Abstract
Developing strategies to counteract imatinib resistance constitutes a challenge in chronic myelogenous leukemia (CML). Therapy with the tyrosine kinase inhibitors nilotinib (AMN107) and dasatinib (BMS-354825) has produced high rates of hematologic and cytogenetic response. Src kinase activation has been linked to Bcr-Abl–mediated leukemogenesis and CML progression. In addition to binding Abl kinase with less stringent conformational requirements than imatinib, dasatinib is a potent Src kinase inhibitor. In the current study, we report on 23 patients with CML (19 of them in accelerated or blastic phases) treated with dasatinib after treatment failure with both imatinib and nilotinib. More than half (13; 57%) of 23 patients responded to dasatinib: 10 (43%) had a complete hematologic response (CHR), including 7 (30%) who had a cytogenetic response (2 complete, 4 partial, and 1 minor). These results suggest that dasatinib may be active in some patients after failure with both imatinib and nilotinib.
Introduction
Although most patients with chronic myeloid leukemia (CML) have a favorable outcome with imatinib, some eventually develop resistance. This occurs through a variety of mechanisms, including Abl kinase domain mutations and amplification or overexpression of Bcr-Abl.1,2 Mutations have been reported in 50% to 90% of patients with imatinib resistance,1-4 mapping to more than 40 different positions.5 In addition to mutations, overexpression of Src-related kinases has been implicated in some cases of imatinib resistance and in Bcr-Abl–mediated leukemogenesis.6-9
Tyrosine kinase inhibitors (TKIs) with enhanced potency against Bcr-Abl and the ability to override resistance mediated by most mutations have been developed. Nilotinib (Tasigna; AMN107; Novartis, Basel, Switzerland) is structurally related to imatinib with 30-fold higher potency against Bcr-Abl.10 Dasatinib (Sprycel; BMS-354825, Bristol-Myers Squibb, New York, NY) is a dual Src- and Abl-kinase inhibitor, approximately 300-fold more potent than imatinib.11 Both nilotinib and dasatinib inhibit the activity of most mutations observed in patients with imatinib resistance, except for T315I,12-14 and have significant activity in patients with CML after imatinib failure. The increased clinical use of these agents will lead to scenarios where patients develop resistance or intolerance to more than 1 TKI. We present the first report of patients treated with dasatinib after sequential failure with both imatinib and nilotinib.
Patients, materials, and methods
Study group
A group of 23 patients with CML who failed therapy with both imatinib and nilotinib were treated with dasatinib between January 2005 and March 2006. Patients were followed with complete blood counts, bone marrow aspirations, cytogenetic analysis, and real-time reverse transcription–polymerase chain reaction (RT-PCR) every 3 months as previously described.15 Approval was obtained from the M. D. Anderson Cancer Center institutional review board (Houston, TX) for the studies in which these patients were included. Informed consent was provided in accordance with the Declaration of Helsinki.
Dasatinib therapy
Dasatinib was administered for a median of 4 months (range, 1 to 10 months). A group of 13 patients (1 in chronic phase [CP], 7 in accelerated phase [AP], and 5 in blastic phase [BP]) received dasatinib at a starting dose of 70 mg twice daily; 9 patients (2 in CP, 1 in second CP, 3 in AP, and 3 in BP) received 140 mg once daily, and 1 patient (in BP) received 120 mg twice daily. Doses were adjusted for toxicity as previously described.16
Results and discussion
When dasatinib was started, 3 patients were in late CP, 1 was in second CP, 10 were in AP, and 9 were in BP. Median age was 58 years (range, 19 to 76 years). Of this group, 2 (9%) patients were in chronic hematologic response (CHR) with no cytogenetic response; 21 had disease not in CHR. Patients had received a median of 4 prior therapies, including imatinib and nilotinib in all patients, interferon-α in 9 (39%) patients, and allogeneic stem cell transplantation in 2 (9%) patients. Patients had received imatinib for a median of 21 months (range, 2 to 61 months) and nilotinib for a median of 8 months (range, 2 to 20 months). All patients received nilotinib at a dose of 400 mg or more twice daily except for 3 patients, who received 400 mg daily. Nilotinib was discontinued due to loss of response in 15 patients and hematologic resistance (HR) in 8. The median Philadelphia chromosome–positive (Ph+) metaphases at study entry were 100% (range, 20% to 100%) in 21 (91%) of 23 assessable patients (2 patients had insufficient metaphases). Ten (4 in AP, 6 in BP) patients had clonal evolution and 1 (in BP) had −7 in 2 Ph− metaphases (18 metaphases were Ph+). The median baseline BCR-ABL/ABL ratio was 72.11% (range, 0.03% to 100%) in 22 evaluable patients. Sequencing of the kinase domain of the BCR-ABL fusion transcript (codons 221 to 500) was performed in 22 patients prior to starting dasatinib, and mutations were identified in 16 (73%) patients, including T315I, Y253F/H, F359V, H396P/R, E255V/K, and G250E (1 in combination with F359I) in 2 patients each, and F311L + E453K, E355G, A433T, and Q252H in 1 patient each.
