Abstract
Membrane-bound receptors generate soluble ligand-binding domains either by proteolytic cleavage of the extracellular domain or alternative mRNA splicing yielding a secreted protein. Mertk (Mer) is in a receptor tyrosine kinase family with Axl and Tyro-3, and all 3 receptors share the Gas6 ligand. Mer regulates macrophage activation, promotes apoptotic cell engulfment, and supports platelet aggregation and clot stability in vivo. We have found that the membrane-bound Mer protein is cleaved in the extracellular domain via a metalloproteinase. The cleavage results in the production of a soluble Mer protein released in a constitutive manner from cultured cells. Significant amounts of the soluble Mer protein were also detected in human plasma, suggesting its physiologic relevance. Cleavage of Mer was enhanced by treatment with LPS and PMA and was specifically inhibited by a tumor necrosis factor α-converting enzyme metalloproteinase inhibitor. As a decoy receptor for Gas6, soluble Mer prevented Gas6-mediated stimulation of membrane-bound Mer. The inhibition of Gas6 activity by soluble Mer led to defective macrophage-mediated engulfment of apoptotic cells. Furthermore, soluble Mer decreased platelet aggregation in vitro and prevented fatal collagen/epinephrine-induced thromboembolism in mice, suggesting a potential therapeutic use for soluble Mer in the treatment of clotting disorders.
Introduction
The Mer (Mertk, Nyk, c-Eyk) receptor tyrosine kinase is a transmembrane receptor consisting of an extracellular domain with 2 immunoglobulin-like and 2 membrane proximal fibronectin III motifs, a transmembrane region, and an intracellular tyrosine kinase domain.1,2 These motifs place Mer in the same tyrosine kinase subfamily as Axl3 and Tyro-3/Sky.4,5 Mer, Axl, and Tyro-3 share the same ligand,6,7 Gas6, which has significant homology to the negative coregulator of the blood coagulation pathway protein S.8
Abnormal expression or activity of the Mer tyrosine kinase may play a role in tumorigenesis. The avian Mer ortholog, eyk, was discovered first as the oncogene in acute avian retrovirus RPL30. The constitutively active tyrosine kinase domain of v-eyk causes fibrosarcomas, endotheliomas, and visceral lymphomatosis in chickens.9,10 Overexpression of murine Mer tyrosine kinase transforms BaF3 lymphocytes11 and overexpression of human Mer transforms NIH3T3 cells.12 Several human cancers overexpress Mer, including mantle cell lymphomas,13 alveolar rhabdomyosarcomas,14 gastric cancer,15 and pituitary adenomas.16 Mer is also ectopically expressed in pediatric T-cell acute lymphoblastic leukemia,17 and a Mer transgenic mouse model with ectopic expression of Mer in thymocytes and lymphocytes develops T-cell lymphoblastic leukemia/lymphoma.18 Axl and Tyro-3 also transform cells in vitro3,4 and are overexpressed in a spectrum of human cancers.
In addition to abnormal function of Mer in cancer, a physiologic role for Mer has recently been described in macrophages. Mer, Axl, and Tyro-3 have been shown to limit the extent of macrophage activation in response to an immune stimulus.19,20 Mer also plays a significant role in the ability of macrophages to clear apoptotic cells,21 and Mer deficiency has been linked to the development of autoimmune disorders in mice. Lack of Mer receptor causing defective macrophage apoptotic cell clearance in the Royal College of Surgeons (RCS) rat has also been implicated in the development of retinitis pigmentosa.22 Interestingly, Mer gene mutations have been defined in a subset of humans with retinitis pigmentosa.23
Furthermore, a physiologic role for the Mer tyrosine kinase has been described for the normal function of platelets. The interaction of Gas6 with Mer, Axl, and Tyro-3 is important in platelet degranulation and aggregation in response to known agonists. Mice lacking either Gas6 or Mer protein have impaired platelet aggregation in vitro and diminished clot stability in vivo.24-26
A delicate balance of ligand interaction with a tyrosine kinase receptor is necessary to maintain normal tyrosine kinase function without causing overactivation, which could result in human disease. One means of regulating tyrosine kinase activation is through proteolytic cleavage of the membrane-bound protein. Through this process, the total number of membrane-bound receptors is reduced. In addition, the soluble cleavage product may function as a decoy receptor and sequester ligand.27 In this study, we show that the Mer receptor tyrosine kinase can be cleaved by a metalloproteinase and that this cleavage results in the release of a soluble Mer (sMer) protein. The production of sMer is significantly increased in response to stimuli such as LPS or PMA. The shed Mer extracellular domain can bind Gas6 and block Gas6-mediated Mer activation. This mechanism of regulating Mer inhibits receptor function in apoptotic cell engulfment and platelet aggregation.
Materials and methods
Animals
Wild-type (C57BL/6J) mice were obtained from Jackson Laboratories (Bar Harbor, ME) and Mer kinase dead (MerKD) mice were a gift from Drs Glenn Matsushima and H. S. Earp (University of North Carolina, Chapel Hill, NC). The care of animals and experimental procedures were in accordance with the guidelines of the University of Colorado Center for Comparative Medicine.
Cell lines
U937, Jurkat Eb-1, HSB-2, K562, and HEK 293 human cell lines and the J774A.1 mouse monocytic cell line were obtained from the American Type Culture Collection (Manassas, VA). Cells were maintained in either RPMI 1640 (U937 and Jurkat), Iscove modified Eagle medium (K562 and HSB-2), or DMEM (J774 and HEK293). All growth media were supplemented with 10% heat-inactivated fetal calf serum (FCS; Atlanta Biologicals, Norcross, GA), 100 U/mL penicillin, 100 U/mL streptomycin, and 2 mM l-glutamine. Lung microvascular endothelial cells (MVECs) were obtained from Cambrex (Walkersville, MD) and grown in EGM-2MV medium (Cambrex).
