Abstract
Immunotherapies using cancer-testis (CT) antigens as targets represent a potentially useful treatment in patients with multiple myeloma (MM) who commonly show recurrent disease following chemotherapy. We analyzed the expression of 11 CT antigens in bone marrow samples from patients with MM (n = 55) and healthy donors (n = 32) using reverse transcriptase–polymerase chain reaction (RT-PCR). CT antigens were frequently expressed in MM with 56% (MAGEC2), 55% (MAGEA3), 35% (SSX1), 20% (SSX4, SSX5), 16% (SSX2), 15% (BAGE), 7% (NY-ESO-1), and 6% (ADAM2, LIPI) expressing the given antigen. Importantly, CT antigens were not expressed in healthy bone marrow. Analyzing patients with MM (n = 66) for antibody responses against MAGEA3, SSX2, and NY-ESO-1, we found strong antibody responses against CT antigens preferentially in patients who had received allogeneic stem cell transplantation (alloSCT). Antibody responses against NY-ESO-1 correlated with NY-ESO-1–specific CD4+ and CD8+ T-cell responses against peptide NY-ESO-151-62 and CD4+ responses against NY-ESO-1121-140 in 1 of these patients. These allogeneic immune responses were not detectable in pretransplantation samples and in the patients' stem cell donors, indicating that CT antigens might indeed represent natural targets for graft-versus-myeloma effects. Immune responses induced by alloSCT could be boosted by active CT antigen–specific immunotherapy, which might help to achieve long-lasting remissions in patients with MM.
Introduction
Multiple myeloma (MM) is a clonal B-cell malignancy with an incidence of approximately 15 000 new cases per year in the United States alone and a median survival of 3 years. MM is characterized by an accumulation of mature plasma cells (PCs) in the bone marrow (BM) leading to bone destruction and BM failure. MM remains essentially incurable by conventional antitumor therapy.1 While the introduction of allogeneic stem cell transplantation (alloSCT) has resulted in higher remission rates and even cures, these therapeutic improvements have been hampered by a significantly increased treatment-related morbidity and mortality. One reason for this is that the immune responses derived from the allogeneic graft are not strictly myeloma-specific and are, therefore, associated with significant immune-mediated side effects.
It has been suggested that donor T-cell–mediated graft-versus-host activities, which result from genetic disparities between donors and recipients, also include graft-versus-leukemia (GVL) effects capable of controlling the underlying malignancy in the host. Clinical observations supporting this idea include decreased relapse risks in patients who develop graft-versus-host disease (GVHD) after allografting, decreased relapse rates in recipients of allogeneic compared with autologous (autoSCT) or syngeneic stem cell transplantation, induction of remission in relapsed patients following donor lymphocyte infusion, and increased relapse risks after use of T-cell–depleted allografts compared with unmodified preparations.2 Minor histocompatibility and tumor-associated proteins such as differentiation antigens have been proposed as targets for GVL effects.2,3
The cancer-testis (CT) class of tumor antigens is a group of currently 44 proteins, the expression of which is characteristically restricted to cancer and the human germ line. Based on their immunogenicity and restricted tissue expression, CT antigens seem ideal targets for active immunotherapies.4 However, MM is considered a malignancy generating strong systemic immunosuppression and little is known about the ability of CT antigens to induce natural immune responses in patients with MM.5-7 Furthermore, the question remains open whether CT antigens might represent targets for graft-versus-myeloma (GVM) effects following alloSCT and whether this mode of therapy might induce spontaneous CT antigen–specific immune responses.
We performed a comprehensive analysis of CT antigen expression in myeloma cell lines, in the BM of patients with MM, and in healthy BM of stem cell donors. Another major goal of our study was to investigate whether the presence of these tumor antigens in the BM of patients with MM would lead to spontaneous humoral and cellular immune responses following alloSCT.
Patients, materials, and methods
Patients and healthy stem cell donors
A total of 106 consecutive consenting patients with MM, 32 healthy stem cell donors, 40 patients with acute myeloid leukemia (AML), and 50 healthy blood donors were included in the study. All patients were admitted for treatment of MM at the University Medical Center Hamburg-Eppendorf and provided informed consent in accordance with the Declaration of Helsinki. The study protocol had received approval by the local ethics committee.
BM samples, sera, and myeloma cell lines
BM samples and sera from consenting patients with MM as well as sera from patients with AML were obtained during routine diagnostic procedures. Samples obtained from consenting healthy donors were part of BM donations used for alloSCT or were collected from blood donors. Mononuclear cells (MNCs) were isolated from BM by density gradient centrifugation and were washed twice with phosphate-buffered saline (PBS). MNCs were lysed using RLT Buffer (Qiagen, Hilden, Germany) and were stored at −80°C until needed. Myeloma cell lines MOLP-8, KMS-12-BM, EJM, IM9, RPMI-8226, NCI-H929, OPM-2, LP-1, and U-266 were obtained from the German Collection of Microorganisms and Cell Cultures (DSMZ, Braunschweig, Germany). Cell lines Brown and SK-007 were provided by the Ludwig Institute for Cancer Research (LICR) New York branch. Cell lines were maintained in RPMI 1640 with 10% fetal calf serum (FCS).
Reverse transcriptase–polymerase chain reaction (RT-PCR)
CT antigen expression was evaluated in 55 patients with at least 10% BM-infiltrating PCs and 51 patients with MM with less than 10% PCs. In addition, PCR analysis was performed on 11 myeloma cell lines as well as on BM or CD34+ stem cells from 32 healthy donors. PCR primers and conditions used were those published in the CT Gene database of the Academy of Cancer Immunology8 and are available as supplementary data (Table S1, available on the Blood website; see the Supplemental Table link at the top of the online article). Negative controls without cDNA and positive control human testis cDNA were integrated in all PCRs.
Immunohistochemistry
The presence of CT antigens in actual tumor tissue was analyzed by immunohistochemistry on formalin-fixed, paraffin-embedded archival tissue as previously described.9-12 Monoclonal antibodies (mAbs) to the following antigens were used: mAb M3H67 (MAGEA3),9 57B (MAGEA4),10 mAb E978 (NY-ESO-1),11 and recently generated mAb CT10#5 (MAGEC2/CT10).12 All mAbs were generated by our group, except mAb 57 which was kindly provided by Dr G. Spagnoli (Basel, Switzerland).13 A heat-based antigen retrieval technique employing citrate buffer (mAb 57B), EDTA (mAbs M3H67, CT10#5), or hipH solution (mAb E978) from DAKO (DAKOcytomation, Carpinteria, CA) was used before primary incubation overnight at 4°C. As a secondary reagent a biotinylated horse antimouse antibody (Vector Labs, Burlingame, CA) followed by an avidini-biotin-complex system (ABC-Elite; Vector Labs) was used except for mAb E978, which was detected with a polymer-based secondary reagent (Powervision system; Immunovision, Brisbane, CA). Diaminobenzidine (DAB; Biogenex, San Ramon, CA) served as chromogen and Gill hematoxylin was used to counterstain. The immunohistochemical staining was graded based on the estimated amount of immunopositive tumor cells as follows: focal, approximately less than 5%; +, 5% to 25%; ++, more than 25% to 50%; +++, more than 50% to 75%; ++++, more than 75%.
