Abstract
CD4+CD25+ regulatory T (Treg) cells play a critical role in the induction and maintenance of peripheral immune tolerance. In experimental transplantation models in which tolerance was induced, donor-specific Treg cells could be identified that were capable of transferring the tolerant state to naive animals. Furthermore, these cells appeared to have indirect allospecificity for donor antigens. Here we show that in vivo alloresponses can be regulated by donor alloantigen-specific Treg cells selected and expanded in vitro. Using autologous dendritic cells pulsed with an allopeptide from H2-Kb, we generated and expanded T-cell lines from purified Treg cells of CBA mice (H2k). Compared with fresh Treg cells, the cell lines maintained their characteristic phenotype, suppressive function, and homing capacities in vivo. When cotransferred with naive CD4+CD25− effector T cells after thymectomy and T-cell depletion in CBA mice that received CBK (H2k+Kb) skin grafts, the expanded Treg cells preferentially accumulated in the graft-draining lymph nodes and within the graft while preventing CBK but not third-party B10.A (H2k+Dd) skin graft rejection. In wild-type CBA, these donor-specific Treg cells significantly delayed CBK skin graft rejection without any other immunosuppression. Taken together, these data suggest that in vitro–generated tailored Treg cells could be considered a therapeutic tool to promote donor-specific transplant tolerance.
Introduction
Transplantation of cells and organs is regarded as the only therapeutic choice for end-stage failure of several organs and has been made possible by the development of powerful immunosuppressive treatments that prevent transplant rejection. However, most of these drugs nonspecifically target the immune response, leading to unwanted side effects, and have limited ability to prevent chronic rejection.1,2 The ultimate goal in transplantation is, therefore, the induction of a sustained state of specific tolerance to donor alloantigens with minimization or complete withdrawal of immunosuppression. Many studies in rodents have shown that it is possible to exploit the mechanisms that normally maintain immune homeostasis and tolerance to self-antigens to induce tolerance to alloantigens.3 Immunologic tolerance involves central and peripheral mechanisms. Central tolerance results from intrathymic deletion of T cells with high avidity for thymically expressed antigens. Peripheral tolerance can be achieved by various mechanisms including deletion of activated/effector T cells, anergy induction, and active regulation of effector T cells.4,5
CD4+ T cells with regulatory function have been shown to play a critical role in the maintenance of transplantation tolerance.6 The CD25+ fraction of CD4+ T cells mediates tolerance on adoptive transfer into a naive host.7-9 CD4+CD25+ regulatory T (Treg) cells are a naturally occurring population of CD4+ T cells that constitutively express the IL-2 receptor α-chain (CD25).10 These cells were shown to be potent suppressors of activated T cells in vitro11,12 and to be crucial for the control of autoreactive T cells13 and of the effector function of alloreactive CD4+ and CD8+ T cells in transplantation models in vivo.8,14 The precise mechanisms by which these Treg cells exert their suppressive function remain to be defined. Surface molecules such as cytotoxic T lymphocyte–associated antigen 4 (CTLA-4),15,16 the glucocorticoid-induced tumor necrosis factor receptor (GITR),17 and cytokines such as TGF-β18-20 and IL-108,21 are thought to play roles in different animal models.
Several studies have implied that the regulation mediated by Treg cells is dependent on a continuous supply of alloantigens8,22-24 or tissue-specific target autoantigens,25,26 suggesting that these cells have specificity for alloantigens or autoantigens. In transplantation, alloreactive CD4+ T cells with indirect allospecificity are thought to play a key role in chronic rejection, and the control of these pathogenic effector cells by donor-specific Treg cells could, therefore, result in transplantation tolerance.27-31 However, the possibility of using Treg cells as immunotherapy for the induction of antigen-specific tolerance is limited by cell number. Indeed, the entire pool of Treg cells accounts for only 5% to 10% of CD4+ T cells in the peripheral blood of healthy persons.10,32,33
In this study, we have explored whether in vivo alloresponses could be regulated by Treg cells with indirect allospecificity selected and expanded in vitro. We show that murine CD4+CD25+ T-cell lines with indirect allospecificity for an allopeptide prevented donor-specific skin allograft rejection mediated by naive effector CD4+ T cells. Our data thus suggest that in vitro–generated “customized” donor alloantigen-specific Treg cells could be used as a therapeutic tool to promote transplantation tolerance.
Materials and methods
Mice
CBA/Ca(H2k), C57BL/6(H2b), (CBA/Ca)×(C57BL/6)F1, BALB/c(H2d), B10.A(H2k+Dd) mice were purchased from Harlan Olac (Bicester, United Kingdom). CBK (CBA/Ca bearing a transgenic Kb molecule)34 mice were bred and maintained in the CBS facility of Hammersmith Campus (London, United Kingdom). Except for thymectomies in which mice between 3 and 6 weeks were used, all experimental procedures were performed on sex-matched mice aged 6 to 12 weeks. Procedures were performed in accordance with the 1986 Home Office Animals Scientific Procedures Act.
Purification of T-cell subsets
A single-cell suspension was obtained by passing spleens and lymph nodes (LNs) through 70-μm cell strainers, and erythrocytes were lysed by ACK buffer (0.15 M NH4Cl/1 mM KHCO3/0.1 mM Na2 EDTA). Cells were then incubated with supernatants of rat anti–mouse hybridoma cultures: anti–H2-Ek,d/Ab,d (M5/114.15.2, TIB-120; ATCC, Manassas, VA), anti-CD8 (YTS169; Therapeutic Immunology Group, Oxford, United Kingdom),35 anti-CD45R/B220 (RA3-3A1/6.1; TIB-146; ATCC), and anti-CD16/32 (2.4G2, HB-197; ATCC), followed by sheep anti–rat DynaBeads (Dynal, Wirral, United Kingdom) before separation in a magnetic field. The purity of the negatively selected CD4+ population was greater than 93% on flow cytometric analysis.
Naive CD4+ cells were selected by their CD45RB expression labeling with rat anti–mouse CD45RB–PE (16A; BD PharMingen, Oxford, United Kingdom) followed by anti–PE MicroBeads (Miltenyi Biotec, Surrey, United Kingdom). Cells were then loaded onto MACS columns (Miltenyi Biotec), and CD4+CD45RBhi retained cells were eluted out of the magnetic field. For selecting CD25+ and CD25− subsets, CD4+ cells were labeled with biotinylated anti-CD25 (7D4; BD PharMingen) followed by Streptavidin MicroBeads (Miltenyi Biotec) and were run through MACS columns. Positively selected CD4+CD25+ cells were greater than 95% pure on flow cytometric analysis.
Generation of dendritic cells
DCs were generated as described36 with some modifications. Fresh bone marrow (BM) cells flushed from femurs and tibias were passed through 70-μm cell strainers, and erythrocytes were lysed in ACK buffer. For higher purity, BM cells were incubated with supernatants from YTS169, YTS191 (Therapeutic Immunology Group),35 M5/114.15.2, and RA3-3A1/6.1 followed by sheep anti–rat DynaBeads (Dynal). After magnetic separation, cells were transferred to 6-well plates in RPMI 10% FCS with 20 ng/mL mouse recombinant granulocyte macrophage–colony-stimulating factor (GM-CSF; kind gift from GlaxoSmithKline, Stevenage, United Kingdom). On days 2 and 4, medium containing small nonadherent cells was removed and replaced with fresh GM-CSF–containing medium.