Antibodies
A monoclonal antibody directed against human Mer extracellular domain was obtained from Caveo Therapeutics (Aurora, CO). A polyclonal antibody against mouse Mer extracellular domain and an antibody against mouse Gas6 were purchased from R&D Systems (Minneapolis, MN). Antibodies from FabGennix (Frisco, TX) were used to detect phosphorylated Mer and the carboxyl terminal domain of Mer. An antibody against p53 was obtained from Novocastra Laboratories (Norwell, MA), and anti-AKT and antiphospho-AKT antibodies were from Cell Signaling Technology (Danvers, MA).
Analysis of Mer protein in cell lysates and conditioned media
To detect constitutively shed Mer ectodomain, J774, HEK293, and A549 cells and MVECs seeded on 10-cm plates were grown to 80% confluence and then placed in serum-free medium. Similarly, cells growing in suspension culture (U937, Jurkat, HSB-2, and K562) were resuspended in serum-free medium at 3 × 106 cells/mL. After 24 hours conditioned media (CM) from the cell cultures was harvested and concentrated 10-fold using a centrifugal filter device (Millipore, Bedford, MA). Aliquots of concentrated CM were digested with 5 U protein N-glycosidase F (PNGase F; New England Biolabs, Ipswich, MA) for 2 hours at 37°C. To prepare cell extracts, cells were washed once in PBS and then resuspended in Mer lysis buffer consisting of 50 mM HEPES, pH7.5, 150 mM NaCl, 10 mM EDTA, 10% glycerol, and 1% Triton X-100 supplemented with 0.1 mM sodium molybdate, 1 mM sodium orthovanadate, and a protease inhibitor cocktail (Roche Diagnostics, Indianapolis, IN). Nuclei and membranes were removed by centrifugation. Cleared cell lysates and CM samples were analyzed by sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE) and immunoblotting.
Human and mouse tissues and primary cells
Mouse serum was prepared from blood collected from the tail vein. To elicit accumulation of macrophages in the peritoneal cavity, mice were given intraperitoneal injections of 1 mL 3% thioglycollate (Sigma-Aldrich, St Louis, MO). Three days after thioglycollate injection, peritoneal macrophages were recovered by lavage. Mouse splenocytes were obtained by passing spleens through a 100-μm cell strainer to yield a single-cell suspension. To analyze membrane-bound and shed Mer, macrophages and splenocytes were resuspended in DMEM without serum and incubated at 37°C for 7 hours. CM was harvested and concentrated, and cells were lysed in Mer lysis buffer.
Human monocytes were isolated from peripheral blood by Percoll-gradient centrifugation followed by adherence to tissue culture-treated plates for 1.5 hours under serum-free conditions. Nonadherent cells were washed off and monocytes were cultured in X-VIVO 10 medium (Cambrex) supplemented with 10% pooled human serum for 7 days to allow differentiation into macrophages. The medium was then removed and replaced with X-VIVO medium without serum. Cells were incubated at 37°C overnight, and CM and cells were analyzed by immunoblotting.
Modifying ectodomain shedding with PMA, LPS, and TAPI-0
J774 cells grown to 80% confluency were placed in DMEM lacking serum. Cells were treated with 50 nM PMA (Sigma-Aldrich) for up to 90 minutes or incubated with 50 ng/mL LPS (Sigma-Aldrich) for up to 6 hours. To inhibit metalloproteinases 5 mM EDTA or 200 μM tumor necrosis factor α (TNF-α) protease inhibitor-0 (TAPI-0; Peptides International, Louisville, KY) was added along with LPS. To investigate the dependence of sMer release on protein synthesis, J774 cells were cultured in the presence of 10 μg/mL cycloheximide (Sigma-Aldrich) for 30 minutes and then incubated in LPS and 10 μg/mL cycloheximide. Cell extracts and CM after each treatment were analyzed by immunoblotting.
For analysis of the Mer carboxyl-terminal cleavage product, elicited peritoneal macrophages from wild-type and MerKD mice were plated at 2.5 × 106 cells/well in 12-well plates and cultured overnight in DMEM containing 1% FCS. Medium was removed and replaced with DMEM without serum and LPS was added to a final concentration of 100 ng/mL. After incubation at 37°C for 5 to 90 minutes, cell lysates were prepared.
Flow cytometry
Surface Mer expression was assessed by staining J774 cells with anti–mouse Mer antibody. J774 cells were blocked in PBS containing 5% FCS for 5 minutes and then stained with anti–mouse Mer antibody for 30 minutes at 4°C. Cells were washed with 2% FCS in PBS followed by incubation with phycoerythrin-conjugated donkey antigoat secondary antibody (Jackson ImmunoResearch, West Grove, PA). Cells were analyzed using a Becton Dickinson (Franklin Lakes, NJ) FACScalibur flow cytometer.
sMer-binding assays
Mouse serum was obtained via tail vein bleed of C57Bl/6 wild-type and C57Bl/6 MerKD mice. Serum was supplemented with 250 nM recombinant mouse Gas6 (rmGas6; R&D Systems) and incubated for 30 minutes at 4°C, followed by addition of anti–mouse Gas6 antibody (R&D Systems) and a second 30-minute incubation. Protein G-Sepharose beads (Invitrogen, South San Francisco, CA) were added and complexes rocked for an additional 3 hours at 4°C. Beads were collected by centrifugation and washed 4 times with cold PBS containing 0.5% NP-40. Bound proteins were subjected to SDS-PAGE and immunoblotting with anti–mouse Mer antibody. For the pull-down assays with recombinant Mer extracellular domain, a chimeric protein was used consisting of mouse Mer extracellular domain or mouse Ret receptor extracellular domain fused to the Fc region of human IgG (Mer/Fc and Ret/Fc, respectively; R&D Systems). Two hundred nanomoles per liter rmGas6 was incubated with 200 nM Mer/Fc or 200 nM Ret/Fc in DMEM for 5 minutes. Protein G–Sepharose beads were added and complexes incubated for an additional 30 minutes. Beads were collected by centrifugation and washed 4 times with cold PBS. Bound proteins were subjected to SDS-PAGE and immunoblotting with anti–mouse Gas6 antibody.