ELISA
Sera for the analysis of serologic immunoreactivity against CT antigens were available from 66 patients with MM, from 4 of these patients' BM donors, from 40 patients with AML, and from 50 healthy blood donors. Enzyme-linked immunosorbent assay (ELISA) for MAGEA3-, SSX2-, and NY-ESO-1–specific IgG antibodies was performed using recombinant full-length protein analyzing serial-diluted sera (1:100, 1:400, 1:1600, 1:6400) as described by Stockert et al14 with minor modifications. Positive cutoff was the mean optical density (OD) of 8 negative controls plus 3 standard deviations at a 1:100 dilution. Positive sera were reanalyzed at the New York branch of the LICR using recombinant proteins of 10 different CT antigens (NY-ESO-1, LAGE1, MAGEA1, MAGEA3, MAGEA4, MAGEA10, SSX1, SSX2, SSX4, XAGE1) and p53 in an ELISA.15
Peptides and viral vectors
20mer peptides (n = 17) overlapping by 10 amino acids (AAs) and spanning the complete NY-ESO-1 sequence consisting of 180 AAs were obtained from Multiple Peptide Systems (San Diego, CA). 12mer peptides overlapping by one AA covering NY-ESO-1 51-70 were obtained from IRIS Biotech (Marktredwitz, Germany). MAGEA3 peptide 271-279 (FLWGPRALV; Multiple Peptide Systems) and NY-CO-58 peptide 151-180 (RPSCPAVAEIPLRMVSEEMEEQVHSIRGSS; Gramsch Laboratories, Schwabhausen, Germany) served as controls for analyses of NY-ESO-1–specific CD8+ and CD4+ responses, respectively. Vaccinia viruses recombinant for full-length NY-ESO-1 and for influenza nucleoprotein (NP) were obtained from THERION Biologics (Cambridge, MA).16
In vitro presensitization of peripheral T cells
Peripheral blood mononuclear cells (PBMCs) were collected using a Ficoll gradient and were frozen in RPMI containing 20% FCS and 10% DMSO in liquid nitrogen until needed. HLA typing of donor PBMCs was done by sequence-specific oligonucleotide probing and sequence-specific priming of genomic DNA. CD4+ and CD8+ T lymphocytes were separated from PBMC magnetic beads (Dynabeads; Dynal, Oslo, Norway) and were separately seeded into round-bottomed 96-well plates (Corning, NY) at a concentration of 5 × 105 cells per well in 200 μL RPMI 1640 with 10% human serum, l-glutamine (2 mM), penicillin (100 U/mL), and streptomycin (100 μg/mL). As antigen-stimulating cells (ASCs) for presensitization, PBMCs depleted of CD4+ and CD8+ T cells were pulsed with 10 μM peptide overnight at 37°C in 500 μL serum-free medium X-VIVO-15 (Bio Whittaker, Verviers, Belgium). Pulsed CD4−/CD8− ASCs were washed, irradiated, and added at a concentration of 1 × 106 ASCs per well to plates containing CD4+ or CD8+ T cells. After 20 hours, IL-2 (10 U/mL; Roche Molecular Biochemicals, Indianapolis, IN) and IL-7 (20 ng/mL; R&D Systems, Minneapolis, MN) were added. Subsequently, one half of the medium was replaced by fresh complete medium containing IL-2 (20 U/mL) and IL-7 (40 ng/mL) twice a week.
Generation and culture of target cells
CD4+ T cells remaining from the initial separation were seeded into 48-well plates (Corning, NY) at 1 × 106 to 2 × 106 cells per well in complete medium supplemented with 10 μg/mL phytohemagglutinin (PHA HA15; Murex, Dartford, United Kingdom). Cells were fed and expanded twice a week with complete medium containing IL-2 (20 U/mL) and IL-7 (40 ng/mL). The activated T-cell APCs (T-APCs) were typically used as target cells after 20 days of culture.15,17 Autologous Epstein-Barr virus (EBV)–transformed B lymphocytes (EBV-B cells) were cultured in RPMI medium with 10% FCS.
ELISPOT assay
Enzyme-linked immunospot (ELISPOT) assays for the determination of antigen-specific effector T cells were performed after a single cycle of antigen-specific stimulation on day 10 of presensitizing culture for CD8+ T cells and on day 20 for CD4+ T cells.17 Flat-bottomed, 96-well nitrocellulose plates (MultiScreen-HA; Millipore, Bedford, MA) were coated with IFN-γ mAb (2 μg/mL, 1-D1K; Mabtech, Stockholm, Sweden) and incubated overnight at 4°C. After washing with RPMI, plates were blocked with 10% human AB type serum for 2 hours at 37°C. Target cells were pulsed overnight at 37°C in 500 μL serum-free medium with 10 μM peptide. In some assays, target cells were infected overnight with 20 pfu/cell vaccinia virus recombinant for NY-ESO-1 or NP. Target cells were washed twice and were resuspended in RPMI medium 1640 without serum. Presensitized CD4+ or CD8+ T effector cells (5 × 104 or 1 × 104) and target cells (5 × 104; T-APC or EBV-B cells) were added to each well and incubated for 20 hours. Plates were then washed thoroughly with water containing 0.05% Tween 20, and anti–IFN-γ mAb (0.2 μg/mL, 7-B6-1-biotin; Mabtech) was added to each well. After incubation for 2 hours at 37°C, plates were washed and developed with streptavidin–alkaline phosphatase (1 μg/mL; Mabtech) for 1 hour at room temperature. After washing, substrate (5-bromo-4-chloro-3-indolyl phosphate/nitroblue tetrazolium; Sigma-Aldrich, Seelze, Germany) was added and incubated for 5 minutes. Spots were then counted using an AID EliSpot reader and EliSpot software version 3.2.3 (Autoimmun Diagnostika, Strassberg, Germany).
Statistical analysis
Spearson rank correlation was used to analyze correlations between patient characteristics and CT gene expression. Results were considered significant if P was less than .05.
Results
CT antigens are frequently expressed in MM and expression correlates with clinicopathologic parameters
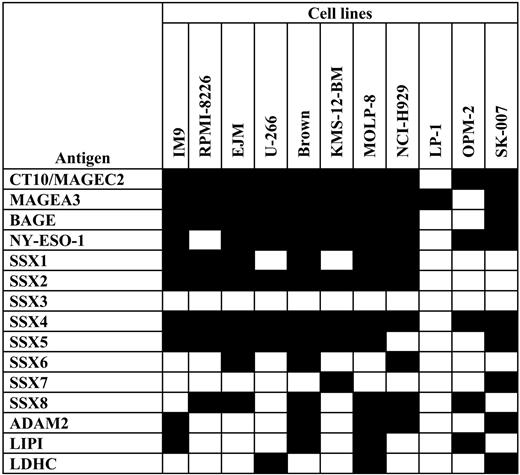
Investigating the expression of 15 CT antigens in myeloma cell lines we observed that malignant PCs very frequently expressed these target structures. Thus, CT10/MAGEC2, MAGEA3, BAGE, and NY-ESO-1 mRNA were detected in about 90% of cell lines (Figure 1).Other CT antigens expressed in the majority of cell lines were several members of the SSX family of genes: SSX1, SSX2, SSX4, SSX5, and SSX8. ADAM2 and LIPI were positive in about 40%. CT antigens less frequently expressed were SSX6, SSX7, and LDHC, and only SSX3 was not expressed in any malignant PC line. As expected for CT antigens, all genes were strongly expressed in healthy testis.
