Abstract
Antiphospholipid antibodies (APLAs) promote inflammatory and procoagulant responses in endothelial cells and monocytes. Previous studies have shown that MyD88, TRAF6, and NF-κB mediate cell activation by APLAs. These intermediates are also used by toll-like receptors (TLRs). We investigated the role of TLRs in the cellular response to APLAs. IgGs were isolated from the plasma of 5 patients with antiphospholipid syndrome along with immunopurified anti–β2-glycoprotein 1 IgG from a sixth patient. Control IgG was obtained from a pool of healthy donor plasmas negative for APLAs. Wild-type mouse embryonic fibroblasts (EFs) and EFs deficient in TLR1, TLR2, TLR4, or TLR6 were incubated with APLAs, anti–β2-glycoprotein 1 IgG, or control IgG. On incubation with the patient IgG, but not control IgG, a significant increase in mRNA levels of the inflammatory marker proteins MCP-1, ICAM-1, and IL-6 as well as IL-6 secretion was observed in wild-type EFs, whereas TLR2-deficient EFs did not respond. Responses in TLR1- and TLR6-deficient EFs were decreased and those in TLR4-deficient EFs comparable to those in wild-type EFs. Overexpression of human TLR2 in the TLR2-deficient EFs restituted the response to patient IgG. Our results imply that TLR2 plays a role in mouse fibroblast activation by APLAs.
Introduction
The antiphospholipid syndrome (APS) is a systemic autoimmune disease characterized by the occurrence of arterial or venous thrombosis, fetal loss, and persistent presence of circulating antiphospholipid antibodies (APLAs) such as lupus anticoagulant (LA), anticardiolipin antibodies, or antibodies against β2-glycoprotein 1 (β2GP1).1,2 These antibodies often coexist and target intravascular proteins, either alone or in complex with anionic phospholipids. In recent years, increasing evidence has revealed cell-based pathogenic mechanisms.3 APLAs, or antibodies to β2GP1, activate endothelial cells (ECs) leading to an increase in leukocyte adhesion molecule and tissue factor expression, chemokine and cytokine secretion, and monocyte adhesion.4-8 Furthermore, APLAs activate monocytes and platelets, the latter in the presence of subactivating doses of thrombin.9-12 The activation of these cells leads to inflammatory and procoagulant responses that may underlie the hypercoagulable state that characterizes APS.13,14 The mechanisms of cell activation by APLAs are still incompletely understood. The activation of ECs is accompanied by the nuclear translocation of NF-κB15,16 and by activation of p38 MAP kinase17,18 and is mediated by MyD88 and TRAF6, which are common adaptor proteins required for cell activation through receptors of the toll-like receptor (TLR) family.19
TLRs are key components of the innate immune response20 and recognize pathogen-associated molecular patterns (PAMPs) produced by microbes or viruses.21 TLR4, in association with CD14 and MD2, specifically binds LPS from Gram-negative bacteria, whereas TLR2 functions as a heterodimer with TLR1 or TLR6 and recognizes a variety of bacterial components such as lipoprotein, peptidoglycan (PGN), zymosan, or macrophage-activating lipopeptide of 2 kDa (MALP-2).22-25 TLRs are type 1 membrane proteins with an extracellular region containing leucine-rich repeats that participate in receptor-ligand interactions.26 The intracellular domain contains a conserved region called the toll/IL-1 receptor homology region (TIR). On TLR stimulation, the TIR domains interact and recruit adaptor proteins such as MyD88 for signal transduction, leading to the nuclear translocation of NF-κB, which increases the expression of inflammatory genes.27 Also, MAP kinase pathways are activated after exposure to TLR ligands.28
The aim of this study was to investigate whether one or more members of the TLR family mediate the inflammatory response to APLAs. As a model system for studying cellular responses to APLAs in the complete absence of a particular TLR, we used mouse embryonic fibroblasts deficient in TLR1, TLR2, TLR4, or TLR6. To detect fibroblast activation we measured changes in expression of 3 inflammatory marker proteins: MCP-1 as a representative inflammatory chemokine, ICAM-1 as a leukocyte adhesion molecule, and IL-6 as an inflammatory cytokine. We observed that the fibroblasts deficient in TLR2 were unresponsive to APLAs and that the expression of human TLR2 in the mouse TLR2-deficient fibroblasts restored the responsiveness to normal levels.