Activation of Mer in cultured cells with Gas6
J774 cells were placed in DMEM lacking serum and plated at 2.5 × 106 cells/well in 12-well plates. After a 2-hour incubation at 37°C, cells were stimulated with 200 nM rmGas6 alone or in combination with 200 nM Mer/Fc or 200 nM Ret/Fc for 10 minutes. Cells were rinsed with PBS and lysed and cell extracts were resolved by SDS-PAGE and evaluated for the presence of phosphorylated Mer and phosphorylated AKT.
Phagocytosis assays
J774 macrophages were plated at 5 × 104 cells/well in 24-well plates. After 48 hours, cells were washed thoroughly with PBS and cultured for 3 hours in DMEM with no added serum. Either Mer/Fc or Ret/Fc was diluted into DMEM containing 6.5% mouse serum and rotated for 2 hours at 4°C. For Gas6 add-back experiments, recombinant human Gas6 (rhGas6; Amgen, Thousand Oaks, CA) was added with Mer/Fc to DMEM with 6.5% mouse serum and incubated 2 hours at 4°C. Jurkat T cells were induced to undergo apoptosis by exposure to UV irradiation at 254 nm for 10 minutes, followed by 3 hours of culture at 37°C. Percent apoptosis was determined by evaluation of nuclear morphology at the light microscope level. Apoptotic Jurkat T cells were pelleted and resuspended in media containing mouse serum in the presence or absence of Mer/Fc, Ret/Fc, or Mer/Fc plus rhGas6. The apoptotic cells were then cocultured with J774 macrophages at a density of 2 × 106 Jurkat T cells/well. Phagocytosis was allowed to proceed for 1.5 hours. The cells were then washed 4 times with ice-cold PBS, fixed, and stained with PROTOCOL Hema 3 Manual Staining System (Fisher Scientific, Houston, TX). Phagocytosis was scored by visual inspection using light microscopy. The investigator was blinded to the identities of the samples during data collection. A minimum of 200 macrophages were counted per well, with 3 replicate wells per condition. Results are displayed as phagocytic index, which is defined as the total number of apoptotic targets ingested divided by the total number of phagocytes counted times 100.
Microscopy
Phagocytosis assays were performed as described with the following modifications. J774 cells were cultured on glass coverslips. Jurkat T cells were labeled with FluoroLink Cy3 reactive dye (GE Healthcare Biosciences, Piscataway, NJ) after UV irradiation. After phagocytosis, cells were fixed and permeabilized with PBS solutions containing either 3% paraformaldehyde and 3% sucrose or 0.2% Triton X-100 (Sigma-Aldrich). Fluorescent labeling of F-actin and DNA was performed using Alexa Fluor 488 phalloidin (Invitrogen) and Hoechst no. 33258 trihydrochloride (Sigma-Aldrich). Cells were visualized using a Leica DMRXMA microscope (Leica Microscopes, Allendale, NJ) and data were collected and processed using SlideBook 3.0 (Intelligent Imaging Innovations, Denver, CO) software.
Statistics
The data were fit with a mixed model (SAS, PROC MIXED). The outcome was phagocytic index. The predictor was treatment, which was analyzed 2 ways: (1) as a class variable and (2) as a continuous variable. For method 1, treatment levels were further compared using the Dunnett procedure.
Platelet aggregation
Blood was drawn into plastic syringes containing 3.2% buffered sodium citrate using a ratio of 9 parts whole blood to 1 part citrate anticoagulant. The whole blood was centrifuged using 135g for 10 minutes at room temperature to prepare platelet-rich plasma (PRP). The PRP was removed and platelet count determined on a Cell-Dyn 4000 (Abbott Diagnostics, Abbott Park, IL). The remaining plasma and cells were centrifuged again at 1500g for 15 minutes at room temperature to prepare platelet-poor plasma (PPP). The PPP was used to dilute the PRP to a final platelet concentration of 200 000/mL. All aggregation studies were performed on a Platelet Aggregation Profiler Model PAP-4D (BioData, Horsham, PA). Briefly, 300 μL PRP was added to each test cuvette along with buffer only, human Mer/Fc (R&D Systems), or Ret/Fc and incubated at 37°C for 5 minutes. Either the ADP or collagen agonists were added and the aggregation recording begun. The total volume of each aggregation test sample including agonist was maintained at 350 μL.
Mouse thrombosis model
Seven- to 9-week-old female C57Bl/6 mice were given 10 μg Mer/Fc in 50 μL PBS or 50 μL PBS alone for vehicle control animals, by tail vein injection. After a 10-minute interval, a mixture of collagen (0.28 mg/kg; Helena Laboratories, Beaumont, TX) and epinephrine (0.029 mg/kg) in a total volume of 50 μL was injected into the tail vein and mice were anesthetized with sodium pentobarbital (60 mg/kg, intraperitoneally). Mortality within 60 minutes after injection was assessed. Lungs were harvested, fixed in formalin overnight, and then sectioned and stained with hematoxylin and eosin. Lung sections were visualized using a Leica DMLB microscope with a 40×/0.75 NA objective.
Results
Ectodomain shedding of the Mer protein
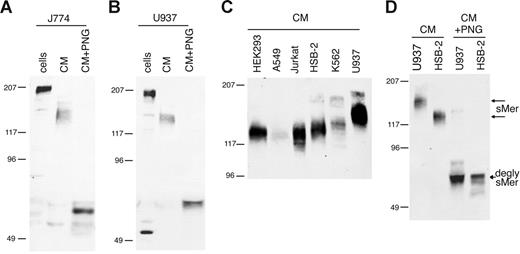
Membrane-bound Mer in macrophage/monocyte cell lines was detected at 205 kDa in mouse J774 cells (Figure 1A) and human U937 cells (Figure 1B), consistent with previous reports of Mer protein size in U937 cells.28 Mer extracellular domain with an apparent molecular weight of 140 to 150 kDa was constitutively shed from J774 and U937 cells and accumulated in the culture medium. Mer is heavily glycosylated in the extracellular domain due to the presence of 13 potential N-linked glycosylation sites.2 When the Mer ectodomain was treated with PNGase F to remove N-glycosylation, the Mer protein detected in the CM was 65 kDa, similar to the predicted size based on amino acid sequence of the extracellular domain.