Expression of CT antigens in myeloma cell lines. Myeloma cell lines (N = 11) were tested for the expression of a variety of CT antigens (N = 15) using RT-PCR. Black fields indicate expression of the given CT antigen in the myeloma cell line; empty fields indicate negative RT-PCR results.
Expression of CT antigens in myeloma cell lines. Myeloma cell lines (N = 11) were tested for the expression of a variety of CT antigens (N = 15) using RT-PCR. Black fields indicate expression of the given CT antigen in the myeloma cell line; empty fields indicate negative RT-PCR results.
Next, we decided to investigate the expression of CT antigens frequently found in myeloma cell lines, in BM from patients with myeloma and from healthy stem cell donors. New criteria for the diagnosis of MM include the presence of at least 10% PCs in the BM.18 Therefore, we selected 55 patients with myeloma who fulfilled these criteria for our study of CT antigen expression. Clinical data of all patients are listed in Table 1. The majority of patients were men, median age was 63 years (range, 36-81 years), and mean percentage of BM-infiltrating PCs was 56.9. Most patients were in advanced stages with IgG kappa being the most common idiotype. At the time of inclusion into the study the majority of patients had been treated with chemotherapy alone as maximal treatment, whereas 13 patients had received autoSCT and 7 patients had undergone alloSCT.
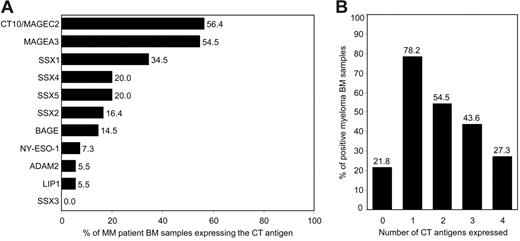
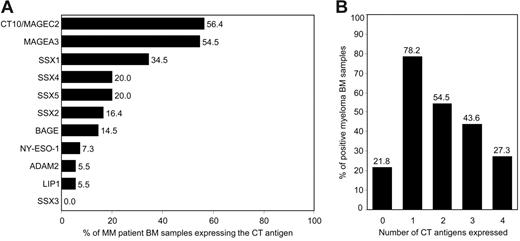
We found that the CT antigen most frequently expressed in PC-infiltrated BM of patients with MM was CT10/MAGEC2, followed by CT antigen MAGEA3 (Figure 2A). Both genes were expressed in the BM of more than half of all patients with myeloma. Members of the SSX family of genes, including SSX1, SSX2, SSX4, and SSX5, were also commonly expressed with SSX1, the most frequently expressed SSX gene, being present in the BM of about one third of all patients. In contrast, SSX3 was not expressed in any of the samples. CT antigen BAGE was positive in 15% of all patients. Our finding of a comparably weak expression of NY-ESO-1 in MM is in line with previous findings by others9,19-21 but is in contrast to observations made by van Rhee et al.5 Genes also less frequently expressed were ADAM2 and LIPI, being present in fewer than 10% of all samples.
Expression of CT antigens in BM samples from patients with MM. Bone marrow samples from patients with MM with at least 10% BM-infiltrating plasma cells (n = 55) were analyzed for the expression of 11 CT antigens (MAGEC2, MAGEA3, SSX1 to SSX5, BAGE, NY-ESO-1, LIPI, and ADAM2) and housekeeping gene GAPDH using RT-PCR. (A) Bars represent percentages of MM BM samples expressing the given CT antigen. (B) Bars represent percentages of samples expressing none of these antigens and samples expressing 1 or more, 2 or more, 3 or more, and 4 or more CT antigens.
Expression of CT antigens in BM samples from patients with MM. Bone marrow samples from patients with MM with at least 10% BM-infiltrating plasma cells (n = 55) were analyzed for the expression of 11 CT antigens (MAGEC2, MAGEA3, SSX1 to SSX5, BAGE, NY-ESO-1, LIPI, and ADAM2) and housekeeping gene GAPDH using RT-PCR. (A) Bars represent percentages of MM BM samples expressing the given CT antigen. (B) Bars represent percentages of samples expressing none of these antigens and samples expressing 1 or more, 2 or more, 3 or more, and 4 or more CT antigens.
The inclusion of a variety of antigens into antigen-specific immunotherapies might keep malignancies from escaping immunosurveillance by downregulating the expression of single target structures. When we analyzed how many of the patients simultaneously expressed different CT antigens, we found that close to 80% of myeloma BM samples expressed at least one of these antigens and more than half of the samples expressed 2 CT antigens or more (Figure 2B). A relatively large number of myeloma samples showed expression of at least 3 CT antigens and one quarter of patient samples even expressed 4 or more antigens, with a maximal coexpression of 7 antigens.
It has previously been proposed that CT antigens might be expressed in healthy BM, especially in mesenchymal stem cells and in nonmalignant CD34+ progenitor cells.22 In order to find out whether CT antigen–specific immunotherapies in MM would have potential to cause significant myelotoxicity derived from BM-targeting immune responses, we examined BM samples from 26 healthy donors for the expression of the 11 CT antigens. Importantly, we did not detect mRNA of any CT antigen in the vast majority of all healthy BM samples. In 4 patients, however, we detected expression of SSX4, a finding that was confirmed by sequencing of the PCR product (data not shown). Although we did not have access to purified normal plasma cells from the majority of our healthy donors, we were able to enrich CD138+ plasma cells from at least one of these subjects. Importantly, the normal plasma cells did not express any of the CT antigens examined. We also analyzed the expression of CT antigens on CD34+ progenitor cells isolated from leukapheresis products or BM of 6 additional healthy stem cell donors. Noticeably, we did not detect expression of any of the 11 CT antigens tested in any of the CD34+ progenitor cell samples (data not shown).
We next investigated whether the expression of CT antigens in MM was related to the patients' clinical characteristics. We did not observe any association between the number of CT antigens expressed in the BM and the patient's sex, heavy or light chain isotype, or the intensity of previous treatments (Table 1). Patients with a cytogenetic abnormality have been indicated to show an increased expression of CT antigen NY-ESO-1,5 however, we did not observe an effect of 13q14 deletion, as determined by fluorescence in situ hybridization (FISH),23 on the number of CT antigens expressed. Importantly, the expression of CT antigens was also not related to the degree of PC infiltration, indicating that a minimum of 10% PCs in the BM was likely to provide sufficient amounts of cDNA for CT antigen expression to be detected. In contrast, we observed a strong and positive correlation between the patient's age and the number of CT antigens expressed, a finding that is in line with observations made in some patients with solid tumors.24 Furthermore, we observed a positive correlation between CT antigen expression and the stage of disease (Table 1). This finding was in line with our observation of a positive association of the number of CT antigens expressed with serum levels of β2-microglobulin, which represents, according to the new International Staging System (ISS), a negative indicator for prognosis in MM.