Cell culture and proliferation assays
All cell cultures were performed in RPMI 1640 (Sigma, Poole, United Kingdom) supplemented with 100 IU/mL penicillin (Gibco-BRL-Invitrogen, Paisley, United Kingdom), 100 μg/mL streptomycin (Gibco), 2 mM L-glutamine (Gibco), 0.01 M HEPES (Gibco), 50 μM 2β-mercaptoethanol (Gibco), and 10% heat-inactivated fetal calf serum (FCS; Seraq, Sussex, United Kingdom), incubated at 37°C in a humidified atmosphere with 5% CO2.
Proliferation assays were performed in triplicate in 96-well plates. Responder T cells (1 × 105 cells/well) were stimulated by allogeneic irradiated DCs (1:10 DC/T-cell ratio), polyclonally with 2 μg/mL soluble anti-CD3 (145-2C11; BD PharMingen), and syngeneic irradiated T cell–depleted splenocytes (antigen-presenting cells [APCs]) (1:1 APC/T cell ratio) or with 5 ng/mL phorbol 12-myristate 13-acetate (PMA; Sigma) and 0.5 μg/mL ionomycin (Sigma). Cell proliferation was measured by [3H]-thymidine incorporation (cpm) at 72 hours of culture. Results are presented as mean ± SD cpm values of triplicate wells.
CTLL-2 bioassay
CTLL-2 (TIB-214; ATCC), a murine cell line that proliferates in response to IL-2 and IL-4, was maintained in culture in RPMI 10% FCS with 10 U/mL recombinant human IL-2 (Boehringer, Mannheim, Germany). For the IL-2 secretion bioassay, 50 μL culture supernatant was harvested at 24 hours of proliferation assay and was plated with 5 × 103 CTLL-2 cells. [3H]-Thymidine incorporation was measured at 24 hours. Results are presented as mean ± SD cpm of triplicate cultures.
Generation of T-cell lines
CD4+CD25− or CD4+CD25+ T cells (2 × 106/mL) purified from CBA were cultured with irradiated immature syngeneic DCs pulsed with 5 μg/mL Kb peptide (sequence 54-68 QEGPEYWERETQKAK; Invitrogen),29 10 U/mL IL-2, and 10 ng/mL mouse IL-7 (R&D Systems, Minneapolis, MN). Cells were split as needed with weekly cycles of restimulation. All experiments were performed with cells obtained 6 to 8 days after restimulation.
Antibodies and flow cytometry
The following fluorochrome-conjugated mAbs and isotype controls were purchased from BD PharMingen: CD4(L3T4), CD25(PC61), CD69(H1.2F3), CD44(IM7), CD45RB(16A), CD62L(MEL-14), CTLA-4(UC10-4F10-11), and Vβ-TCR Kit. Purified anti–mouse GITR was purchased from R&D Systems, and anti–mouse CCR7 and anti–goat fluorochrome-conjugated secondary antibodies were purchased from Calbiochem (La Jolla, CA). Flow cytometric analysis was performed on FACSCalibur (Becton Dickinson, Mountain View, CA) using CellQuest software (Becton Dickinson). Anti–IL-10– (JES5-2A5; BD PharMingen) and anti–TGF-β1,2,3 (1D11; R&D Systems)–purified antibodies were used in blocking experiments.
ELISA
Cytokine levels in supernatants were detected by 2-site sandwich ELISA using BD PharMingen (IFN-γ, IL-10) or R&D System antibody pairs (TGF-β). Samples were assayed in duplicate and were quantitated by comparison with standard curves obtained with purified recombinant cytokines. For TGF-β, samples and controls were acidified for 10 minutes with 1 M HCl and then were neutralized with 1.2 M NaOH/0.5 M HEPES before they were added to ELISA plates. Results are presented as the means of duplicates.
Intracellular cytokine staining
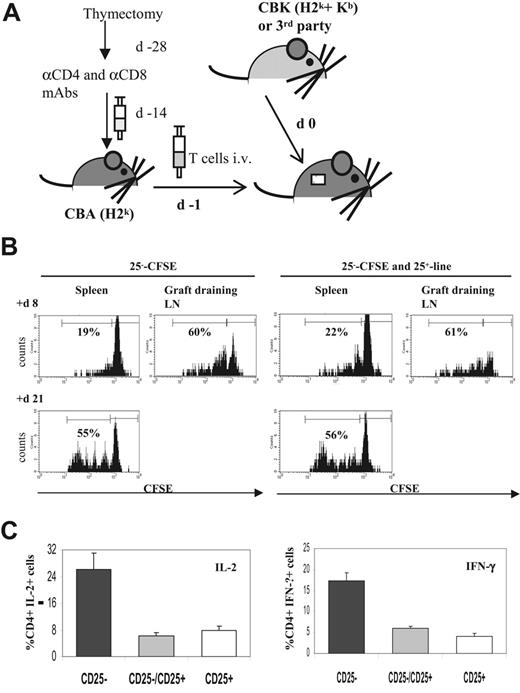
For each animal, cells were pooled from spleen and graft-draining LNs, resuspended at 4 × 106cells/mL in RPMI 10% FCS, restimulated with 50 ng/mL PMA and 0.5 μg/mL ionomycin for 4 hours in the presence of 10 μg/mL Brefeldin A (Sigma), harvested, surface stained, fixed for 10 minutes in ice-cold 4% paraformaldehyde (Sigma), washed, and permeabilized with 0.5% saponin (Sigma) in PBS/2% FCS/0.1% Na azide before intracellular staining. Flow cytometric analysis was performed within 6 hours. IL-2 (JES6-5H4), IFN-γ (XMG1.2), and IL-10 (JES5-16E3) PE-conjugated mAbs were purchased from BD PharMingen with isotype controls.
CFSE labeling
Individual cell proliferation was measured by serial dilution of the intracellular dye CFSE, as described.37 In brief, after 2 washes in PBS, 10 × 106 cells were incubated with 5 μM CFSE (Molecular Probes, Leiden, The Netherlands) diluted in PBS for 10 minutes at 37°C followed by washing with ice-cold PBS and 10% FCS.
RT-PCR analysis
Total RNA was extracted with the RNeasy Kit (Qiagen, Valencia, CA) from 5 × 106 snap-frozen cell pellets kept at −80°C. cDNA was reverse transcribed from 5 μL RNA using reverse transcriptase and oligo-dT in 20 μL final volume. PCR reactions were carried out using 1 μL cDNA template, 30 cycles with 57°C annealing. The following primers were used: Foxp3 sense, GGCCCTTCTCCAGGACAGA; Foxp3 antisense, GAAGAGTGTTGGTCCGGTGAAC; β-actin sense, CCAGCCTTCCTTCTTGGGTA; β-actin antisense, CTAGAAGCACTTGCGGTGCA (Sigma-Genosys; Sigma-Aldrich, Cambridge, United Kingdom).
Skin digestion
Full-thickness skin grafts and control tail skins were cut into fragments and digested 2 × for 30 minutes each at 37°C using 100 U/mL collagenase (Sigma). The obtained cells were washed and layered on mouse Lympho-Sep separation Media (Harlan Sera-Lab, Loughborough, United Kingdom). The lymphocyte-enriched population was recovered after centrifugation and was analyzed by flow cytometry.
