A are plotted as percentage PeP3b cells transplanted versus percentage PeP3b cells engrafting. Error bars indicate the mean ± standard deviation (SD) of experiments using 3 to 5 mice.
Interestingly, WT cells have a moderate competitive advantage, even within the thrombopoietin-deficient environment, when they are transplanted in a higher proportion (c-mpl−/−–to–WT ratios of 2:1 and 1:1), which may be better visualized when the percentage of PeP3b (WT) cells transplanted is plotted against the percentage of WT engraftment (Figure 2B). Given the contour of the dose/response curve, we wondered if some thrombopoietin-producing cells were present in both the c-mpl−/− and the WT donor marrow cell inoculum and transplanted along with donor HSCs. Assuming these cells were relatively short-lived, their impact on multilineage WT progenitors in the recipient animal would be time limited, resulting in an initial, but not a continued, exponential amplification.
To test this hypothesis, we transplanted marrow cells obtained 3 months after the primary transplantation into secondary irradiated tpo−/− recipients. If our hypothesis was correct, thrombopoietin-producing nonhematopoietic cells should be rare or absent, and ratios of c-mpl−/− to WT cells that more closely reflected the ratio of input marrow cells (eg, 2:1 and 1:1, or 33% and 50% WT) should emerge. The results, 27.9% ± 2.1% and 65.2% ± 13%, respectively, are as hypothesized. Although c-Mpl is present on HSCs,5 its dominating effect is on the proliferation of the earliest multilineage progenitor cells.
In final studies, we performed nonmyeloablative transplantation using AMD3100, a specific CXCR4 antagonist, as a preparative regimen to mobilize HSCs, open niches, and allow the competitive engraftment of endogenous circulating HSCs and infused competitor HSCs.16 Previously, we have shown that transplanting 40 × 106 ROSA26 (WT) marrow cells into PeP3b (WT) mice results in 1.0% ± 0.2% donor cell engraftment without AMD3100 exposure, whereas 4.6% ± 1.1% donor cell engraftment occurs when the marrow cells are transplanted 2 hours after the recipient mice are injected subcutaneously with AMD3100 (5 mg/kg). This is the percentage that is expected if 11% of the marrow niches were vacated and the HSCs present among the 40 × 106 infused marrow cells competed equally with mobilized HSCs to reoccupy these sites (see discussion in Chen et al16 ). A similar study was performed using c-mpl−/− recipient mice after initial experiments confirmed that their ability to mobilize progenitors was equivalent to PeP3b mice. The fold change in CFU-GMs per mL of blood 2 hours after AMD3100 was 6.4 ± 3.0 and 4.6 ± 1.0 in PeP3b mice and c-mpl−/− mice, respectively.
Specifically, c-mpl−/− mice received a transplant of 40 × 106 ROSA26 (WT) marrow cells after AMD3100 or mock (saline) administration as the only preparative regimen. Three months later, blood and marrow cells were phenotyped and the frequency of donor HSCs was assayed by the transplantation of marrow cells into tpo−/− irradiated secondary recipients (Table 2). The percentage of WT HSCs as determined by this assay was 5.4 ± 1.6 and 1.7 ± 0.5, respectively, values that are identical to those in the similar studies of WT recipient mice.16 These data argue that HSC number, not just frequency, is relatively normal in c-mpl−/− mice.
Our nonmyeloablative transplantation studies (Table 2) also show that differentiating cells derived from WT HSCs have a profound growth advantage versus c-mpl−/− clones in the thrombopoietin-rich (c-mpl−/−) environment and thus confirm the results of our competitive repopulation studies in which WT and c-mpl−/− marrow were transplanted into irradiated WT recipients (Figure 2A-B, experiment C).
Discussion
In this report, we study the consequences of Tpo–c-Mpl signaling using experimental strategies that allow us to observe the behavior of HSCs independently of the behaviors of derivative cells. Our studies show that c-mpl−/− mice have normal numbers of HSCs but that these cells compete ineffectively in a standard competitive repopulation assays because multipotent progenitor cells cannot optimally expand. From a technical standpoint, the data argue that caution is required when interpreting the results of competitive transplantation assays involving cells with biologically distinct genotypes.