Immunohistochemical analysis shows high expression of CT antigens in extramedullary plasmocytoma
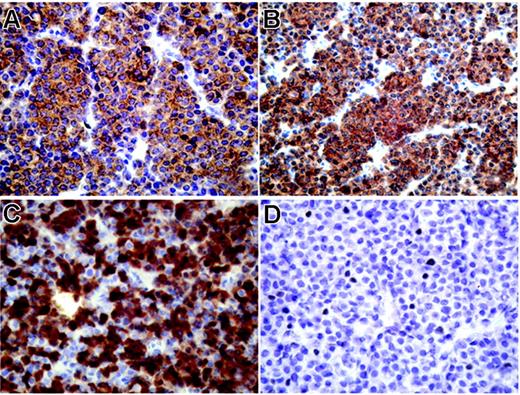
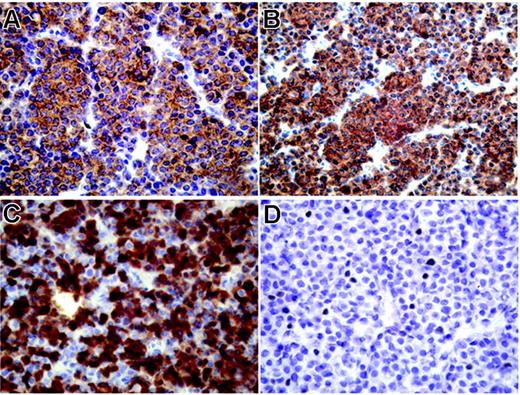
We next aimed at conforming our finding of a high mRNA expression of several CT antigens in myeloma on the protein level. However, myeloma bone marrow samples suitable for immunohistochemical analysis were not available for most of our patients. Therefore, we applied immunohistochemistry to an archived extramedullary plasmocytoma from one of our patients (BMT47). Interestingly, the tumor was strongly and homogeneously positive for NY-ESO-1, MAGEA3, and MAGEA4, with more than 75% immunopositive tumor cells for each antigen (Figure 3A-C). We also observed expression of CT10/MAGEC2, although this protein showed only a focal expression pattern (Figure 3D). Immunostaining was predominantly cytoplasmic for MAGE-A3 and MAGE-A4, whereas NY-ESO-1 was also present in the nucleus and CT10/MAGEC2 showed solely a nuclear expression (Figure 3).
Strong expression of CT antigens in extramedullary myeloma using immunohistochemistry. Immunohistochemical staining of an extramedullary plasmocytoma (patient BMT47) with mAb M3H67 to MAGEA3 (A), mAb 57Bm to MAGEA4 (B), mAb E978 to NY-ESO-1 (C), and mAb CT10#5 to MAGE-C2/CT10 (D), diaminobenzidine chromogen. Primarily cytoplasmic expression of MAGEA3 and MAGEA4 in all tumor cells (A, B), mixed cytoplasmic and nuclear presence of NY-ESO-1 in a majority of plasmocytoma cells, and nuclear expression of CT10/MAGEC2 in single tumor cells.
Strong expression of CT antigens in extramedullary myeloma using immunohistochemistry. Immunohistochemical staining of an extramedullary plasmocytoma (patient BMT47) with mAb M3H67 to MAGEA3 (A), mAb 57Bm to MAGEA4 (B), mAb E978 to NY-ESO-1 (C), and mAb CT10#5 to MAGE-C2/CT10 (D), diaminobenzidine chromogen. Primarily cytoplasmic expression of MAGEA3 and MAGEA4 in all tumor cells (A, B), mixed cytoplasmic and nuclear presence of NY-ESO-1 in a majority of plasmocytoma cells, and nuclear expression of CT10/MAGEC2 in single tumor cells.
CT antigens induce a systemic humoral immune response in patients with MM following alloSCT
Given the frequent expression of CT antigens in patients with MM we analyzed whether the same patients had developed an IgG antibody response against 3 of these proteins (MAGEA3, SSX2, NY-ESO-1). Sera were available from a total of 66 patients, of whom 15 had at least 10% malignant PCs in their BM and the remaining patients being in therapy-induced near-complete remission with less than 10% PCs but positive immunofixation. Nine of the patients had received chemotherapy alone, 22 had received autoSCT, and 35 had received alloSCT as maximal treatment.
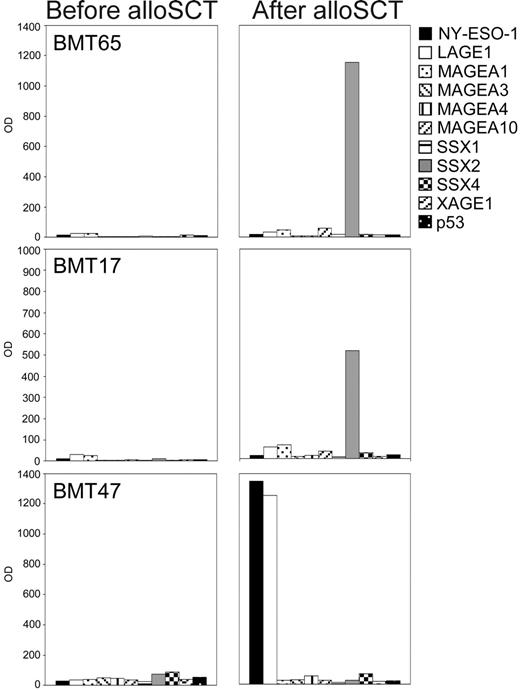
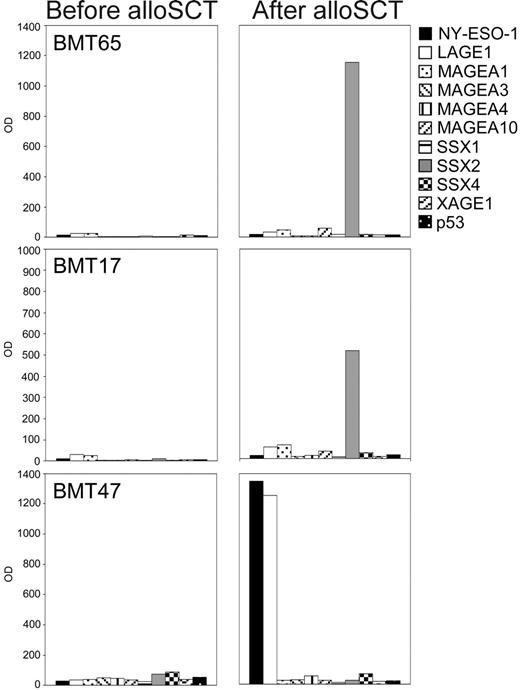
Whereas none of the 50 sera from healthy controls showed any such antibody responses, we detected IgG antibodies against CT antigens in 10 of the myeloma patients' sera. One patient who had received autoSCT as maximal treatment had antibodies against NY-ESO-1 and MAGEA3. Remarkably, all of the 9 remaining antibody-positive patients belonged to the group who had undergone alloSCT (Figure 4). These patients had received alloSCT between 14 and 64 months (mean 34.6 months) before their sera were first analyzed. The responses consisted of IgG antibodies against SSX2 in 3 cases and of responses against MAGEA3 and NY-ESO-1 in 2 cases, respectively. In addition, 2 patients showed simultaneous antibody responses against CT antigens MAGEA3 and NY-ESO-1. We were able to obtain stored sera that had been collected a few days before alloSCT from 7 of the 9 antibody-positive patients. Noticeably, none of these patients showed antibody responses against any of the CT antigens in their pretransplantation sample (Figure 4).