Mer extracellular domain is released into the medium of cultured cell lines. (A) J774 (mouse) or (B) U937 (human) monocytic cells were grown overnight in serum-free medium. CM was collected and concentrated 10-fold, cells were lysed, and a sample of each CM was deglycosylated with PNGase F. (C) Concentrated CM from overnight cultures of human cell lines. (D) Concentrated CM from HSB-2 and U937 cells untreated and digested with PNGase F. Cell lysates (cells), CM, and PNGase-digested medium (CM + PNG) were analyzed by SDS-PAGE and immunoblotting with antibodies against mouse (A) or human (B-D) Mer extracellular domain. Untreated sMer (sMer) and deglycosylated sMer (degly sMer) are indicated in panel D.
Mer extracellular domain is released into the medium of cultured cell lines. (A) J774 (mouse) or (B) U937 (human) monocytic cells were grown overnight in serum-free medium. CM was collected and concentrated 10-fold, cells were lysed, and a sample of each CM was deglycosylated with PNGase F. (C) Concentrated CM from overnight cultures of human cell lines. (D) Concentrated CM from HSB-2 and U937 cells untreated and digested with PNGase F. Cell lysates (cells), CM, and PNGase-digested medium (CM + PNG) were analyzed by SDS-PAGE and immunoblotting with antibodies against mouse (A) or human (B-D) Mer extracellular domain. Untreated sMer (sMer) and deglycosylated sMer (degly sMer) are indicated in panel D.
sMer was released into the medium from all cultured cell lines tested that express membrane-bound Mer. The shed Mer ectodomain was detected at different molecular weights in the media from different cell lines (Figure 1C). To demonstrate that the size differences noted in the shed Mer ectodomain are due to differences in glycosylation, we treated the U937 and HSB-2 Mer ectodomains (150 kDa and 125 kDa, respectively) with PNGase F. After removal of N-linked glycosylation, the shed Mer ectodomain from both of these cell lines migrated at the same size (Figure 1D).
In addition to detecting the Mer ectodomain in CM from cultured lines, we found sMer in CM from mouse peritoneal macrophage and spleen primary cells (Figure 2A). Furthermore, a significant amount of sMer was present in the serum of wild-type C57Bl/6 mice. The serum from a MerKD mouse,29 which lacks expression of any Mer protein, did not have detectable levels of sMer. Membrane-bound Mer was detected on the surface of human primary cells, including monocyte-derived macrophages (205 kDa), MVECs (185 kDa), and platelets (165 kD). sMer was shed from these primary cells into the CM (Figure 2B). sMer was also abundant in human plasma obtained commercially as plasma pooled from healthy donors (Figure 2B). Free-flowing plasma drawn in a manner to minimize coagulation was also obtained from a healthy donor to confirm that sMer was present in both human plasma and serum. There was no apparent increase in sMer noted following clotting of the plasma and analysis of the serum. Shedding of membrane-bound Mer from monocytes/macrophages, platelets, and endothelial cells likely contributes to sMer present in plasma.
sMer is shed from primary cells in culture and is detected in mouse and human blood. (A) Elicited peritoneal macrophages and dissociated splenocytes from wild-type mice were cultured in serum-free DMEM for 7 hours. Cell lysates and conditioned medium from the macrophages and splenocytes, and 5 μL serum from wild-type mice or mice with the Mer gene disrupted (KD) were analyzed by SDS-PAGE and immunoblotting. (B) Human monocyte-derived macrophages (Μφ), MVECs, platelets, and plasma samples were examined for the presence of Mer by Western blotting. Cultured monocyte-derived macrophages and MVECs express 205- and 185-kDa Mer glycoform, respectively, and sMer was present in CM from these cells. A 165-kDa Mer protein was detected in pelleted platelets, and sMer extracellular domain proteins of 110 to 140 kDa were abundant in pooled plasma from healthy donors (George King Bio-Medical, Overland Park, KS) and in plasma from free-flowing blood (plasma), and subsequently after clotting, in serum from the same donor (serum).
sMer is shed from primary cells in culture and is detected in mouse and human blood. (A) Elicited peritoneal macrophages and dissociated splenocytes from wild-type mice were cultured in serum-free DMEM for 7 hours. Cell lysates and conditioned medium from the macrophages and splenocytes, and 5 μL serum from wild-type mice or mice with the Mer gene disrupted (KD) were analyzed by SDS-PAGE and immunoblotting. (B) Human monocyte-derived macrophages (Μφ), MVECs, platelets, and plasma samples were examined for the presence of Mer by Western blotting. Cultured monocyte-derived macrophages and MVECs express 205- and 185-kDa Mer glycoform, respectively, and sMer was present in CM from these cells. A 165-kDa Mer protein was detected in pelleted platelets, and sMer extracellular domain proteins of 110 to 140 kDa were abundant in pooled plasma from healthy donors (George King Bio-Medical, Overland Park, KS) and in plasma from free-flowing blood (plasma), and subsequently after clotting, in serum from the same donor (serum).
PMA and LPS increase proteolytic cleavage of Mer
Ectodomain shedding of proteins can often be accelerated in the presence of PMA or LPS.30 After treatment of J774 cells with 50 ng/mL LPS for 4 hours or 50 nM PMA for 45 minutes, a reduction in membrane-bound Mer protein was detected by flow cytometry (Figure 3A-B). Following treatment with LPS or PMA, Western blot analysis of the cells and CM showed that decreased full-length Mer was present in the cells over time as increased shed Mer ectodomain was detected in medium (Figure 3C-D). In the LPS-treated cells, sMer was detected at 1 hour and increased through the 6-hour time point. The shedding response to PMA was readily detected at 15 minutes and continued to increase through the 1-hour time point.