Thymectomy and skin grafting
For thymectomy, an incision was made on the anterior part of the neck, and the thymus was removed as 2 intact lobes by application of negative pressure through a glass pipette inserted in the anterior mediastinum. For skin transplantation, full-thickness donor tail skin was grafted on beds prepared on the lateral flanks of recipients. Graft sites were protected under sterile gauze covered by plaster removed on day 10. Grafts were observed daily afterward and were considered rejected when no viable skin remained. Graft survival between 2 groups was compared with the log-rank test.
In vivo T-cell depletion and adoptive transfer
Two weeks after thymectomy, CD4+ and CD8+ cells were depleted in vivo by intraperitoneal injection of YTS191 (2 × 500 μg/mouse) and YTS169 (2 × 250 μg/mouse) purified from hybridoma culture supernatants. Fourteen days later, mice underwent reconstitution with the intravenous injection of T cells, and transplantation was performed the next day.
Production of retroviral supernatants and retroviral transduction
Retroviral supernatants were obtained 24 hours after transfection of pMIGR-GFP into Pheonix-Eco packaging cell lines with a calcium-phosphate transfection system (Invitrogen) and were stored at −80°C. The transduction of CD25+ lines was performed 48 hours after restimulation with Kb-peptide–pulsed CBA DCs. Cells were transferred to 24-well plates in RPMI 10% FCS, 10 U/mL IL-2, and 4 μg/mL polybrene (Sigma), and a 50% final dilution of viral supernatant was added. Plates were spun for 20 minutes at 450g at RT. This was followed by 36-hour culture to allow infection to occur, and then the viral supernatant was washed away. The cells were further cultured for 3 days in IL-2–supplemented medium. GFP+ cells were sorted by FACS and expanded as described.
Results
Induction and characterization of murine CD4+CD25+ regulatory T-cell lines with indirect allospecificity
Previously, we showed that human Treg cells with indirect allospecificity for an allopeptide could be generated by stimulating purified CD4+CD25+ T cells from peripheral blood with autologous monocyte-derived DCs pulsed with the peptide.38 To extend this study in vivo, we purified CD4+CD25+ and CD4+CD25− T cells from spleen and LNs of CBA mice (H2k). Expression of CD25, CD45RB, and CD62L on the isolated cells is shown in Figure 1A. We then stimulated the CD4+CD25+ and CD4+CD25− T cells with autologous BM-derived DCs pulsed with a 15-amino acid immunodominant peptide of the major histocompatibility complex (MHC) class I Kb molecule.29 CD4+CD25+ (CD25+ line) and CD4+CD25− (CD25− line) T-cell lines were maintained in culture by weekly restimulation with the peptide-pulsed irradiated DCs in the presence of 10 U/mL exogenous IL-2. The CD25+ line could be expanded up to 5-fold weekly and maintained for up to 1 year in continuous culture.
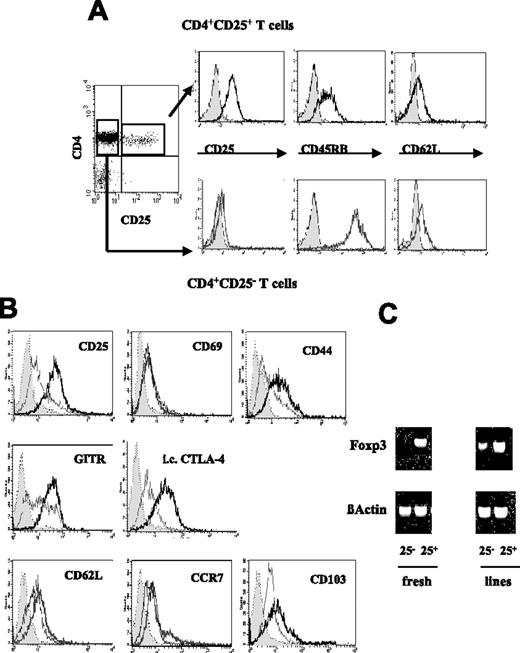
Phenotype of in vitro–expanded CD4+CD25+ and CD4+CD25− T-cell lines. CD4+ T cells were isolated from spleens and pooled LNs of nonmanipulated CBA mice and separated in CD25+ and CD25− subsets. (A) Representative flow cytometry histograms showing the level of surface expression of CD25, CD45RB, and CD62L on the selected cells used to generate the T-cell lines. (B) Surface expression of CD25, CD69, CD44, GITR, CD62L, CCR7, CD103, and intracellular expression of CTLA-4 was compared on the in vitro–expanded CD25− (gray line) and CD25+ line (black line) with the respective isotype controls (gray area). (C) Foxp3 mRNA was detected by RT-PCR in freshly isolated CD4+CD25− and CD4+CD25+ T cells and in the expanded CD25− and CD25+ line. The amount of β-actin mRNA was used as control. The phenotypic characterization of the T-cell lines was carried out more than 6 days after restimulation (ie, in the resting phase). Results are representative of 4 independent experiments.
Phenotype of in vitro–expanded CD4+CD25+ and CD4+CD25− T-cell lines. CD4+ T cells were isolated from spleens and pooled LNs of nonmanipulated CBA mice and separated in CD25+ and CD25− subsets. (A) Representative flow cytometry histograms showing the level of surface expression of CD25, CD45RB, and CD62L on the selected cells used to generate the T-cell lines. (B) Surface expression of CD25, CD69, CD44, GITR, CD62L, CCR7, CD103, and intracellular expression of CTLA-4 was compared on the in vitro–expanded CD25− (gray line) and CD25+ line (black line) with the respective isotype controls (gray area). (C) Foxp3 mRNA was detected by RT-PCR in freshly isolated CD4+CD25− and CD4+CD25+ T cells and in the expanded CD25− and CD25+ line. The amount of β-actin mRNA was used as control. The phenotypic characterization of the T-cell lines was carried out more than 6 days after restimulation (ie, in the resting phase). Results are representative of 4 independent experiments.
The phenotype of the expanded CD25+ line was analyzed by flow cytometry and compared with that of the CD25− line and freshly isolated Treg cells. As shown in Figure 1B, the CD25+ line retained a high level of expression of CD25, GITR, and the inhibitory molecule CTLA-4, comparable to fresh Treg cells. Although these molecules were also induced on the CD25− line, levels of expression remained higher on the CD25+ line. The CD25+ line also expressed high levels of CD62L, the chemokine receptor CCR7, and CD103 (αEβ7-integrin).39-41
We next used RT-PCR to examine the expression of transcription factor Foxp3 on these cells, which has been described as a master gene for control of the development and function of naturally occurring Treg cells.42 As shown in Figure 1C, the cultured CD25+ line expressed a similar level of Foxp3 mRNA compared with freshly isolated Treg cells. Foxp3 mRNA could not be detected on fresh CD4+CD25− T cells but was induced in the CD25− line, albeit at a significantly lower level compared with the CD25+ line. This could reflect the existence of a small population of cells with regulatory potential within the CD4+CD25− subset that could be expanded by repeated stimulation with immature DCs.43,44 Furthermore, a study by Hori et al42 has shown that a small population within the CD4+CD25− subset (mainly the CD45RBlo fraction) expresses Foxp3 at a low level. Although it is less potent than its CD4+CD25+-Foxp3+ counterpart, this population was shown to have suppressive properties in vivo in experimental models of colitis and transplantation.45,46
Expanded Treg cells retained their suppressive properties in vitro
We next addressed whether in vitro–expanded Treg cells could retain their suppressive functions, a key question. As shown in Figure 2A, the CD25+ line remained hypoproliferative in response to TCR polyclonal activation and suppressed proliferation (Figure 2A), IL-2 production, and IFN-γ production (Figures 2B-C, respectively) by cocultured freshly isolated CD4+CD25− T cells. Suppression was dose dependent, and the CD25+ line became more potent than freshly isolated Treg cells on a cell per cell basis, causing 90% inhibition of proliferation at a 1:8 CD25+/CD25− cell ratio. As seen with fresh Treg cells,11 the anergic state and the suppressive function of the CD25+ line could be abolished by the addition of exogenous IL-2 and soluble anti-CD28 antibody (Figure 2D) and by stimulation with PMA and ionomycin (data not shown).