CT antigen–specific antibody responses in patients with multiple myeloma following allogeneic stem cell transplantation. Sera from patients with MM (n = 66) were screened for IgG antibodies against 3 CT antigens (MAGEA3, SSX2, NY-ESO-1) in an ELISA. Sera showing positive results were sent to the New York branch of the Ludwig Institute for Cancer Research for confirmation of the results using recombinant proteins of 10 different CT antigens (NY-ESO-1, LAGE1, MAGEA1, MAGEA3, MAGEA4, MAGEA10, SSX1, SSX2, SSX4, XAGE1) and p53 in an ELISA. Optical density (OD) results (serum dilution 1:100) of the ELISA are given for 3 representative antibody-positive patients with MM, with patients BMT65 and BMT17 showing high-titered antibodies against SSX2, and patient BMT47 showing a strong antibody response against NY-ESO-1 and its homologue LAGE following allogeneic stem cell transplantation. Importantly, pre–bone marrow transplantation samples of all patients who had undergone alloSCT were negative for CT antigen–specific IgG antibodies.
CT antigen–specific antibody responses in patients with multiple myeloma following allogeneic stem cell transplantation. Sera from patients with MM (n = 66) were screened for IgG antibodies against 3 CT antigens (MAGEA3, SSX2, NY-ESO-1) in an ELISA. Sera showing positive results were sent to the New York branch of the Ludwig Institute for Cancer Research for confirmation of the results using recombinant proteins of 10 different CT antigens (NY-ESO-1, LAGE1, MAGEA1, MAGEA3, MAGEA4, MAGEA10, SSX1, SSX2, SSX4, XAGE1) and p53 in an ELISA. Optical density (OD) results (serum dilution 1:100) of the ELISA are given for 3 representative antibody-positive patients with MM, with patients BMT65 and BMT17 showing high-titered antibodies against SSX2, and patient BMT47 showing a strong antibody response against NY-ESO-1 and its homologue LAGE following allogeneic stem cell transplantation. Importantly, pre–bone marrow transplantation samples of all patients who had undergone alloSCT were negative for CT antigen–specific IgG antibodies.
Although pretransplantation plasma samples of the patients' stem cell donors were not available to us, we were able to obtain fresh plasma samples from 4 of these patients' donors. Healthy donors generally do not show CT antigen–specific antibodies in their plasma.5,6,25,26 However, we still evaluated the patients' donors for the presence of anti–CT antigen antibodies in order to exclude the possibility that anti–CT antigen immunity had simply been transplanted into the patients. We found that all of the donors were still in complete health and none of them showed antibody responses against any of the CT antigens. Importantly, at the time their response was detectable, all patients with myeloma had fully engrafted and showed more than 99% chimerism in their BM as analyzed by patient-specific real-time PCR, indicating that the antibody response was indeed entirely allogeneic. These findings led us to the conclusion that the allogeneic humoral immune response seemed to have developed in the patients at some point in time following alloSCT.
One of the antibody-positive patients (BMT47) had relapsed shortly before his antibody response against NY-ESO-1 was detected but the BM-infiltrating PCs, while they expressed MAGEA3, SSX1, and SSX4, were negative for NY-ESO-1. At the same time, an extramedullary plasmocytoma, showed strong expression of NY-ESO-1 and other CT antigens on the protein level (Figure 3), indicating that in this patient the immune response was indeed driven by antigen expressed in the recurrent disease.
In contrast, at the time we detected their CT antigen–specific antibody response, 8 of the 9 positive patients who had received alloSCT were still in near-complete remission without detectable expression of any CT antigens in their BM. These findings suggest that in the majority of antibody-positive patients the allogeneic humoral immune response had persisted in the absence of a detectable antigenic stimulus even after remission of the disease had been achieved.
Due to the very restricted expression pattern of CT antigens we considered it very unlikely that the strong and commonly observed antibody responses in our patients were simply part of a general autoimmune phenomenon in patients who had undergone alloSCT. Nevertheless, we analyzed post-alloSCT sera from 40 patients with acute myeloid leukemia (AML), which is considered an entity lacking expression of most CT antigens,20,27,28 for CT antigen–specific antibodies. All 40 patients with AML had experienced full engraftment and complete immune reconstitution at the time the sera were collected. Analyzing them for IgG antibodies against NY-ESO-1, MAGEA3, and SSX2 we did not observe any CT antigen–specific immune responses, underlining the conclusion that in our patients with myeloma the transplantation-induced immune response was indeed most likely myeloma-specific and was not simply a consequence of autoimmune reactions.
NY-ESO-1–specific T-cell responses in a patient with MM following alloSCT
Strong and persistent IgG antibody responses usually require the presence of antigen-specific help of T cells, and T cells are important for an effective antitumor response.29,30 We, therefore, performed a preliminary analysis of CT antigen–specific CD4+ and CD8+ T-cell responses in one of the NY-ESO-1 antibody–positive patients who had undergone alloSCT. As shown above, this patient had relapsed shortly before his antibody response was detected and evidenced strong expression of NY-ESO-1 in an extramedullary plasmocytoma (Figure 3).