Materials and methods
Reagents
Mouse TNF-α (mTNF-α) was from R&D Systems (Minneapolis, MN); highly purified LPS from Escherichia coli R515, Pam3-Cys-Ser-(Lys)4 (Pam3CSK4), and MALP-2 were from Alexis (Lausen, Switzerland). Monoclonal mouse anti–human TLR2 antibodies (clone TL2.1) and rat anti–mouse TLR4/MD2 antibodies (clone MTS510) were from Alexis; mouse anti–mouse TLR2 antibodies (clone TL2.5) were from HyCult Biotechnology (Uden, The Netherlands). These antibodies efficiently block the activity of the respective TLRs. Isotype-matched control antibodies were from Ancell (Bayport, MN); R-PE-conjugated F(ab)′2 goat anti–mouse (H+L) IgG was from Jackson ImmunoResearch Laboratories (West Grove, PA).
Fibroblast cell culture
Mouse embryonic fibroblasts (EFs) deficient in TLR1 (TLR1−/−EFs), TLR2 (TLR2−/−EFs), TLR4 (TLR4−/−EFs), or TLR6 (TLR6−/−EFs) as well as wild-type EFs (WT-EFs) were a kind gift of Dr O. Takeuchi (Research Institute for Microbial Disease, Osaka University, Osaka, Japan).22,23,29,30 All mice had the same genetic background. Cells were grown in DMEM with 4.5 g/L glucose, GlutaMax I (Gibco BRL-Life Technologies, Rockville, MD), and sodium pyruvate, supplemented with 100 IU/mL penicillin, 100 μg/mL streptomycin, and 10% decomplemented fetal bovine serum (FBS; Gibco BRL-Life Technologies, Rockville, MD). Cells were tested for mycoplasma contamination using the mycoAlert Kit (Cambrex Bio Science, Verviers, Belgium). Briefly, cell supernatants were incubated with lysis buffer containing a substrate for mycoplasma-specific cytosolic ATP-generating enzymes. ATP generation is measured by a luciferase-based assay. All cells were negative for mycoplasma. Cells were used between passages 3 and 10 after isolation.
Patients and isolation of IgG
Six patients were selected at the Hemostasis Unit of the University Hospital of Geneva. The selection criteria were that (1) they were diagnosed for APS according to the Sydney criteria,1 (2) their plasma was positive for anti-β2GP1 antibodies by enzyme-linked immunosorbent assay (ELISA),31 and (3) the purified IgG fractions stimulated WT-EFs and human umbilical vein endothelial cells as described previously.32 The clinical and laboratory characteristics of the patients are shown in Table 1. Plasma samples were obtained after approval by the institutional ethics committee of the University Hospital of Geneva, in accordance with the Declaration of Helsinki and written informed consent was obtained from all the patients included in the study. IgG fractions (APLAs) were isolated from patient plasma on protein-A CL-4B Sepharose (Amersham Pharmacia Biotech, Uppsala, Sweden) and protein levels measured by the BCA protein assay (Pierce, Rockford, IL). The IgGs were tested after purification for β2GP1 binding at 500 μg/mL.31 Negative control antibodies were an IgG pool from 10 healthy donor plasmas. Endotoxin levels were measured by the Limulus Amebocyte Lysate Endochrome Assay (Charles River Laboratories, Charleston, SC), and found to be below the detection limit (0.5 EU/mg) for all IgG fractions.
For patient 6, IgGs directed against β2GP1 were purified from total IgG by passage over a 5-mL column of β2GP1 conjugated to Affi-Gel-Hz (Bio-Rad Laboratories, Hercules, CA; 5 mg β2GP1/mL gel). After washing, bound IgG was eluted using 0.2 M glycine, pH 2.5. The pH of fractions was neutralized by addition of 1:7 vol/vol of 1 M Tris, pH 9.0. The immunopurified IgG (β2GP1-IgG) was quantified by BCA assay, controlled by sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE), and tested for its ability to bind to β2GP1 by ELISA.31 The β2GP1-IgG was also tested negative for endotoxin.
Fibroblast activation
Fibroblasts were grown to confluence in 24-well plates. The culture medium was changed prior to the addition of the following stimuli: 100 ng/mL mTNF-α, 1 μg/mL LPS, 100 ng/mL Pam3CSK4, 100 ng/mL MALP-2, 500 μg/mL APLA or control IgG, or 25 μg/mL β2GP1-IgG. After 4 or 8 hours of stimulation at 37°C, cell culture supernatants were collected for IL-6 quantification and mRNA isolated from the cells. In some experiments blocking antibodies to human or murine TLR2 or murine TLR4 were added at 10 μg/mL to the medium 20 minutes prior to addition of APLAs.