Expanded CD4+CD25+ T cells become more potent suppressors than fresh Treg cells while remaining anergic in vitro. Freshly isolated CD4+CD25− responder T cells (1 × 105; CD25−) were cultured alone, with 1:1 freshly isolated CD4+CD25− (25−/25−), with decreasing ratios (25−/25+; ratios 1:1, 1:0.5, 1:0.25) of freshly isolated CD4+CD25+ T cells (⊡), or with CD25+ line cells (▪) in the presence of soluble anti-CD3 and 1 × 105 APCs. (A) T-cell proliferation was measured after 3 days of culture. (B-C) Supernatants were collected at days 1 and 2 of cocultures for the determination of IL-2, as measured by CTLL-2 proliferation (B) and IFN-γ by ELISA (C). (D) Freshly isolated CD4+CD25− T cells and CD25+ line (25+) (1 × 105) (25−) were cultured alone or were cocultured at a 1:1 ratio in the presence of soluble anti-CD3 antibody and APCs (□). To reverse suppression, 10 U/mL IL-2 (⊡) or 20 μg/mL purified anti-CD28 antibody (▪) was added to the cultures, and T-cell proliferation was measured at 72 hours. Results are representative of 4 independent experiments. Error bars reflect mean ± SD of triplicate cultures.
Expanded CD4+CD25+ T cells become more potent suppressors than fresh Treg cells while remaining anergic in vitro. Freshly isolated CD4+CD25− responder T cells (1 × 105; CD25−) were cultured alone, with 1:1 freshly isolated CD4+CD25− (25−/25−), with decreasing ratios (25−/25+; ratios 1:1, 1:0.5, 1:0.25) of freshly isolated CD4+CD25+ T cells (⊡), or with CD25+ line cells (▪) in the presence of soluble anti-CD3 and 1 × 105 APCs. (A) T-cell proliferation was measured after 3 days of culture. (B-C) Supernatants were collected at days 1 and 2 of cocultures for the determination of IL-2, as measured by CTLL-2 proliferation (B) and IFN-γ by ELISA (C). (D) Freshly isolated CD4+CD25− T cells and CD25+ line (25+) (1 × 105) (25−) were cultured alone or were cocultured at a 1:1 ratio in the presence of soluble anti-CD3 antibody and APCs (□). To reverse suppression, 10 U/mL IL-2 (⊡) or 20 μg/mL purified anti-CD28 antibody (▪) was added to the cultures, and T-cell proliferation was measured at 72 hours. Results are representative of 4 independent experiments. Error bars reflect mean ± SD of triplicate cultures.
To determine whether the suppressive function of the CD25+ line was mediated by secreted soluble cytokines such as IL-10 or TGF-β, a transwell coculture was grown. As shown in Figure 3A, proliferation was suppressed only when the CD25+ line and the CD25− line were cocultured but not when both subsets were separated by a semipermeable membrane, indicating that the suppression required cell–cell contact. Furthermore, in response to TCR stimulation, no significant amount of IL-10 or active TGF-β was detected in the supernatants of the CD25+ line cultured alone or in CD25+/CD25− cocultures, as measured by ELISA (data not shown). We also observed that the addition of neutralizing antibodies against these cytokines had no effect (Figure 3B), suggesting that IL-10 and TGF-β were not involved in the suppression by CD25+ line.
Suppressive function of the CD25+ line is mainly cell contact mediated in vitro. (A) Freshly isolated CD4+CD25− T cells (25−) and CD25+ line (25+) (5 × 105) were cultured alone or were cocultured at a 1:1 ratio for 3 days with soluble anti-CD3 and 5 × 105 APCs in 24-well plates. When cocultured, the T cells either were in contact in the same well (25− and (25+)) or were separated by a transwell membrane with the CD25+ line in the upper well (25− and 25+), in which case APCs were put in both the upper and the lower wells. At day 3, [3H]-thymidine was added to the bottom wells to measure proliferation. (B) Freshly isolated CD4+CD25− T cells (25−) and CD25+ line (25+) (1 × 105) were cultured for 3 days alone or were cocultured at a 1:1 ratio in the presence of soluble anti-CD3 antibody and APCs (□). Anti–TGF-β–purified (⊡) or anti–IL-10–purified (▪) blocking antibodies were added to the cultures at 20 μg/mL. Results are representative of 3 independent experiments. Error bars reflect mean ± SD of triplicate cultures.
Suppressive function of the CD25+ line is mainly cell contact mediated in vitro. (A) Freshly isolated CD4+CD25− T cells (25−) and CD25+ line (25+) (5 × 105) were cultured alone or were cocultured at a 1:1 ratio for 3 days with soluble anti-CD3 and 5 × 105 APCs in 24-well plates. When cocultured, the T cells either were in contact in the same well (25− and (25+)) or were separated by a transwell membrane with the CD25+ line in the upper well (25− and 25+), in which case APCs were put in both the upper and the lower wells. At day 3, [3H]-thymidine was added to the bottom wells to measure proliferation. (B) Freshly isolated CD4+CD25− T cells (25−) and CD25+ line (25+) (1 × 105) were cultured for 3 days alone or were cocultured at a 1:1 ratio in the presence of soluble anti-CD3 antibody and APCs (□). Anti–TGF-β–purified (⊡) or anti–IL-10–purified (▪) blocking antibodies were added to the cultures at 20 μg/mL. Results are representative of 3 independent experiments. Error bars reflect mean ± SD of triplicate cultures.
CD25+ line has antigen specificity for the Kb peptide
It has been suggested that, like CD4+CD25− T cells, naturally occurring Treg cells can recognize a broad variety of antigens.47 To investigate the specificity of the CD25+ line for the Kb allopeptide, we first analyzed T-cell receptor (TCR) Vβ usage by flow cytometry. Freshly isolated Treg cells displayed heterogeneous Vβ usage, indicating that they are polyclonal. In comparison, the CD25+ line showed a more limited Vβ repertoire (Figure 4A), indicating that the CD25+ line became oligoclonal during prolonged culture. We next addressed whether the CD25+ line could suppress antigen-specific T-cell responses. The CD25+ and CD25− lines were cocultured at various ratios and stimulated with autologous mature DCs pulsed with Kb peptide. As shown in Figure 4B, the CD25+ line suppressed the proliferation of effector CD4+CD25− T cells specific for the Kb peptide. Similar suppression was observed when the CD25+ line was cocultured with CD4+CD25− T cells isolated from CBA(H2k) mice, which had rejected a CBK(H2k+Kb) skin graft (data not shown). It has been shown that naive CD4+ T cells give a measurable indirect response to MHC class I alloantigens.48 By taking advantage of this observation, the CD25+ line suppressed the proliferation of naive CD4+CD25− T cells when stimulated by CBK DCs but not by third-party B10.A(H2k+Dd) DCs (Figure 4C). Taken together, these data imply that the CD25+ line has specificity for the Kb peptide.