LPS or PMA stimulates release of Mer ectodomain. Surface expression of Mer on J774 cells treated with 50 ng/mL LPS for 4 hours (A) or treated with 50 nM PMA for 45 minutes (B) was evaluated by flow cytometry. Expression on untreated cells at each time point is shown for comparison. (C) J774 cells were incubated in serum-free medium with or without 50 ng/mL LPS or (D) treated with 50 nM PMA for the indicated times. Mer and actin present in cell lysates and sMer released into the medium at each time point were analyzed by immunoblotting. (E) The carboxyl-terminal portion of cleaved Mer remains cell-associated. Elicited peritoneal macrophages from MerKD or wild-type mice were cultured overnight, then placed in serum-free medium and treated with 100 ng/mL LPS for the indicated times. Cells were lysed, and proteins from equal numbers of cells were separated by SDS-PAGE and immunoblotted with an antibody against the C-terminus of Mer. Membrane-bound Mer receptor (200 kDa), which is absent from MerKD macrophages, decreases with time of LPS treatment. The C-terminal 60-kDa fragment of Mer that accumulates with LPS treatment is indicated by an asterisk.
LPS or PMA stimulates release of Mer ectodomain. Surface expression of Mer on J774 cells treated with 50 ng/mL LPS for 4 hours (A) or treated with 50 nM PMA for 45 minutes (B) was evaluated by flow cytometry. Expression on untreated cells at each time point is shown for comparison. (C) J774 cells were incubated in serum-free medium with or without 50 ng/mL LPS or (D) treated with 50 nM PMA for the indicated times. Mer and actin present in cell lysates and sMer released into the medium at each time point were analyzed by immunoblotting. (E) The carboxyl-terminal portion of cleaved Mer remains cell-associated. Elicited peritoneal macrophages from MerKD or wild-type mice were cultured overnight, then placed in serum-free medium and treated with 100 ng/mL LPS for the indicated times. Cells were lysed, and proteins from equal numbers of cells were separated by SDS-PAGE and immunoblotted with an antibody against the C-terminus of Mer. Membrane-bound Mer receptor (200 kDa), which is absent from MerKD macrophages, decreases with time of LPS treatment. The C-terminal 60-kDa fragment of Mer that accumulates with LPS treatment is indicated by an asterisk.
Ectodomain shedding of Mer results in a cell-associated carboxyl-terminal peptide
Proteolytic cleavage of membrane-bound Mer would predictably yield an amino terminal fragment sMer as well as a carboxyl terminal fragment that remains cell-associated. A polyclonal antibody against the Mer carboxyl terminus was used to detect the cell-associated portion of Mer in elicited mouse peritoneal macrophages following LPS-induced shedding (Figure 3E). Western blot analysis revealed the presence of a 60-kDa Mer protein in LPS-treated macrophages from wild-type mice. The 60-kDa protein increased in concentration during the first 30 minutes of LPS treatment (a 2.3-fold increase over 30 minutes) before reaching a plateau in accumulation of the cleaved product. The 60-kDa molecular weight of the C-terminal product was the predicted size of the intracellular domain of Mer based on amino acid sequence. Although nonspecific bands are present in the lysates from both MerKD and wild-type mice, the 60-kDa intracellular domain fragment was not detected in LPS-treated macrophages from MerKD mice.
Mer shedding is blocked by TAPI, an inhibitor of the TACE metalloproteinase
To confirm that sMer was a result of cleavage of an already synthesized membrane-bound protein, we treated J774 macrophages with cycloheximide to block new protein synthesis. The blockage of protein synthesis was demonstrated by loss of p53 protein in cell lysates following cycloheximide treatment. In contrast, cycloheximide did not alter LPS-induced shedding of Mer (Figure 4A). Further confirmation that the sMer detected was a result of protease cleavage is shown in Figure 4B. Treatment with EDTA, which chelates cations necessary for metalloproteinase function, partially inhibited LPS-induced shedding of Mer. In addition, LPS-induced shedding was virtually eliminated in the presence of TAPI-0. TAPI-031-34 is an inhibitor of TNF-α–converting enzyme (TACE), a member of the ADAM family35 of metalloproteinases, which cleaves the membrane-bound precursor form of TNF-α to generate soluble TNF-α. The related Axl protein has been shown to be cleaved by TACE36 as well as by ADAM10.37 The inhibition of LPS shedding by TAPI-0 suggests that membrane-bound Mer, like Axl, can be cleaved by TACE or a TACE-like protease to generate sMer.
LPS-induced production of sMer is independent of protein synthesis and is blocked by metalloproteinase inhibitors. (A) A 6-cm plate of J774 cells was cultured in the presence of 10 μg/mL cycloheximide for 30 minutes, rinsed with serum-free medium, and then incubated in serum-free medium containing 10 μg/mL cycloheximide and 50 ng/mL LPS for 1 hour. Two additional untreated plates were incubated in serum-free medium with or without 50 ng/mL LPS for 1 hour. sMer released into the medium and Mer and p53 in cell lysates were monitored by Western blot. (B) J774 cells were treated with 50 ng/mL LPS to stimulate the cleavage of the Mer extracellular domain. EDTA (5 mM), 200 μM TAPI-0, or DMSO (vehicle used to dissolve TAPI) was added to cell cultures as indicated for 2 hours. Mer remaining in cells and sMer released into the medium after each treatment were detected by Western blot.