Two techniques to control for fibroblast viability were used. In one, we checked for morphologic changes under microscopy at the start and the end of the incubation period. In addition, we performed colorimetric viability assays using the CellTiter 96 Aqueous One solution Cell proliferation Assay (MTS) kit from Promega (Madison, WI) to verify for each lineage that the cell number was equivalent in confluent wells.
qRT-PCR
Total RNA was extracted from cells and treated with DNAseI using the RNeasy Micro kit (Qiagen, Hilden, Germany). Total RNA (1 μg) was used for reverse transcription using the ImPromII reverse transcription system and oligo-d(T)15 primers (Promega). Negative controls were processed in parallel without reverse transcriptase.
Mouse MCP-1, ICAM-1, and IL-6 mRNA levels were determined by quantitative reverse transcription-polymerase chain reaction (qRT-PCR) using cDNA obtained from the reverse transcription reactions as template, with an Applied Biosystems Prism 7000 instrument and SYBR-Green master mix reagent (Applied Biosystems, Foster City, CA). Mouse β-actin was used as the housekeeping gene. Data were analyzed using the ΔCT method and Applied Biosystems Prism software, according to the manufacturer's instructions. Primer sequences are given in Table S1 (available on the Blood website; see the Supplemental Table link at the top of the online article). For each primer pair, we checked that a single temperature dissociation curve was obtained and that the amplification product had the expected size.
PCR analysis for genotyping the TLR-deficient EFs
Genomic DNA (gDNA) from 2 × 105 TLR-deficient EFs or WT-EFs was extracted using the Protrans DNA kit (Endotell, Allschwil, Switzerland). The detection of TLR1, TLR2, TLR4, and TLR6 gene sequences was performed by PCR amplification using the conditions and primers described in Table S1. The PCR products were analyzed on 1% agarose gel (Q-Biogene, Irvine, CA).
IL-6 quantification by ELISA
IL-6 was quantified in the cell culture supernatant using the mouse IL-6 ELISA kit (R&D Systems) and Maxisorp (Nunc, Roskilde, Denmark) microtiter plates, according the manufacturer's protocol. Absorbances were measured at 450 nm and IL-6 concentrations determined using an internal standard curve. IL-6 secretion was expressed as the ratio of IL-6 concentration measured in stimulated-fibroblast supernatant versus IL-6 concentration detected in nonstimulated-fibroblast supernatant.
Stable expression of human TLR2 in mouse TLR2-deficient fibroblasts
Preparation of lentiviral vectors for human-TLR2.
The human TLR2 cDNA (a gift from Dr T. Espevik, Institute of Cancer Research and Molecular Biology, Trondheim, Norway) was cloned into the lentiviral plasmid pLoxEWiresEGFP (a gift from Dr P. Salmon, University Medical Center, Geneva33 ), which carries the enhanced green fluorescent protein (EGFP) downstream of an internal ribosomal entry site (IRES). Both EGFP- and TLR2-encoding sequences are under the control of the human elongation factor 1 α (EF1α) promoter and are expressed on the same mRNA. Empty pLoxEGFP plasmid was used as plasmid for control lentivirus preparation. For lentiviral vector production 2 other plasmids were used: pCMVR8.91 encodes HIV structural genes and pMD.G bears the VSV-G envelope cDNA (gifts from Dr P. Salmon).
Lentiviral vectors were produced by cotransfection of 293T cells with the 3 plasmids, essentially as described before.34,35 Sixteen hours after transfection, the medium was changed. Forty-eight hours later, the supernatant containing the lentiviral particles was collected, centrifuged (900g, 5 minutes), passed through 0.45-μm sterile filters, concentrated 100 times using an Amicon Ultra-15, 100 000 MCO filter (Millipore, Billerica, MA), and stored at −80°C. The recombinant DNA work and production of lentiviral vectors were done according to the Swiss Federal Guidelines for Work with Genetically Modified Organisms.
Transduction of TLR2-deficient fibroblasts.