Antigen specificity of the in vitro expanded Treg cells. (A) Vβ TCR-usage of the alloantigen-stimulated Treg cells. Fresh CD4+CD25+ T cells (⊡) and CD25+ line (▪) were stained with 15 different FITC-labeled anti–TCR Vβ mAbs and analyzed by flow cytometry. (B) CD25− line (1 × 105; 25−) were cultured for 3 days alone, with 1:1 CD25− line (25−/25−), with decreasing ratios (25−/25+; ratios, 1:1, 1:0.5, 1:0.25) of CD25+ line, in the presence of syngeneic irradiated DCs pulsed with Kb peptide. (C) Freshly isolated CD4+CD25− responder (25−) or CD25+ line cells (1 × 105) were cultured alone or were cocultured 1:1 for 4 days in the presence of soluble anti-CD3 antibody and APCs (□), irradiated mature syngeneic CBA DCs (H2k) (⊡), CBK DCs (H2k+Kb) (▪), or B10.A DCs (H2k+Dd) (). Results are representative of 3 independent experiments. Error bars reflect mean ± SD of triplicate cultures.
Antigen specificity of the in vitro expanded Treg cells. (A) Vβ TCR-usage of the alloantigen-stimulated Treg cells. Fresh CD4+CD25+ T cells (⊡) and CD25+ line (▪) were stained with 15 different FITC-labeled anti–TCR Vβ mAbs and analyzed by flow cytometry. (B) CD25− line (1 × 105; 25−) were cultured for 3 days alone, with 1:1 CD25− line (25−/25−), with decreasing ratios (25−/25+; ratios, 1:1, 1:0.5, 1:0.25) of CD25+ line, in the presence of syngeneic irradiated DCs pulsed with Kb peptide. (C) Freshly isolated CD4+CD25− responder (25−) or CD25+ line cells (1 × 105) were cultured alone or were cocultured 1:1 for 4 days in the presence of soluble anti-CD3 antibody and APCs (□), irradiated mature syngeneic CBA DCs (H2k) (⊡), CBK DCs (H2k+Kb) (▪), or B10.A DCs (H2k+Dd) (). Results are representative of 3 independent experiments. Error bars reflect mean ± SD of triplicate cultures.
In vivo suppressive function of the CD25+ line
To study the in vivo suppressive properties of the CD25+ line, we transferred a T-cell adoptive model into syngeneic T cell–depleted CBA mice carrying CBK skin allografts, as illustrated in Figure 5A. When compared with the transfer of CFSE-labeled naive CD4+CD25− T cells alone, cotransfer of the CD25+ line had no effect on cell division and homing of CD4+CD25− T cells in response to the CBK skin allograft (Figure 5B). We next measured cytokine production by alloreactive CD4+CD25− T cells at different time points after CBK skin graft. At day 30, cell suspensions were obtained from spleen and graft-draining LNs of T cell–depleted CBA mice that underwent transfer with CD4+CD25− T cells alone, CD4+CD25− T cells cotransferred with the CD25+ line, or only the CD25+ line. These cells were briefly restimulated in vitro, and cytokine production was measured by intracellular flow cytometric analysis (Figure 5C). In recipients that underwent transfer with naive CD4+CD25− T cells alone, 26.1% ± 4.9% CD4+ T cells produced IL-2, and 17.4% ± 1.9% CD4+ T cells produced IFN-γ. At the same time point after transplantation, the production of these TH1 cytokines was significantly lower in recipients that underwent cotransfer with the CD25+ line (6.3% ± 1.1% for IL-2, 5.9% ± 0.5% for IFN-γ) or transfer with the CD25+ line only (8% ± 1% for IL-2, 4.2% ± 0.5% for IFN-γ). These results indicated that the CD25+ line primarily affected the effector function of alloreactive CD4+CD25− T cells rather than their proliferation in response to allograft.
In vivo suppressive function of the Kb-specific CD25+ line. (A) In vivo adoptive transfer of T cells and transplant model. T-cell subsets were transferred to T cell–depleted sex-matched syngeneic CBA recipients. For T-cell depletion, mice underwent thymectomy and 2 weeks later were given intraperitoneal injections of anti-CD4– and anti-CD8–depleting mAbs. Fifteen days later, CD4+CD25− CD45RBhi T cells were injected intravenously alone or with CD25+ line 1 day before (d −1) recipient mice underwent skin allograft (d 0). (B) In vivo trafficking. Naive CFSE-labeled CD4+CD25− T cells (25−-CFSE) (1 × 106) were injected intravenously alone or were coinjected with 2 × 106 unlabeled CD25+ line or 2 × 106 unlabeled CD25+ line injected alone in T cell–depleted CBA mice the day before CBK skin grafts. Trafficking and divisions of the transferred effector T cells were assessed for individual animals by flow cytometric analysis of CFSE dilutions on separate cell suspensions from spleen and graft-draining LNs at various time points after transplantation (here shown at days 8 and 21). The histograms correspond to a minimum of 30 000 acquired events in a mixed lymphocyte gate consisting of live CD4+ T cells. Data shown are from 1 of 5 representative mice per group. (C) At day 30 after transplantation, cells were isolated from spleens and graft-draining LNs of recipients in the 3 groups: CD4+CD25− T cells injected alone (▪), CD4+CD25− T cells coinjected with CD25+ line (⊡), or CD25+ line injected alone (□). Cells were briefly restimulated in vitro before intracellular staining for the indicated cytokine (IL-2, left panel; IFN-γ, right panel). The frequency of cytokine-positive cells among gated whole CD4+ T cells was determined for individual animals. Results shown are mean ± SD from 4 representative animals per group.
In vivo suppressive function of the Kb-specific CD25+ line. (A) In vivo adoptive transfer of T cells and transplant model. T-cell subsets were transferred to T cell–depleted sex-matched syngeneic CBA recipients. For T-cell depletion, mice underwent thymectomy and 2 weeks later were given intraperitoneal injections of anti-CD4– and anti-CD8–depleting mAbs. Fifteen days later, CD4+CD25− CD45RBhi T cells were injected intravenously alone or with CD25+ line 1 day before (d −1) recipient mice underwent skin allograft (d 0). (B) In vivo trafficking. Naive CFSE-labeled CD4+CD25− T cells (25−-CFSE) (1 × 106) were injected intravenously alone or were coinjected with 2 × 106 unlabeled CD25+ line or 2 × 106 unlabeled CD25+ line injected alone in T cell–depleted CBA mice the day before CBK skin grafts. Trafficking and divisions of the transferred effector T cells were assessed for individual animals by flow cytometric analysis of CFSE dilutions on separate cell suspensions from spleen and graft-draining LNs at various time points after transplantation (here shown at days 8 and 21). The histograms correspond to a minimum of 30 000 acquired events in a mixed lymphocyte gate consisting of live CD4+ T cells. Data shown are from 1 of 5 representative mice per group. (C) At day 30 after transplantation, cells were isolated from spleens and graft-draining LNs of recipients in the 3 groups: CD4+CD25− T cells injected alone (▪), CD4+CD25− T cells coinjected with CD25+ line (⊡), or CD25+ line injected alone (□). Cells were briefly restimulated in vitro before intracellular staining for the indicated cytokine (IL-2, left panel; IFN-γ, right panel). The frequency of cytokine-positive cells among gated whole CD4+ T cells was determined for individual animals. Results shown are mean ± SD from 4 representative animals per group.