These observations also have clinical implications. Why c-mpl−/− mice have thrombocytopenia, but not pancytopenia, yet children with CAMT develop marrow failure by age 2 to 11 years10–12 has previously been attributed to differences in the kinetics of murine and human HSCs or their different propensities to acquire additional defects with aging.1,12 Our data provide an alternative explanation: the deficient proliferation of differentiating HSC clones. A decrease in the proliferative capacity of differentiating clones should be more problematic for man (70 kg; eg, HSCs must generate 2.5 × 1011 red cells per day) than mouse (25 g; eg, HSCs must generate only 3.2 × 108 red cells per day).18 Similarly, a decrease in the proliferative capacity of differentiating clones should be more problematic for an older, larger child than a younger, smaller child. It is likely that multipotent progenitor cells that lack c-MPL cannot divide and differentiate sufficiently to accommodate for this increased demand.
The studies in which WT and c-mpl−/− marrow were transplanted into irradiated WT recipients (Figure 2A-B) and the nonmyeloablative transplantation studies (Table 2) clearly demonstrate that differentiating cells derived from WT HSCs have a profound growth advantage (vs c-mpl−/− clones) in the thrombopoietin-rich (WT or c-mpl−/−) environment, since in both settings there are much higher percentages of WT granulocytes than of WT HSCs. Because a similar advantage should exist for WT clones in patients with CAMT, a clinical benefit might derive from the transplantation and engraftment of small numbers of gene-corrected HSCs.
Our studies of c-Mpl function may also provide new insights into the pathogenesis of the myeloproliferative disorders. c-Mpl signals via the activation of the Jak2 kinase. The unregulated expression of c-Mpl or thrombopoietin or the expression of an activated form of Jak2 causes myeloproliferative disorders in murine models (reviewed in Kaushansky1 ); an acquired mutation of JAK2 (V617P) is associated with the human myeloproliferative disorders polycythemia vera, essential thrombocytosis, and idiopathic myelofibrosis19,20 ; and recently, rare patients with idiopathic myelofibrosis have been described who have gain of functions in c-MPL (ie, W515L, W515K).21 Our results argue that in these disorders, the predominant pathophysiologic consequence of interference with JAK2 signaling may occur at the level of multipotent progenitor cells and not HSCs. Although it is likely that HSCs contain the mutation, the kinetics of these cells may be minimally impacted.
Observations in the fourth human myeloproliferative disorder, chronic myelogenous leukemia (CML), support this concept. CML results from a balanced translocation of chromosomes 9 and 22, resulting in the fusion protein BCR-ABL, which has intrinsic tyrosine kinase activity, and the constitutive activation of comparable signal transduction pathways. CML patients treated with imatinib, a specific BCR-ABL kinase inhibitor, appear to regain normal hematopoiesis and many obtain a molecular remission in which BCR-ABL transcripts are not detected by quantitative reverse transcription–PCR (RT-PCR; sensitivity ∼1/105 cells). However, if imatinib is stopped, the myeloproliferative hematopoiesis quickly recurs, demonstrating that aberrant HSCs persist and that the preferential expansion of BCR-ABL–positive cells distal to HSCs, and not HSC expansion, is responsible for the neoplastic phenotype.22,23
Together our data argue that the molecular mechanisms that regulate differentiating clones are distinct from those impacting HSC growth. In addition, they demonstrate that disordered, as well as normal, hematopoiesis can be significantly regulated at the level of early multipotent progenitor cells. This further reinforces the need for careful experimental strategies that can distinguish an effect on HSC survival and replication, and hence HSC number, from an effect on the survival, replication, and number of the earliest multipotent progenitor cells that derive from HSCs.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This work was supported by RO1 HL046598 from the National Institutes of Health. The authors thank Allan Dimaunahan for help in the preparation of this manuscript and Thalia Papayannopoulou for her insightful review.
National Institutes of Health
Authorship
Contribution: J.L.A. designed the experiments, analyzed data, and wrote the manuscript; and J.C. performed research and analyzed data.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Janis L. Abkowitz, Professor of Medicine, Head, Division of Hematology, Adjunct Professor of Genome Sciences, University of Washington, Box 357710, Seattle, WA 98195-7710; e-mail: janabk@u.washington.edu.