TLR2−/−EFs were seeded at low density (30% confluence) in 6-well plates. The lentivirus for expression of human TLR2 (hTLR2) or the control lentivirus was added dropwise onto TLR2−/−EF monolayers (20 μL/well). After overnight incubation, the culture medium was changed and cells were grown to confluence. Expression of EGFP was used as a marker to select hTLR2-expressing cells or control EGFP-expressing cells using a fluorescent activated cell sorter (FACSVantage; Becton Dickinson, San Jose, CA). TLR2−/−EFs transduced with hTLR2 or vector control were sorted 3 times to obtain at least 95% EGFP+ cells in both populations. The expression of hTLR2 in these cells was analyzed by qRT-PCR. The cell surface expression of hTLR2 in the transduced TLR2−/−EFs was analyzed by flow cytometry using 10 μg/mL mouse anti–human TLR2 antibodies (clone TL2.1) and R-PE-conjugated F(ab)′2 goat anti–mouse IgG.
All cells were tested for mycoplasma infection and found to be negative. The stability of EGFP expression was controlled by measuring EGFP expression in the transduced cells by flow cytometry over several weeks of cell culture. No decrease in fluorescence intensity was observed.
Statistical analysis
Data for fibroblast stimulation are expressed as mean ± standard error (SE) values of at least 5 independent experiments per patient IgG. Statistical analyses for determination of the significance of the differences in the responses of the TLR-deficient cells to the various APLA samples were performed on log-transformed data to obtain homoscedasticity of the variances. Then, comparisons were performed using the 2-way ANOVA test and Bonferroni correction for multiple comparison was applied.
Results
Characterization of TLR-deficient mouse fibroblasts
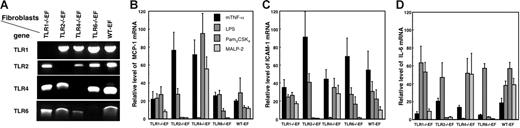
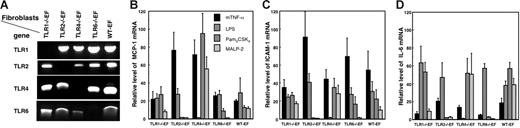
To investigate the cellular response to APLAs, in the absence of individual TLRs, we used mouse EFs isolated from TLR-deficient mice. The genotype of the EFs was verified by PCR and their response to TLR agonists measured. Figure 1 A shows that the TLR1−/−EFs, TLR2−/−EFs, TLR4−/−EFs, and TLR6−/−EFs, indeed, lack the deleted TLR DNA, but bear the other TLR genes. WT-EFs had all 4 TLR genes intact. We verified the response of the different EF lines to several key agonists: Pam3CSK4, a triacylated lipopeptide that reacts preferentially with the TLR2/TLR1 heterodimer; MALP-2, a diacylated lipopeptide specific for TLR2/TLR6; and highly purified LPS for TLR4. Cell stimulation was measured by qRT-PCR for MCP-1, ICAM-1, and IL-6 mRNA. Figure 1 shows that: (1) all cells responded to mTNF-α as a positive control; (2) all, except the TLR4−/−EFs, responded to LPS; (3) TLR2−/−EFs did not respond to Pam3CSK4 and MALP-2; (4) TLR6−/−EFs did not respond to MALP-2; and (5) TLR1−/−EFs responded to Pam3CSK4 at the concentrations used. The latter result is in accordance with previous studies showing that Pam3CSK4 stimulation is only partially dependent on TLR1.30,36 Taken together, these results validate the genotype and phenotype of the TLR-deficient fibroblasts.
Genotype and agonist responses of TLR-deficient and WT-EFs. gDNA was extracted from the TLR-deficient and wild-type EFs and amplified by PCR using primers for amplification of TLR1, TLR2, TLR4, or TLR6 DNA as described in Table S1. The position of the amplified bands was as expected for the size of the different PCR products (A). To analyze their functional characteristics, the fibroblasts were stimulated for 4 hours with 100 ng/mL Pam3CSK4 (a TLR1/TLR2 agonist), 100 ng/mL MALP-2 (a TLR2/TLR6 agonist), 1 μg/mL LPS (a TLR4 agonist), or 100 ng/mL mTNF-α. MCP-1 (B), ICAM-1 (C), and IL-6 (D) mRNA were quantified by qRT-PCR and expressed as relative mRNA level, which corresponds to the ratio of mRNA in cells incubated with agonists over that in cells incubated without agonists. The mRNA expression level of the samples was normalized to β-actin mRNA. Data are mean values ± SE of 3 independent experiments.