CD25+ line divides and migrates to the allograft draining LNs and within the graft after adoptive transfer in vivo
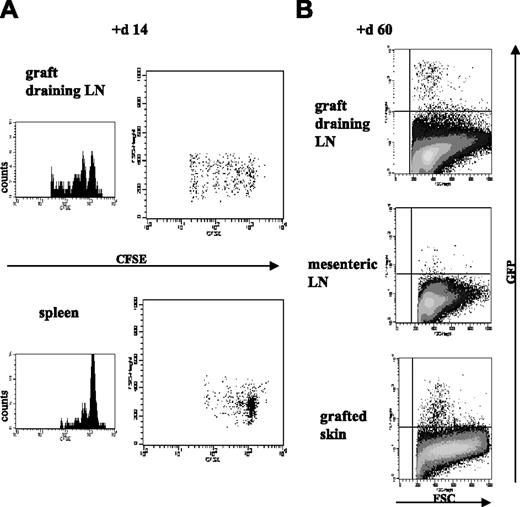
As shown in Figure 1, in vitro–expanded Treg cells maintained surface expression of CCR7, CD62L, and CD103. To investigate the in vivo trafficking of the in vitro–expanded Treg cells, CFSE-labeled CD25+ line cells were injected intravenously, either alone or with naive CD4+CD25− T cells, into T cell–depleted syngeneic CBA the day before the mice received CBK skin grafts. Survival and trafficking of the labeled CD25+ line in spleen, graft-draining LNs, and mesenteric LNs were analyzed at different time points after transplantation. As shown in Figure 6A, although the CD25+ line was hyporesponsive in vitro, the cells were able to survive and expand in vivo. Furthermore, they homed preferentially to spleen and graft-draining LNs, and few labeled CD25+ lines could be detected in distant mesenteric LNs. In addition, when comparing spleen and graft-draining LN-infiltrating cells at different time points, we observed that donor-specific Treg cells initially predominantly accumulated and divided in graft-draining LNs (Figure 6A), indicating a role for antigens in the local environment in the in vivo homeostasis and trafficking of antigen-specific Treg cells.
After adoptive transfer, in vitro–cultured Treg cells survive, traffic to secondary lymphoid organs, and preferentially expand at sites of antigenic challenge in vivo. Unlabeled naive CD4+CD25− T cells (1 × 106) were coinjected in T cell–depleted CBA mice with 2 × 106 CD25+ line labeled with 5 μM CFSE (A) or with 2 × 106 GFP-CD25+ line (B). The day after adoptive transfer, recipients received skin grafts from CBK donors. Survival, trafficking, and divisions of the transferred Treg cells were assessed for individual animals by flow cytometric analysis of separate cell suspensions from spleen, graft-draining LNs, and distant mesenteric LNs at various time points after transplantation—days 7, 14, and 21 for CFSE analysis (here shown at day 14) and days 20 and 60 for GFP analysis (here shown at day 60). For the animals that underwent cotransfer with the GFP-CD25+ line, cell suspensions were also obtained from the grafted tissue and distant nongrafted skin for flow cytometric analysis. Histograms correspond to a minimum of 30 000 acquired events in a mixed lymphocyte gate consisting of live CD4+ T cells. Data are shown from 1 of 5 representative mice per group.
After adoptive transfer, in vitro–cultured Treg cells survive, traffic to secondary lymphoid organs, and preferentially expand at sites of antigenic challenge in vivo. Unlabeled naive CD4+CD25− T cells (1 × 106) were coinjected in T cell–depleted CBA mice with 2 × 106 CD25+ line labeled with 5 μM CFSE (A) or with 2 × 106 GFP-CD25+ line (B). The day after adoptive transfer, recipients received skin grafts from CBK donors. Survival, trafficking, and divisions of the transferred Treg cells were assessed for individual animals by flow cytometric analysis of separate cell suspensions from spleen, graft-draining LNs, and distant mesenteric LNs at various time points after transplantation—days 7, 14, and 21 for CFSE analysis (here shown at day 14) and days 20 and 60 for GFP analysis (here shown at day 60). For the animals that underwent cotransfer with the GFP-CD25+ line, cell suspensions were also obtained from the grafted tissue and distant nongrafted skin for flow cytometric analysis. Histograms correspond to a minimum of 30 000 acquired events in a mixed lymphocyte gate consisting of live CD4+ T cells. Data are shown from 1 of 5 representative mice per group.
The use of the intracellular dye CFSE allowed the study of the trafficking of the CD25+ line only in the first weeks after transplantation. To investigate the long-term survival of these Treg cells in vivo, we used a GFP-labeled CD25+ line (GFP-CD25+ line). Cells from the established CD25+ line were transduced with a GFP-expressing retroviral vector. After transduction, GFP-positive cells were sorted by flow cytometry and expanded using the same protocol described. The GFP-CD25+ line retained its phenotypic and potent in vitro–suppressive properties (data not shown). The GFP-CD25+ line was cotransferred with naive CD4+CD25− T cells into T cell–depleted syngeneic CBA recipients carrying CBK skin grafts. We then used flow cytometry to examine GFP-positive cells in secondary lymphoid organs (graft-draining LNs, mesenteric LNs, spleen) and in the grafted tissue and distant nongrafted skin at different times after transplantation. As shown in Figure 6B, at day 60 after transplantation, the transferred allospecific Treg cells preferentially accumulated in the graft-draining LNs but not in mesenteric LNs, and they could be detected in significant numbers within the allograft itself. No GFP-labeled cells were detected in distant nongrafted skin (data not shown).
Expanded Treg cells induce donor-specific transplantation tolerance
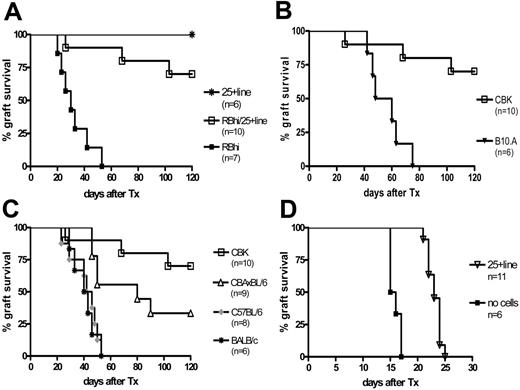
To address whether the Kb-specific CD25+ line could induce donor-specific transplantation tolerance, T cell–depleted CBA recipients received CBK skin grafts after thymectomy. One day before transplantation, naive CD4+CD25−CD45RBhi T cells were injected into syngeneic CBA recipients alone or with the CD25+ line at a 2:1 CD25+/RBhi cell ratio (Figure 5A). As shown in Figure 7A, all allografts were rejected in recipients that underwent transfer with CD4+CD25−CD45RBhi T cells (median survival time [MST], 30 days), and 7 of 10 CBK skin allografts (P < .001) were accepted in recipients that underwent cotransfer with CD4+CD25−CD45RBhi and the CD25+ line (graft survival, more than 120 days) in the absence of any added immunosuppression. One hundred fifty days after transplantation, skin grafts in these mice had grown hair, no retraction or necrotic zones developed, and results of histologic examination were normal (data not shown). When freshly isolated CD4+CD25+ T cells were coinjected with CD4+CD25−CD45RBhi T cells at a 2:1 CD25+/RBhi cell ratio, all mice rejected their grafts at a pace similar to that of mice receiving CD4+CD25−CD45RBhi T cells alone (data not shown).