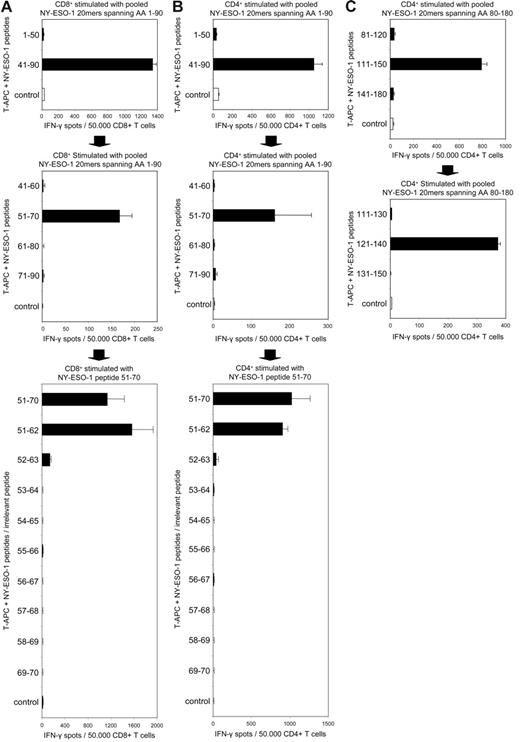
Using a panel of overlapping 20mer peptides spanning the complete sequence of CT antigen NY-ESO-1, we stimulated peripheral CD4+ and CD8+ T cells of patient BMT47 and analyzed them for peptide-specific responses in an ELISPOT assay. Although we did not observe CD8+ responses against epitopes in NY-ESO-1 region 91-180 (data not shown), stimulating the patient's cytotoxic T lymphocytes (CTLs) with peptides spanning NY-ESO-1 1-90 we detected a strong CD8+ T-cell response against 20mer NY-ESO-151-70 (Figure 5A). Interestingly, using the same pool of peptides for stimulation we also detected a CD4+ response against this peptide (Figure 5B). We next used a panel of overlapping 12mer peptides covering NY-ESO-1 sequence 51-70 in order to further define both epitopes and we were able to demonstrate that the CD8+ as well as the CD4+ response were both directed against NY-ESO-151-62 (Figure 5A-B). The strong responses against NY-ESO-151-62 were accompanied by a second CD4+ response that turned out to be specific for NY-ESO-1121-140 (Figure 5C).
CT antigen NY-ESO-1 elicits a strong allogeneic CD4+ and CD8+ T-cell response in a patient with MM who received allogeneic stem cell transplantation. ELISPOT assays for the determination of NY-ESO-1–specific CD4+ and CD8+ T cells in MM patient BMT47 were performed after a single cycle of antigen-specific stimulation using a series of overlapping 20mer peptides spanning the full sequence of NY-ESO-1. Stimulating CD4+ as well as CD8+ T cells with a pool of peptides covering NY-ESO-1 1-90, a CD8+ (A) and a CD4+ (B) response against NY-ESO-1 region 51-50 were detected. Using T-APCs pulsed with single 20mer peptides as targets, we found that both responses were directed against NY-ESO-151-70 (A, B). Using 12mer peptides overlapping by a single AA and spanning NY-ESO-1 region 51-70, we were able to further characterize the CD8+ and CD4+ responses as both being directed against NY-ESO-151-62 (A, B). Using pooled 20mer peptides covering NY-ESO-1 80-180, a second CD4+ response directed against NY-ESO-1 region 111-150 was detected (C). This CD4+ response was further defined as being specific for NY-ESO-1121-140 (C). MAGEA3 peptide 271-279 and NY-CO-58 peptide 151-180 served as control peptides (control) for analyses of NY-ESO-1–specific CD8+ and CD4+ responses, respectively. Bars show the mean spot number of duplicate ELISPOT experiments, with error bars indicating SEM.
CT antigen NY-ESO-1 elicits a strong allogeneic CD4+ and CD8+ T-cell response in a patient with MM who received allogeneic stem cell transplantation. ELISPOT assays for the determination of NY-ESO-1–specific CD4+ and CD8+ T cells in MM patient BMT47 were performed after a single cycle of antigen-specific stimulation using a series of overlapping 20mer peptides spanning the full sequence of NY-ESO-1. Stimulating CD4+ as well as CD8+ T cells with a pool of peptides covering NY-ESO-1 1-90, a CD8+ (A) and a CD4+ (B) response against NY-ESO-1 region 51-50 were detected. Using T-APCs pulsed with single 20mer peptides as targets, we found that both responses were directed against NY-ESO-151-70 (A, B). Using 12mer peptides overlapping by a single AA and spanning NY-ESO-1 region 51-70, we were able to further characterize the CD8+ and CD4+ responses as both being directed against NY-ESO-151-62 (A, B). Using pooled 20mer peptides covering NY-ESO-1 80-180, a second CD4+ response directed against NY-ESO-1 region 111-150 was detected (C). This CD4+ response was further defined as being specific for NY-ESO-1121-140 (C). MAGEA3 peptide 271-279 and NY-CO-58 peptide 151-180 served as control peptides (control) for analyses of NY-ESO-1–specific CD8+ and CD4+ responses, respectively. Bars show the mean spot number of duplicate ELISPOT experiments, with error bars indicating SEM.
We next analyzed the peptide specificity of all 3 T-cell responses performing peptide titration experiments (Figure 6A). Although these experiments were performed using polyclonal T-cell lines and not peptide-specific T-cell clones, the patient's NY-ESO-1 CD4+ and CD8+ T cells, at least in the case of the NY-ESO-151-62–specific cell lines, were able to recognize the antigen in nanomolar concentrations, thus demonstrating the high avidity and specificity of these T-cell responses.
NY-ESO-1–specific T cells are of high avidity, recognize the naturally processed antigen, and have cytolytic potential in a patient with MM after alloSCT. We analyzed the peptide specificity of the CD4+ T-cell responses against NY-ESO-151-70 and NY-ESO-1121-140 and of the CD8+ response against NY-ESO-151-70, performing an ELISPOT in combination with peptide titration experiments (A). Following a single cycle of antigen-specific stimulation with peptides NY-ESO-151-70 or NY-ESO-1121-140, patient BMT47's T cells were able, especially in the case of the NY-ESO-151-62–specific cell lines, to recognize the antigen in nanomolar concentrations and in a highly specific fashion. Irrelevant peptides are shown as an empty circle (NY-ESO-151-70–specific CD4+), triangle (NY-ESO-151-70–specific CD8+), and diamond (NY-ESO-1121-140–specific CD4+) and were used at a concentration of 10 μmol/mL. (A) When we analyzed whether the patient's NY-ESO-151-62–specific CTLs would demonstrate cytolytic potential in a granzyme B ELISPOT, we observed that the patient's CD8+ T cells indeed secreted this cytoloytic molecule upon exposure to autologous EBV-B cells pulsed with NY-ESO-1 peptide but not the irrelevant control (B). Furthermore, CD4+ as well as CD8+ T cells specific for NY-ESO-151-62 not only recognized this peptide but also produced IFN-γ in response to the naturally processed antigen in form of autologous EBV-B cells infected with vaccinia virus recombinant for full-length NY-ESO-1 (VV NY-ESO-1) (C). Irrelevant VV recombinant for influenza nucleoprotein (VV NP) was used as a control. Bars show the mean spot number of duplicate ELISPOT experiments, with error bars indicating SEM.