Dasatinib was started after a median of 65 months (range, 9 to 233 months) from CML diagnosis. A group of 19 patients started dasatinib after nilotinib discontinuation. Four patients received other therapies between nilotinib and dasatinib: 2 patients received systemic chemotherapy; 1 patient received a combination of idarubicin, low-dose cytarabine, and imatinib for BP; and 1 patient received imatinib combined with the heat shock protein 90 inhibitor KOS-953. The median time from the discontinuation of nilotinib to the start of dasatinib was 1.3 months (range, 0.2 to 6.1 months).
Responses occurred in 13 (57%) patients (Table 1). A CHR (normalization of peripheral blood and disappearance of all signs and symptoms of leukemia for at least 4 weeks) was achieved in 10 (43%) patients (1 in CP, 8 in AP, and 1 in BP), of whom 7 (30%) achieved a cytogenetic response: 2 achieved complete cytogenetic response (CGCR; 0% Ph+), 4 achieved partial cytogenetic response (CGPR; 1% to 35% Ph+), and 1 achieved minor cytogenetic response (mCGR; 36%-90% Ph+). One patient with CHR and no cytogenetic response had a disappearance of clonal evolution consisting of t(3;17). One of the patients who achieved CGCR was in AP and had a F359V mutant, and the other was in BP with a E255V/K mutant (Figure 1). Responses occurred in 13 (81%) of 16 patients harboring mutations, including all 4 with P-loop mutations. None of the patients with T315I responded. Responses are ongoing (as of September 2006) in 8 (62%) of 13 responders. A number (20 [87%]) of patients have had at least 1 quantitative RT-PCR measurement after start of therapy, and 13 (65%) patients had a decrease of their BCR-ABL/ABL ratio: less than 1 log in 6 patients (1 in second CP, 3 in AP, and 2 in BP) and 1 to 2 log in 7 patients (1 in CP, 1 in second CP, 4 in AP, and 1 in BP). Ten patients did not respond to dasatinib: 9 had HR, and 1 had disease progression.
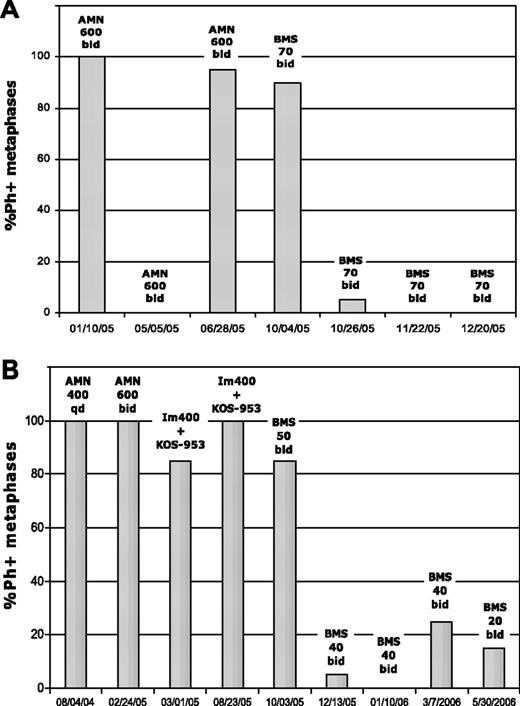
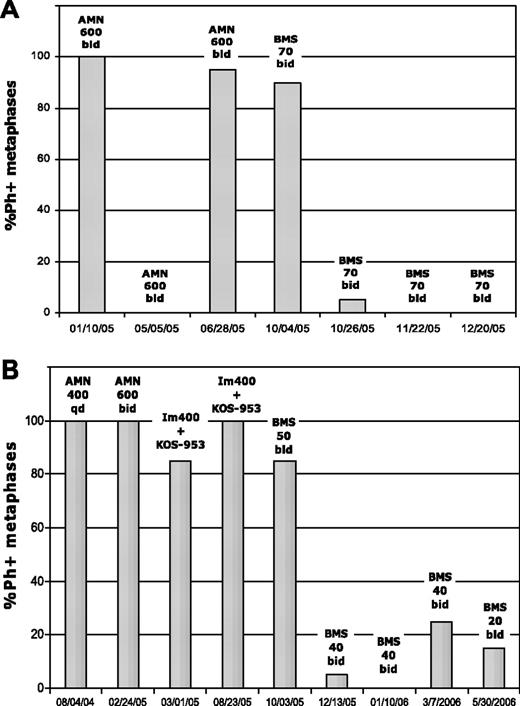
Complete cytogenetic response in 2 patients treated with dasatinib after imatinib and nilotinib failure. Patient A was in BP and harbored the P-loop mutant E255V/K. This patient was taken off the study in January 2006 to undergo bone marrow transplantation in complete cytogenetic response. Patient B was in accelerated phase and harbored the F359V mutation at dasatinib therapy. KOS-953 indicates the heat shock protein 90 inhibitor 17-allylamino-17-demethoxygeldanamycin (17-AAG); Im, imatinib; AMN, nilotinib; BMS, dasatinib; qd, daily; and bid, twice daily.