In vitro–expanded Treg cells prevent CBK but not third-party skin allograft rejection mediated by naive CD4+CD25− T cells. (A) CD4+CD25−CD45RBhi T cells (0.5 × 106) (▪, n = 7) or CD25+ line (1 × 106) (*, n = 6) were injected alone or were coinjected (□, n = 10) in T cell–depleted CBA mice the day before the recipients received CBK skin grafts. (B-C) CD4+CD25−CD45RBhi T cells (0.5 × 106) and CD25+ line (1 × 106) were coinjected in T cell–depleted CBA mice the day before the recipients underwent CBK (□, n = 10; B-C) or B10.A (▾, n = 6; B), BALB/c (*, n = 6; C), C57BL/6 (♦, n = 8; C), or (CBA/Ca) × (C57BL/6)F1 (▵, n = 9; C) skin allograft. (D) Wild-type CBA recipients did not receive any cells but received control PBS intravenously (▪, n = 6), or they underwent transfer with 2 × 106 CD25+ line (▿, n = 11) the day before CBK skin graft.
In vitro–expanded Treg cells prevent CBK but not third-party skin allograft rejection mediated by naive CD4+CD25− T cells. (A) CD4+CD25−CD45RBhi T cells (0.5 × 106) (▪, n = 7) or CD25+ line (1 × 106) (*, n = 6) were injected alone or were coinjected (□, n = 10) in T cell–depleted CBA mice the day before the recipients received CBK skin grafts. (B-C) CD4+CD25−CD45RBhi T cells (0.5 × 106) and CD25+ line (1 × 106) were coinjected in T cell–depleted CBA mice the day before the recipients underwent CBK (□, n = 10; B-C) or B10.A (▾, n = 6; B), BALB/c (*, n = 6; C), C57BL/6 (♦, n = 8; C), or (CBA/Ca) × (C57BL/6)F1 (▵, n = 9; C) skin allograft. (D) Wild-type CBA recipients did not receive any cells but received control PBS intravenously (▪, n = 6), or they underwent transfer with 2 × 106 CD25+ line (▿, n = 11) the day before CBK skin graft.
To investigate whether the regulation mediated by the CD25+ line was donor specific, skin grafts from B10.A (H2k+Dd) (MHC class I mismatched), BALB/c (H2d), and C57BL/6 (H2b) (MHC I and II mismatched) mice were transplanted into CBA mice. The CD25+ line did not prevent the rejection of B10.A skin grafts mediated by CD4+CD25−CD45RBhi effector T cells (MST, 54 days) (Figure 7B). Similarly, as shown in Figure 7C, all BALB/c and C57BL/6 skin grafts were rejected (P = .001) at an MST of 41.5 and 44 days, respectively. Furthermore, the CD25+ line significantly prolonged (CBA/Ca) × (C57BL/6)F1 skin graft survival (MST, 80 days), and 3 of 9 recipients had healthy hair growth 120 days after transplantation, suggesting that the CD25+ line was capable of mediating “linked suppression” in vivo.27 Taken together, these results indicate that the CD25+ line expanded in vitro with Kb peptide–pulsed DCs could regulate the indirect pathway CD4+ T-cell alloresponse and a direct alloresponse through linked suppression in vivo and could induce donor-specific transplantation tolerance.
CD25+ line delays CBK skin graft rejection in wild-type CBA recipients in the absence of any other immunosuppression
Data presented in Figure 7A-C demonstrate that the antigen-expanded Treg cells were capable of inducing donor-specific transplantation tolerance in T cell–depleted recipients after thymectomy in which graft rejection was mediated by adoptive transfer of a limited number of effector CD4+ T cells. To test whether the in vitro–expanded Treg cells had the capacity to regulate alloresponses in a more clinically relevant model, we grafted CBK skin onto wild-type CBA mice. As shown in Figure 7D, Kb-specific CD25+ line cells delayed graft rejection in the absence of any other immunosuppression. MST of CBK skin grafts was 15.5 days in sex-matched wild-type CBA recipients. Transfer of 2 × 106 Kb-specific CD25+ line 1 day before transplantation prolonged CBK skin graft survival with an MST of 23 days (P < .001). These data suggest that in vitro–generated and –expanded alloantigen-specific Treg cells have the therapeutic potential to promote transplantation tolerance.
Discussion
A better understanding of the role of naturally occurring Treg cells in the maintenance of immune homeostasis has prompted researchers to investigate the potential of these regulatory T cells in the prevention or treatment of T cell–mediated diseases. For a successful therapeutic use of Treg cells in the induction of immune tolerance, antigen-specific Treg cells are needed that can specifically control autoimmune diseases or transplant rejection while allowing protective immune responses to pathogens. Furthermore, because of the low precursor frequency of alloantigen cross-reactive Treg cells expected to be found in a healthy population without previous antigen exposure, strategies are needed to expand this specific population in vivo or to generate and culture antigen-specific Treg cells in large number in vitro before adoptive transfer. Although recent data have highlighted the potential of immunotherapy using in vitro–expanded antigen-specific Treg cells in mouse models of graft-versus-host disease49-51 and diabetes,52-54 few data exist in the field of solid organ transplantation.55
In clinical transplantation, acute allograft rejection can usually be successfully prevented or treated with current immunosuppressive regimens, but inexorable late loss because of chronic rejection remains a serious threat. Clinical data have indicated that the indirect pathway of alloresponse is the main driver for chronic graft rejection.56-59 Thus, the control of CD4+ T cells with indirect antidonor allospecificity would help to achieve transplantation tolerance.60 Previously, we showed that alloreactive CD4+ T cells with indirect allospecificity for an HLA-A2 peptide could be generated from a patient who rejected an HLA-A2–mismatched transplant.61 Furthermore, we failed to detect the regulation of direct antidonor alloreactive CD4+ T cells,30 whereas depletion of CD4+CD25+ T cells revealed significant indirect antidonor alloresponses in a fraction of stable patients after renal transplantation,31 suggesting that Treg cells arise in some recipients with stable transplant function. To address this notion, we have recently shown that it is possible to generate CD4+CD25+ regulatory T-cell lines with indirect specificity for an HLA-A2 peptide from peripheral blood Treg cells.38 These cultured cells retained their potent suppressive functions in vitro. Here we extended our study in a mouse system and showed that Treg cells with indirect allospecificity for an MHC class I alloantigen can be selected from the pool of naturally occurring Treg cells and expanded in vitro in large numbers in the presence of IL-2 without modification of their characteristic phenotype and suppressive functions. We demonstrated that these donor-specific Treg cells regulated alloresponses of effector CD4+ T cells in vivo and that donor-specific transplantation tolerance could be achieved by adoptive transfer of these Treg cells in a T cell–depleted skin transplant model after thymectomy.
The need for in vitro–expanded Treg cells to be antigen specific for use in adoptive cell immunotherapy has been highlighted in transplantation models8 and by recent studies in experimental diabetes models.52-54 It has been reported that islet-specific Treg cells expanded from nonobese diabetic (NOD) mice were more efficient than polyclonal Treg cells in suppressing autoimmune diabetes. Transfer of a small number, such as 5000 expanded islet autoantigen-specific Treg cells, blocked autoimmunity caused by diabetogenic T cells in NOD mice, whereas injection of 105 polyclonal Treg cells had no effect. Treg cells specific for a single islet autoantigen could suppress diabetes caused by effector T cells reactive to a repertoire of autoantigens.53 In line with these data, Treg cells specific for a single MHC class I epitope effectively induced indefinite survival of skin grafts expressing the same alloantigens but not skin grafts carrying different alloantigens. These results indicate that in vitro–expanded allopeptide-specific Treg cells are capable of promoting donor-specific transplantation tolerance.