NY-ESO-1–specific T cells are of high avidity, recognize the naturally processed antigen, and have cytolytic potential in a patient with MM after alloSCT. We analyzed the peptide specificity of the CD4+ T-cell responses against NY-ESO-151-70 and NY-ESO-1121-140 and of the CD8+ response against NY-ESO-151-70, performing an ELISPOT in combination with peptide titration experiments (A). Following a single cycle of antigen-specific stimulation with peptides NY-ESO-151-70 or NY-ESO-1121-140, patient BMT47's T cells were able, especially in the case of the NY-ESO-151-62–specific cell lines, to recognize the antigen in nanomolar concentrations and in a highly specific fashion. Irrelevant peptides are shown as an empty circle (NY-ESO-151-70–specific CD4+), triangle (NY-ESO-151-70–specific CD8+), and diamond (NY-ESO-1121-140–specific CD4+) and were used at a concentration of 10 μmol/mL. (A) When we analyzed whether the patient's NY-ESO-151-62–specific CTLs would demonstrate cytolytic potential in a granzyme B ELISPOT, we observed that the patient's CD8+ T cells indeed secreted this cytoloytic molecule upon exposure to autologous EBV-B cells pulsed with NY-ESO-1 peptide but not the irrelevant control (B). Furthermore, CD4+ as well as CD8+ T cells specific for NY-ESO-151-62 not only recognized this peptide but also produced IFN-γ in response to the naturally processed antigen in form of autologous EBV-B cells infected with vaccinia virus recombinant for full-length NY-ESO-1 (VV NY-ESO-1) (C). Irrelevant VV recombinant for influenza nucleoprotein (VV NP) was used as a control. Bars show the mean spot number of duplicate ELISPOT experiments, with error bars indicating SEM.
Granzyme B is found exclusively in the cytoplasmic granules of cytolytic cells, and is important for granule-mediated killing induced by CTLs.31 When we analyzed whether the NY-ESO-151-62–specific CTLs would demonstrate cytolytic potential in a granzyme B ELISPOT assay, we observed that the patient's CD8+ T cells indeed secreted this molecule upon exposure to NY-ESO-1 (Figure 6B). Furthermore, CD4+ as well as CD8+ T cells specific for NY-ESO-151-62 not only recognized the peptide but also produced IFN-γ in response to the naturally processed antigen in the form of autologous EBV-B cells infected with vaccinia virus recombinant for full-length NY-ESO-1 (Figure 6C).
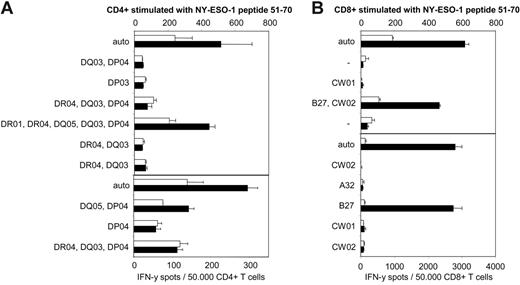
We next analyzed the HLA restriction pattern of all CD4+ and CD8+ T-cell responses using partially matched EBV-B cell lines as peptide-pulsed targets. While the CD4+ response against NY-ESO-151-62 seemed to be HLA-DQ5–restricted (Figure 7A), the CD8+ response against the same peptide was restricted by HLA-B27 (Figure 7B). We have, so far, not definitively identified the HLA restriction pattern of the second CD4+ response, directed against NY-ESO-1121-140. This response, however, seemed to be presented by a number of different HLA class II molecules, including HLA-DR1, HLA-DQ5, and possibly also HLA-DR4 (data not shown).
Identification of HLA restriction patterns of CD4+ and CD8+ responses against NY-ESO-151-62. Patient BMT47's CD4+ and CD8+ T cells stimulated with peptide NY-ESO-151-62 were tested in ELISPOT assays against partially matched allogeneic EBV-B cells pulsed with the same peptide. Patient BMT47 expressed the following alleles: HLA-A32 homozygous, HLA-B27 homozygous, HLA-CW01/HLA-CW02 for class I molecules, and HLA-DR01/HLA-DR04, HLA-DP03/HLA-DP04, and HLA-DQ03/HLA-DQ05 for class II molecules. The allele(s) the EBV lines were partially matched for are shown. Minus signs indicate completely mismatched targets and “auto” indicates autologous EBV-B cell targets. Only HLA-DQ5–expressing EBV-B cell lines were capable of presenting NY-ESO-151-62 to the CD4+ T-cell line (A), whereas the CD8+ response against the same peptide was clearly restricted by HLA-B27 (B). Black bars indicate specific responses against the NY-ESO-1 peptides whereas white bars show background responses against control peptides MAGEA3 271-279 for CD8+ and NY-CO-58 151-180 for CD4+ (mean spot numbers of duplicate experiments). Error bars indicate SEM.
Identification of HLA restriction patterns of CD4+ and CD8+ responses against NY-ESO-151-62. Patient BMT47's CD4+ and CD8+ T cells stimulated with peptide NY-ESO-151-62 were tested in ELISPOT assays against partially matched allogeneic EBV-B cells pulsed with the same peptide. Patient BMT47 expressed the following alleles: HLA-A32 homozygous, HLA-B27 homozygous, HLA-CW01/HLA-CW02 for class I molecules, and HLA-DR01/HLA-DR04, HLA-DP03/HLA-DP04, and HLA-DQ03/HLA-DQ05 for class II molecules. The allele(s) the EBV lines were partially matched for are shown. Minus signs indicate completely mismatched targets and “auto” indicates autologous EBV-B cell targets. Only HLA-DQ5–expressing EBV-B cell lines were capable of presenting NY-ESO-151-62 to the CD4+ T-cell line (A), whereas the CD8+ response against the same peptide was clearly restricted by HLA-B27 (B). Black bars indicate specific responses against the NY-ESO-1 peptides whereas white bars show background responses against control peptides MAGEA3 271-279 for CD8+ and NY-CO-58 151-180 for CD4+ (mean spot numbers of duplicate experiments). Error bars indicate SEM.
Although we did not have access to frozen pre–bone marrow transplantation peripheral blood lymphocyte (PBL) samples from the patient's related donor, we were able to obtain fresh PBL samples from the donor at the time we conducted our analysis. In repeated experiments we did not detect any NY-ESO-1–specific CD8+ or CD4+ T-cell responses in the donor's peripheral blood (data not shown), indicating that the T-cell response had indeed developed in the patient.
Importantly, in patient BMT47 CD4+ and CD8+ T-cell responses against all 3 epitopes were detectable in repeated analyses over a period of 5 months, proving the high persistence of this immune response. Furthermore, patient BMT47 had achieved 100% T-cell chimerism in the BM, indicating that these T-cell responses were indeed allogeneic.
In summary, our data indicate that CT antigens like NY-ESO-1 are able to elicit strong and persistent T-cell responses in patients with MM who have undergone alloSCT. These entirely allogeneic and high avidity CD4+ and CD8+ T-cell responses seem to develop in the patient at some point after transplantation and recognize the naturally processed tumor antigen with high efficiency.
Discussion
CT antigens represent ideal targets for immunotherapeutic approaches based on their restricted tissue expression and immunogenicity. Here, we present a comprehensive analysis of CT antigen expression in patients with MM, showing that these patients' BM infiltrated by malignant PCs shows a high degree of expression of a number of CT antigens and that this malignancy might, therefore, represent a prime candidate for the application of immunotherapies directed against CT antigens.