Complete cytogenetic response in 2 patients treated with dasatinib after imatinib and nilotinib failure. Patient A was in BP and harbored the P-loop mutant E255V/K. This patient was taken off the study in January 2006 to undergo bone marrow transplantation in complete cytogenetic response. Patient B was in accelerated phase and harbored the F359V mutation at dasatinib therapy. KOS-953 indicates the heat shock protein 90 inhibitor 17-allylamino-17-demethoxygeldanamycin (17-AAG); Im, imatinib; AMN, nilotinib; BMS, dasatinib; qd, daily; and bid, twice daily.
Dose reductions were required in 31 (58%) patients due to grades 3 to 4 toxicity. Nearly half (11 [48%]) of the patients discontinued dasatinib due to resistance in 8 patients (1 in AP, 7 in BP), progression to BP in 1 patient (in second CP), shortness of breath in 1 patient (in AP), or stem cell transplantation (SCT) in 1 patient (in BP). After a median follow-up of 34 weeks (range, 4 to 55 weeks), 7 (30%) patients died (1 in second CP, 6 in BP) and 11 (48%) continued dasatinib. BCR-ABL mutations were present in all 13 responders, and resequencing of the BCR-ABL transcript during dasatinib therapy was performed in 11 (85%) patients, showing disappearance of mutations in 4 patients (1 patient with 2 mutations, G250E + F359I, lost F359I on dasatinib), new mutations in 2 patients, and persistence of the same mutations in 5 patients.
These results suggest activity of dasatinib in patients with CML after failure with both imatinib and nilotinib, with hematologic and cytogenetic response rates of 57% and 30%, respectively. Considering that 19 (83%) patients were in AP or BP at the time of dasatinib start, these results are comparable with those obtained in phase 2 studies of dasatinib in AP or BP after imatinib failure, in which the hematologic and cytogenetic response rates ranged from 40% to 59% and 36% to 54%, respectively.17-20 Therefore, failure with nilotinib in addition to imatinib may not significantly affect the expected response to dasatinib. More important, these results suggest that there may not be significant cross-resistance between these novel TKIs. Whether these responses are due to the higher potency of dasatinib against the Abl kinase,11 its inhibitory effect on Src family kinases,11 or other mechanisms remains to be determined. As these agents become available, lack of cross-resistance, if confirmed, would be of significant value in enhancing therapeutic options with sequential TKIs for patients with CML. Investigating the response to nilotinib after dasatinib failure is also warranted.
We conclude that dasatinib therapy may induce response in some patients with CML and resistance to both imatinib and nilotinib, suggesting lack of cross-resistance between nilotinib and dasatinib in at least some instances. Longer follow-up of a larger series of patients is needed to determine the long-term impact of these responses.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Conflict-of-interest disclosure: J.C., H.K., and M.T. received research support from Novartis and Bristol-Myers Squibb. S.O'B. received research support from Bristol-Myers Squibb. F.G. received research support from Novartis. C.N. is an employee of Bristol-Myers Squibb.
Contribution: A.Q.-C. wrote the manuscript, and analyzed data; H.K. designed research, analyzed data, contributed to the research, and revised the manuscript; D.J. performed mutation analysis and revised the manuscript; C.N. designed research and revised the manuscript; S.O'B. contributed to the research and revised the manuscript; F.G. contributed to the research and revised the manuscript; M.T. designed research, contributed to the research, and revised the manuscript; and J.C. wrote the manuscript, designed research, analyzed data, contributed to the research, and revised the manuscript.