Consistent with previous in vitro analysis of the effector function of naturally occurring Treg cells, our data showed that the suppressive function of the CD25+ line was not mediated by secreted soluble inhibitory cytokines but was cell contact dependent. This also demonstrated that the in vitro expansion protocol had not modified the intrinsic properties of Treg cells and that the expanded CD25+ line must be distinguished from other described “induced” regulatory T cells, such as IL-10–producing Tr1 cells.43,44,62 In various models, Treg cells appeared to mediate immunoregulation through cell contact–dependent mechanisms, either by direct T-cell/T-cell interactions through surface molecules11,63 or by initial cognate interaction with the APCs.64 It was shown that coculture with activated Treg cells leads to reduced amounts of costimulatory molecules on DCs and B cells,65 inhibiting their immunogenic properties. We have also made similar observations with anergic T cells.66 “Tolerogenic” DCs would, in turn, induce other regulatory cells, thus contributing to the maintenance of tolerance in vivo.
One of the key findings of this report is the preserved in vivo homeostasis, trafficking pattern, and effector function of in vitro–cultured Treg cells. Although hyporesponsive to TCR activation in vitro, the transferred antigen-specific Treg cells survived and expanded in vivo in response to the allograft without losing their capacity to regulate the rejection process. Our study also highlighted less well-described in vivo properties of Treg cells. A defining feature of Treg cells is their ability to inhibit the proliferation of other T-cell populations in vitro by inducing cell cycle arrest rather than by preventing initial responder T-cell activation because the up-regulation of early activation markers is not affected.63 We did not observe any limitation of the proliferation and accumulation of effector T cells in the presence of Treg cells in vivo in response to the allograft, suggesting that Treg cells did not interfere with the induction of the immune response. However, when cotransferred with naive CD4+CD25− T cells, Treg cells inhibited downstream effector functions in response to alloantigens, as reflected by a lower production of TH1 cytokines. With the use of an adoptive transfer model of monospecific CD8+ T cells into tolerant skin graft recipients, Lin et al67 showed that Treg cells were able to censor CD8+ T cell–mediated graft rejection by hindering their cytotoxicity and IFN-γ production without modifying their expansion and survival. Together these data suggest that, in the presence of Treg cells, alloreactive T cells may persist but may be prevented from developing effector functions.
Another important finding is that, after adoptive transfer into allograft recipients, these Treg cells migrated appropriately and accumulated where the alloantigen was present. The characterization of the in vivo homing properties of in vitro–expanded cells has crucial implications for future clinical use of antigen-specific, in vitro–“customized” Treg cells in the prevention or treatment of organ-specific immune disorders. Indeed, it appears in various autoimmune diseases and transplantation models that the preferential homing of naturally occurring Treg cells is dictated by the sites at which antigen-induced inflammatory responses take place.9,25,26 It remains to be determined whether the long-term maintenance of immunologic tolerance is mediated by continuously expanding Treg cells in response to locally persistent specific antigens or if the induction of other regulatory cells or cytokines is in turn facilitated in the “regulated” local environment. Our data suggest that the maintenance of tolerance results, at least in part, from the expansion of donor-specific Treg cells and that the alloantigen plays an important role in this homeostasis.
The observation that in vitro–expanded donor-specific Treg cells were not functionally modified by the culture conditions, were able to induce dominant transplantation tolerance in a T cell–depleted skin graft model after thymectomy, and were capable of prolonging skin allograft survival in wild-type recipients in the absence of any other immunosuppression offers great hope and has important implications for clinical therapeutics. That Kb-specific Treg cells could not prolong C57BL/6 allograft survival in T cell–depleted CBA mice, combined with the data obtained with the wild-type CBA recipients, reflected the strength of the posttransplantation early direct pathway response against fully allogeneic cells, overwhelming the regulatory mechanisms. It has been shown that, after transplantation, the immunosuppressive agent rapamycin can significantly reduce the number of effector alloreactive T cells by deletion, leaving a small pool of residual alloreactive T cells that could be regulated by Treg cells.68 Furthermore, recent data have shown that rapamycin can selectively expand Treg cells in vitro and in vivo.69,70 Thus, the combination of short-term immunosuppression by rapamycin during the early phase after transplantation and adoptive transfer of in vitro–expanded alloantigen-specific Treg cells at the later stage could be a clinically applicable strategy to achieve donor-specific transplantation tolerance.5,60
In conclusion, we believe that the data presented here constitute an important step toward the clinical applicability of immunotherapy using in vitro–expanded antigen-specific Treg cells to induce donor-specific transplantation tolerance. The same strategies could be exploited in the treatment of autoimmune diseases in which the target self-antigens are known.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Conflict-of-interest disclosure: The authors declare no conflicting financial interests.
Contribution: D.G. designed and performed the research, collected and analyzed the data, and wrote the paper; S.J. designed and performed the research; J.T., M.I.G., and C.M. performed the research; and R.I.L. designed the research, analyzed the data, and wrote the paper.
Acknowledgments
This work was supported by the Swiss National Science Foundation (D.G., C.M.), the Medical Research Council (R.I.L.), and the British Heart Foundation (R.I.L., S.J.).


![Figure 3. Suppressive function of the CD25+ line is mainly cell contact mediated in vitro. (A) Freshly isolated CD4+CD25− T cells (25−) and CD25+ line (25+) (5 × 105) were cultured alone or were cocultured at a 1:1 ratio for 3 days with soluble anti-CD3 and 5 × 105 APCs in 24-well plates. When cocultured, the T cells either were in contact in the same well (25− and (25+)) or were separated by a transwell membrane with the CD25+ line in the upper well (25− and 25+), in which case APCs were put in both the upper and the lower wells. At day 3, [3H]-thymidine was added to the bottom wells to measure proliferation. (B) Freshly isolated CD4+CD25− T cells (25−) and CD25+ line (25+) (1 × 105) were cultured for 3 days alone or were cocultured at a 1:1 ratio in the presence of soluble anti-CD3 antibody and APCs (□). Anti–TGF-β–purified (⊡) or anti–IL-10–purified (▪) blocking antibodies were added to the cultures at 20 μg/mL. Results are representative of 3 independent experiments. Error bars reflect mean ± SD of triplicate cultures.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/109/2/10.1182_blood-2006-05-025460/4/m_zh80020706340003.jpeg?Expires=1763859355&Signature=qMahe0uW5qAZiQ9Lk6vwb0WXtvNA7Frm9E8WwEJFGT88p2SlUfUZpWGRWQjtuEL9EkN3BWOv3VoclnJmEZs-XY~0N8IgGufGLOzeCFZ8RBT-j5NdueH80cRG984DCyfFkjnvFVWibi2xVj-7yo2rKfUJRXiYz6Rafg-q7AlgYWdLne9lr4UC4i9C~JL6k0ZndUHx1iIttvqaji0zJ5N~doF4WAB56V6gpTfwlBg-NNPVvOMlvp2HTfXap2UAY5-masvf5Vor4hSqA72dPeStirPDlb4Lr82R6AWI-RMTEDHXi8ASuhcGoE4ajKmkivMEhe5EIPMpunPunr-Esxr7HA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)