Similar to myeloma cell lines, PC-infiltrated BM of patients with myeloma highly expressed CT antigens such as CT10/MAGEC2, MAGEA3, and members of the SSX family of genes. Our finding of a significant expression of the latter 2 antigens confirm previous findings,19,21,32 but the expression of CT10/MAGEC2 has, so far, only been analyzed in a limited number of human tumor types33-38 and its expression pattern in hematologic malignancies has been largely unknown. We show here for the first time that CT10/MAGEC2 might represent one of the most frequently expressed CT antigens in myeloma, with more than 56% of patients expressing this antigen. The comparably high expression of CT10/MAGEC2 in MM may be of relevance for its potential use in cancer vaccines, since CT10/MAGEC2 has previously been shown to be capable of eliciting spontaneous antibody33,37,38 and CD8+ T-cell responses39 in patients with CT10/MAGEC2-positive solid tumors.
It has recently been reported that mesenchymal stem cells and normal human BM may express CT antigens NY-ESO-1, MAGE-A, and SSX.22 This finding is in apparent contrast to previous studies showing that CT antigens are not expressed in normal BM or CD34+ progenitors.6,19,21,40-42 Here, we performed the largest analysis of CT antigen expression in healthy BM to date and found no expression of any CT antigens in the vast majority of BM and CD34+ progenitor samples, indicating that no myelotoxicity should be expected from immunotherapeutic approaches targeting these antigens. SSX4, however, was expressed in a minority of samples, suggesting that this SSX gene family member might be responsible for previous findings of pan-SSX expression in healthy BM.22
In our current study, we have analyzed the majority of our myeloma patients' bone marrow samples on the RNA level. Previous studies examining the expression of CT antigens in myeloma, however, indicated a strong correlation between RNA and protein expression.5,21,43 Here, we provided evidence for strong protein expression of several CT antigens, including NY-ESO-1, in an extramedullary plasmocytoma of one of our patients. Interestingly, this patient also showed a strong immune response against NY-ESO-1, supporting the idea that the RNA expression of these genes in myeloma translates into biologically relevant levels of protein. Finally, we have demonstrated in an exemplary matter protein expression of CT10/MAGEC2 in myeloma, a CT antigen whose high expression we have described here for the first time in this malignancy. The high incidence and concordance of CT10/MAGE-C2 protein and mRNA expression in myeloma is corroborated by a separate analysis (A.A.J. and Hearn J. Cho, manuscript in preparation).
An indirect way to show that CT antigens expressed in MM on the mRNA level are also translated into protein is to demonstrate immune responses against these antigens. We show here for the first time a high frequency of anti–CT antigen antibody responses in patients with MM who had received alloSCT. In 9 (26%) of 35 of these patients an allogeneic humoral immune response had developed after transplantation. Remarkably, in 4 of these patients these antibodies were specific for NY-ESO-1, a CT antigen we found to be comparably infrequently expressed in myeloma, suggesting that NY-ESO-1 might play an important role in the immunology of MM and supporting the idea that NY-ESO-1 is one of the most immunogenic CT antigens.
Interestingly, the vast majority of antibody-positive patients were still in near-complete remission and none of them showed BM expression of the corresponding CT antigen. This observation is in contrast to findings in patients with solid tumors, suggesting that persistent expression of the antigen is required for the maintenance of a CT antigen–specific antibody response.25,44 We suggest a number of possible explanations for our observation of high-titered antibody responses against single CT antigens in the absence of expression of the given gene in the patient's BM. First, a BM aspirate represents only a very small fraction of the patient's total BM and small numbers of malignant PCs expressing the CT antigen might still be present in other areas within the BM compartment. Second, although this explanation seems unlikely, it is conceivable that malignant PCs expressing the respective CT antigen are residing in extramedullary tissues and that expression of the antigen is therefore not detectable by BM sampling. Last, it also seems conceivable that the CT antigen was indeed at some point significantly expressed by BM-residing PCs but that these cells were subsequently eliminated by an effective GVM immune response, the IgG antibody titers representing a remnant of this immune attack. The latter idea would be in line with findings in the field of infectiology where, following infection or vaccination with microbial components, persistent levels of specific antibodies are detectable in the human serum for decades. These antibodies are constantly produced by memory B cells even in the absence of antigen, and effectively protect against reinfection.45
In addition to a high-titered antibody response against NY-ESO-1, one of the patients studied had developed NY-ESO-1–specific CD4+ and CD8+ T-cell responses following alloSCT. One CD4+ response was directed against NY-ESO-1121-140, which might be identical to one of the class II epitopes previously identified in this region of NY-ESO-1.46-50 The second CD4+ response was specific for NY-ESO-151-62 and was restricted by HLA-DQ5, representing a newly defined NY-ESO-1 epitope. Interestingly, we also observed a CD8+ T-cell response against NY-ESO-151-62, suggesting that this region of the NY-ESO-1 sequence may be of high immunogenicity in vivo. The CTL response we observed represents the first HLA-B27–restricted epitope of NY-ESO-1 and it might be that this epitope is related to NY-ESO-153-62, previously described as being HLA-A31 restricted.51 Importantly, CD4+ as well as CD8+ responses against NY-ESO-151-62 both recognized the naturally processed antigen and secreted granzyme B in response to NY-ESO-1.
For more than 2 decades, the principle goal in the field of alloSCT has been to segregate beneficial GVL from life-threatening GVHD. Any progress in this area depends on the identification of relevant structures targeted by GVL mechanisms. CT antigens, however, have so far not been considered such targets.3 We suggest that screening patients with MM who underwent alloSCT for immune responses might be a promising way to identify biologically relevant targets for alloSCT and other immunotherapies. The data presented here strongly indicate that CT antigens may belong to this category of targets.
The question remains, how might alloSCT induce a natural immune response against CT antigens? First, chemotherapy applied prior to transplantation might lead to increased tumor necrosis and, subsequently, to a release of tumor antigen contributing to the development of immune responses against CT antigens.44 Second, it has been clear for some time now that lymphodepletion by chemotherapeutic regimen boosts antitumor immunity exerted, for example, by adoptively transferred tumor-reactive T cells in mouse and in humans.52,53 Enhanced activation and availability of APC, increased access to homeostatic cytokines through elimination of cytokine sinks, and eradication of the suppressive influence of regulatory T cells appear to be the underlying mechanisms in this paradigm.53 Third, the allogeneic immune environnment itself might provide enough “danger signals” to guarantee a persistent and effective immune response and might keep self-limiting immune mechanisms from downregulating the antitumor immune response.
In conclusion, CT antigens frequently expressed in MM represent promising candidates for cancer-specific immunotherapy in patients with myeloma. The application of polyvalent cancer vaccines, especially in the allogeneic setting, could help to boost and also broaden a posttransplantation anti–CT antigen immune response and might contribute to preventing recurrences in patients with MM.
Authorship
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Djordje Atanackovic, Department of Medicine II, Oncology/Hematology, University Medical Center Hamburg-Eppendorf, Martinistrasse 52, 20246 Hamburg, Germany; e-mail: d.atanackovic@uke.uni-hamburg.de.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
This work was supported by grants from the Erich und Gertrud Roggenbuck-Stiftung, Eppendorfer Krebs- und Leukämiehilfe eV, and from the Cancer Research Institute (D.A.).