LPS-induced production of sMer is independent of protein synthesis and is blocked by metalloproteinase inhibitors. (A) A 6-cm plate of J774 cells was cultured in the presence of 10 μg/mL cycloheximide for 30 minutes, rinsed with serum-free medium, and then incubated in serum-free medium containing 10 μg/mL cycloheximide and 50 ng/mL LPS for 1 hour. Two additional untreated plates were incubated in serum-free medium with or without 50 ng/mL LPS for 1 hour. sMer released into the medium and Mer and p53 in cell lysates were monitored by Western blot. (B) J774 cells were treated with 50 ng/mL LPS to stimulate the cleavage of the Mer extracellular domain. EDTA (5 mM), 200 μM TAPI-0, or DMSO (vehicle used to dissolve TAPI) was added to cell cultures as indicated for 2 hours. Mer remaining in cells and sMer released into the medium after each treatment were detected by Western blot.
sMer binds and sequesters Gas6
Receptor shedding occurs by cleavage in a region immediately amino terminal to the transmembrane region.38 Many shed receptors can effectively bind the ligand for the membrane-bound receptor and, consequently, prevent activation of the membrane-bound receptor.27,38-40 Endogenous sMer from the serum of wild-type mice was able to bind Gas6, as shown in immunoprecipitation experiments with Gas6 followed by immunoblotting for Mer. The Mer/Gas6 complex was not detected when Gas6 was immunoprecipitated from the control MerKD mouse serum (Figure 5A). Using a chimeric recombinant protein consisting of the extracellular domain of murine Mer fused to the Fc domain of human immunoglobulin G (Mer/Fc), we also demonstrated the ability of the extracellular domain of recombinant Mer to bind the ligand Gas6 in pull-down assays. Mer/Fc was shown to bind Gas6 in assays in which Mer/Fc-Gas6 complexes were pulled down with protein G-Sepharose beads and bound Gas6 was detected by immunoblotting. A Ret/Fc control (extracellular domain of Ret fused to the Fc domain of human immunoglobulin G) did not bind Gas6 (Figure 5B). Furthermore, Gas6 complexed with Mer/Fc was unable to cause autophosphorylation of membrane-bound Mer in J774 macrophages (Figure 5C) or activate the Mer downstream target AKT (Figure 5D).41
The soluble ectodomain of Mer binds to Gas6 and inhibits Gas6 signaling. (A) Gas6/Mer complexes in wild-type C57Bl/6 serum were detected by immunoprecipitating with a mouse anti-Gas6 antibody and by performing a Western blotting with an anti–mouse Mer antibody. The Gas6/Mer complexes were not observed in the control serum from C57Bl/6 MerKD mice. (B) A chimeric recombinant protein consisting of the mouse Mer extracellular domain fused to the Fc region of human IgG was incubated with recombinant mouse Gas6 (rmGas6). As a control for nonspecific binding, a parallel experiment was performed with a Ret receptor ectodomain/Fc chimera and mGas6. Complexes pulled down with protein G Sepharose beads were run on SDS-PAGE gels, and bound Gas6 was detected by immunoblotting with anti–mouse Gas6 antibody. Also shown is the input rmGas6. (C) J774 cells were starved in serum-free medium and then treated for 10 minutes with 200 nM mGas6 and 200 nM Mer/Fc or Ret/Fc as indicated. Cell lysates were analyzed for phospho-Mer and total Mer content. (D) J774 cells incubated with or without mGas6 and Mer/Fc as in panel C and phospho-AKT and total AKT were monitored.
The soluble ectodomain of Mer binds to Gas6 and inhibits Gas6 signaling. (A) Gas6/Mer complexes in wild-type C57Bl/6 serum were detected by immunoprecipitating with a mouse anti-Gas6 antibody and by performing a Western blotting with an anti–mouse Mer antibody. The Gas6/Mer complexes were not observed in the control serum from C57Bl/6 MerKD mice. (B) A chimeric recombinant protein consisting of the mouse Mer extracellular domain fused to the Fc region of human IgG was incubated with recombinant mouse Gas6 (rmGas6). As a control for nonspecific binding, a parallel experiment was performed with a Ret receptor ectodomain/Fc chimera and mGas6. Complexes pulled down with protein G Sepharose beads were run on SDS-PAGE gels, and bound Gas6 was detected by immunoblotting with anti–mouse Gas6 antibody. Also shown is the input rmGas6. (C) J774 cells were starved in serum-free medium and then treated for 10 minutes with 200 nM mGas6 and 200 nM Mer/Fc or Ret/Fc as indicated. Cell lysates were analyzed for phospho-Mer and total Mer content. (D) J774 cells incubated with or without mGas6 and Mer/Fc as in panel C and phospho-AKT and total AKT were monitored.
Because the related Axl and Tyro-3 transmembrane receptors also bind Gas6, we analyzed mouse and human plasma for the presence of soluble forms of these receptors. Consistent with previously published results from other groups,37,42,43 soluble Axl (sAxl) glycoforms were detected in mouse and human plasma ranging in size from 65 to 80 kDa (data not shown). In contrast, soluble Tyro-3 could not be detected in human plasma (data not shown). Similar to our findings with sMer, sAxl was capable of binding Gas637 and an Axl/Fc construct was able to block Gas6-mediated activation of full-length Mer (data not shown). Thus, sMer and sAxl both have the capability of sequestering Gas6 and preventing Gas6-mediated signaling.
sMer inhibits clearance of apoptotic cells
Mer is required for the normal function of macrophages to efficiently clear apoptotic cells. Macrophages from MerKD mice display a profound defect in apoptotic cell engulfment.21,44 This phenomenon is believed to contribute to the development of a lupuslike autoimmunity in MerKD mice. To test for a possible role of sMer in macrophage function, we examined the ability of J774 macrophages to ingest apoptotic Jurkat T cells in the absence or presence of Mer/Fc. Mer/Fc (50 nM) significantly diminished the ability of J774 cells to ingest apoptotic Jurkat T cells (Figure 6A-B). Add-back of 10 nM Gas6 reversed the inhibitory effect of 50 nM Mer/Fc on apoptotic cell clearance (Figure 6C). Significantly, a Ret/Fc control did not yield a similar effect on macrophage function (Figure 6D).
Mer/Fc inhibits apoptotic cell engulfment. (A) Fluorescence analysis of phalloidin-stained J774 cells (green) engulfing Cy3-labeled apoptotic Jurkat T cells (red) in the absence (top) or presence (bottom) of 50 nM Mer/Fc. DNA is stained with Hoechst and appears blue. Bar represents 10 μm. (B) In vitro engulfment assay using J774 macrophages incubated with apoptotic Jurkat T cells in the absence or presence of increasing doses of Mer/Fc protein (n = 3) or (C) Mer/Fc plus 10 nM rhGas6 (n = 2). (D) Engulfment by cells incubated with Mer/Fc or Ret/Fc control. Results are expressed as percent of untreated control and reflect the phagocytic index of the samples assayed. The phagocytic indices of untreated controls never fell below 25.5. *P < .001.
Mer/Fc inhibits apoptotic cell engulfment. (A) Fluorescence analysis of phalloidin-stained J774 cells (green) engulfing Cy3-labeled apoptotic Jurkat T cells (red) in the absence (top) or presence (bottom) of 50 nM Mer/Fc. DNA is stained with Hoechst and appears blue. Bar represents 10 μm. (B) In vitro engulfment assay using J774 macrophages incubated with apoptotic Jurkat T cells in the absence or presence of increasing doses of Mer/Fc protein (n = 3) or (C) Mer/Fc plus 10 nM rhGas6 (n = 2). (D) Engulfment by cells incubated with Mer/Fc or Ret/Fc control. Results are expressed as percent of untreated control and reflect the phagocytic index of the samples assayed. The phagocytic indices of untreated controls never fell below 25.5. *P < .001.
sMer inhibits platelet aggregation in vitro and protects against fatal thromboembolism in vivo
Because an effect of sMer was detected in macrophages via inhibition of membrane-bound Mer, we assayed whether sMer could also affect Mer activity and function in platelets. A physiologic role in the normal functioning of platelets has been shown using mice lacking either Mer or Gas6 protein. Both genetically engineered strains of mice exhibit decreased platelet aggregation in response to known agonists and decreased clot stability.25,26 We examined the effect of the addition of Mer/Fc in standard platelet aggregation assays with ADP (Figure 7A-B) and collagen (Figure 7D) as agonists. Using agonist concentrations of 2 μM ADP or 4 μM ADP, the addition of Mer/Fc decreased platelet aggregation in a concentration-dependent manner. A similar result was found with collagen as the agonist. The diminished platelet aggregation was not evident with a Ret/Fc control (Figure 7C). The Ret/Fc, in contrast, caused slightly more platelet aggregation at the higher Ret/Fc concentration.
Mer extracellular domain inhibits platelet aggregation induced by ADP and collagen and protects mice against collagen-epinephrine–induced thrombosis. (A-D) In vitro platelet aggregation was performed using human PRP and was analyzed on a BioData aggregometer. (A-B) Aggregation response of platelets in response to 2 μM ADP (A) or 4 μM ADP (B) following preincubation with different concentrations of Mer/Fc. (C) Platelet aggregation induced by 4 μM ADP after pretreatment with Ret/Fc. (D) Aggregation of platelets in response to 10 μg/mL collagen with and without preincubation with Mer/Fc. Squares on the x-axis represent 15-second intervals. Data shown are representative of 3 independent experiments. (E) Survival of mice pretreated with Mer/Fc (n = 10) or saline (PBS control, n = 8) after collagen-epinephrine injection. Protection from fatal thromboembolism by Mer/Fc was significant (P < .022, log-rank test). (F-G) Hematoxylin and eosin staining of lungs from control mouse (F) or Mer/Fc-pretreated mouse (G) after collagen-epinephrine injection. Extensive platelet thromboembolism is seen in control mice (arrows) compared to the Mer/Fc-pretreated mice. Scale bar represents 100 μm.
Mer extracellular domain inhibits platelet aggregation induced by ADP and collagen and protects mice against collagen-epinephrine–induced thrombosis. (A-D) In vitro platelet aggregation was performed using human PRP and was analyzed on a BioData aggregometer. (A-B) Aggregation response of platelets in response to 2 μM ADP (A) or 4 μM ADP (B) following preincubation with different concentrations of Mer/Fc. (C) Platelet aggregation induced by 4 μM ADP after pretreatment with Ret/Fc. (D) Aggregation of platelets in response to 10 μg/mL collagen with and without preincubation with Mer/Fc. Squares on the x-axis represent 15-second intervals. Data shown are representative of 3 independent experiments. (E) Survival of mice pretreated with Mer/Fc (n = 10) or saline (PBS control, n = 8) after collagen-epinephrine injection. Protection from fatal thromboembolism by Mer/Fc was significant (P < .022, log-rank test). (F-G) Hematoxylin and eosin staining of lungs from control mouse (F) or Mer/Fc-pretreated mouse (G) after collagen-epinephrine injection. Extensive platelet thromboembolism is seen in control mice (arrows) compared to the Mer/Fc-pretreated mice. Scale bar represents 100 μm.
To determine whether sMer affects platelet function and clot formation in vivo, the effect of sMer on platelet function was evaluated in a collagen/epinephrine mouse model of thrombosis.25,26 Wild-type mice were initially given via tail vein injection either 10 μg MerFc or a similar volume of PBS as control. Five minutes later, pulmonary thromboembolism was induced by injection of a mixture of collagen and epinephrine. Respiratory distress and death were monitored in anesthetized mice over a 1-hour period after collagen/epinephrine injection. In mice pretreated with Mer/Fc, 7 of 10 mice (70%) survived compared to 2 of 8 mice (20%) treated with PBS control (P = .022; Figure 7E). All deaths occurred in the first 15 minutes following collagen/epinephrine injection. Lungs were harvested at death or after the 1-hour observation period from all mice and histopathologic sections were analyzed for the presence of pulmonary emboli. More than 2-fold more pulmonary clots were detected in the lung sections from mice given PBS relative to mice treated with Mer/Fc (Figure 7F-G).
Discussion
Membrane-bound receptors generate soluble receptors using alternative mRNA splicing to remove the transmembrane region or through proteolytic cleavage and release of the receptor ectodomain. Proteolytic cleavage has been found to regulate the activity and number of cytokine receptors, the density of cell surface adhesion molecules, and the release of soluble growth factors.40 We now report that the receptor Mer tyrosine kinase can be proteolytically cleaved by a metalloproteinase to produce a sMer receptor, capable of binding the ligand Gas6.
Inhibition of Mer shedding with TAPI-0 suggests that the protease responsible for cleavage of the extracellular domain of the Mer receptor tyrosine kinase is TACE (ADAM17). TACE was the first member of ADAM family to be characterized35 due to its ability to cleave the membrane-bound precursor form of TNF-α, releasing the soluble TNF-α from cells. Using mass spectrometric analysis of tryptic fragments differentially evident in TACE+/+ versus TACE−/− cell lines, TACE has also been shown to process the Mer-related protein, Axl, as well as other proteins.34 Primary amino acid sequence of the membrane proximal or “stalk” region is not crucial as stalk length for TACE cleavage.45-48 The defined TACE cleavage site in Axl is a 14 amino acid region immediately amino terminal to the transmembrane domain.43 This membrane proximal region of Mer shows little sequence homology with Axl.
Soluble receptors exert biologic effects through altering the interaction between ligand and membrane-bound receptors. Soluble receptors and their membrane-bound counterparts can act agonistically, as is the case with the IL-6 receptor.49 However, the majority of soluble receptors act antagonistically and function as decoys to sequester soluble ligands. Soluble receptors may also have different effects that are dependent on concentration. Although low levels of soluble TNF receptor enhance the actions of TNF-α, high concentrations of soluble TNF receptor inhibit TNF-α activity.50 sMer primarily functions in an antagonistic role to full-length Mer, as sMer binds to the ligand Gas6 and inhibits Gas6-mediated Mer activation. In some instances, sAxl may function in a cooperative role with sMer, because both proteins can sequester Gas6.
Gas6 contains an amino terminal region with glutamic acid residues termed the Gla domain, a central domain with epidermal growth factor repeats, and a C-terminal sex hormone-binding globulin domain. The Gla domain of Gas6 undergoes vitamin K-dependent γ-carboxylation. This process mediates the interaction of coagulation factors with negatively charged phospholipid. Gas6 specifically binds phosphatidylserine in a manner dependent on calcium and γ-carboxyglutamic acid residues within the Gla domain.51 The ability of the carboxyl terminal domain of Gas6 to bind Mer52 and the amino terminal domain of Gas6 to bind phosphatidylserine on the surface of apoptotic cells provides a mechanism through which Mer may promote apoptotic cell clearance. Consistent with this argument, macrophages from MerKD mice have defects in the engulfment of apoptotic thymocytes, although the macrophages are capable of effectively binding the apoptotic thymocytes.21 The MerKD mice also develop a lupuslike autoimmunity,21,44 supporting the concept that failure to remove apoptotic cells may provide an immunogenic stimulus for autoimmune disease. Our data suggest that sMer plays a role in regulating macrophage clearance of apoptotic cells. Additional studies will be necessary to determine whether sMer levels are altered in patients with autoimmune disease.
In a similar manner, we use platelets to demonstrate that sMer directly affects the activity of full-length Mer in another cell type and that this specific tyrosine kinase inhibition significantly alters cell function. Mer is known to be important in normal platelet function because mice lacking a Mer receptor26 or Gas625 have diminished platelet aggregation in response to agonist. In this report, we demonstrate that sMer has a similar effect on platelet aggregation in vitro and, furthermore, the dysfunctional platelet aggregation leads to an effect on clot stability in vivo as wild-type mice were protected against collagen/epinephrine-induced pulmonary thromboembolism. These findings are consistent with reports that MerKD and Gas6−/− mice have in vivo protection from collagen/epinephrine-induced pulmonary thromboembolism and inhibition of ferric chloride-induced thrombosis.25,26 In both animal models, the protective effects of platelet inhibition were not accompanied by an increase in bleeding tendencies or alteration in coagulation parameters.
Platelet activation at the site of endothelial injury is thought to include an initial phase in which a monolayer of platelets is activated in response to local exposure of collagen plus von Willebrand factor. A second phase necessary for clot stability involves the recruitment of additional platelets and the formation of stable platelet to platelet contacts. The loss of Mer tyrosine kinase impairs the second phase or stabilization phase, in part through decreased platelet granule secretion of soluble agonists.24 Because Mer-Gas6 interactions are important in the later phases of platelet activation, a soluble protein that blocks the receptor-ligand binding is appealing as a strategy in the treatment of patients with thrombophilia. The use of sMer as an antiplatelet therapeutic may provide a means of preventing thrombus formation without increasing bleeding risks.
Authorship
Contribution: S.S., K.D.K, and J.B.L. designed and performed research as well as analyzed data; S.S. assisted with manuscript writing; X.L. performed research and analyzed data; B.C.V. contributed vital reagents;. P.M.H. analyzed data; and D.K.G. analyzed data and wrote the manuscript.
Conflict-of-interest disclosure: B.C.V. is employed by Amgen, whose product Gas6 was studied in the present work. D.K.G. and S.S. have submitted a patent application related to the work described in the present study.
Correspondence: Douglas K. Graham, University of Colorado at Denver and Health Sciences Center, Pediatrics, Mail Stop 8302, PO Box 6511, Aurora, CO 80045; e-mail: doug.graham@uchsc.edu.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This work was supported by the Research Scholar Award from the Research Institute at the Children's Hospital, Denver, CO (D.K.G) and grant GM061031 from the National Institutes of Health (K.D.K and P.M.H.). The authors would like to thank Dr James DeGregori for his insightful comments, Kim Hill and Jamie Tackett for animal expertise in these experiments, and Matthew Strand for his assistance with the statistical analysis.