Genotype and agonist responses of TLR-deficient and WT-EFs. gDNA was extracted from the TLR-deficient and wild-type EFs and amplified by PCR using primers for amplification of TLR1, TLR2, TLR4, or TLR6 DNA as described in Table S1. The position of the amplified bands was as expected for the size of the different PCR products (A). To analyze their functional characteristics, the fibroblasts were stimulated for 4 hours with 100 ng/mL Pam3CSK4 (a TLR1/TLR2 agonist), 100 ng/mL MALP-2 (a TLR2/TLR6 agonist), 1 μg/mL LPS (a TLR4 agonist), or 100 ng/mL mTNF-α. MCP-1 (B), ICAM-1 (C), and IL-6 (D) mRNA were quantified by qRT-PCR and expressed as relative mRNA level, which corresponds to the ratio of mRNA in cells incubated with agonists over that in cells incubated without agonists. The mRNA expression level of the samples was normalized to β-actin mRNA. Data are mean values ± SE of 3 independent experiments.
Response of TLR-deficient mouse fibroblasts to APLAs
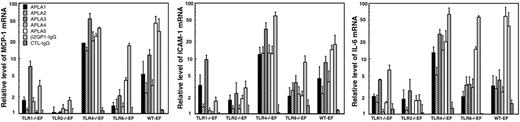
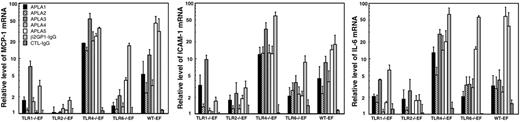
We investigated by qRT-PCR to what extent one or more TLRs are involved in the response of mouse fibroblasts to APLAs. Preliminary experiments were performed to determine the minimum concentration of APLA able to activate WT-EFs and the time dependence of the responses. The minimal concentration of IgG inducing a response was 250 μg/mL for the APLA and 10 μg/mL for the β2GP1-immunopurified IgG. Time-course experiments showed that a maximum response was obtained between 4 to 16 hours of stimulation. To limit eventual positive or negative feedback responses due to release of regulatory proteins by the fibroblasts, most of the experiments were done after 4 hours of stimulation. We incubated EFs with 500 μg/mL of an IgG fraction purified from the plasma of 5 patients with APS (APLA1-5) and with 25 μg/mL of an IgG fraction that was immunopurified on β2GP1 (β2GP1-IgG) from the plasma of a sixth patient with APS. Fibroblast stimulation was performed in the presence of 10% FBS, which provides plasma cofactors such as β2GP1. Cell activation was detected by measuring the changes in mRNA levels of MCP-1, ICAM-1, and IL-6. Results are expressed as relative mRNA level in cells incubated with IgG compared to cells incubated without IgG. Each individual APLA induced a significant increase in MCP-1, ICAM-1, and IL-6 mRNA levels in the WT-EFs (Figure 2). The average responses of WT-EFs to the 6 APLAs is given in Table 2. No increase was observed for TLR2−/−EFs (Figure 2; Table 2), even with APLA concentrations as high as 1500 μg/mL. The lack of response of TLR2−/−EFs to APLAs was not due to a delay in the response to the antibodies. Indeed, no stimulation of the TLR2−/− EFs was observed at any time point between 4 and 24 hours after incubation with 25 μg/mL immunopurified β2GP1-IgG or 500 μg/mL APLA from 2 patients, whereas in WT-EFs a strong response was observed (Table 3).
Stimulation of TLR-deficient and WT-EFs by APLAs. TLR-deficient and wild-type EFs were incubated for 4 hours with 500 μg/mL APLAs from 5 patients or with 25 μg/mL β2GP1 immunopurified IgG (β2GP1-IgG) obtained from a sixth patient. MCP-1, ICAM-1, and IL-6 mRNAs were quantified by qRT-PCR and expressed as relative mRNA levels, which correspond to the ratio of mRNA in cells incubated with APLAs over that in cells incubated without APLAs. Values are expressed as mean ± SE of at least 5 independent experiments per patient IgG.
Stimulation of TLR-deficient and WT-EFs by APLAs. TLR-deficient and wild-type EFs were incubated for 4 hours with 500 μg/mL APLAs from 5 patients or with 25 μg/mL β2GP1 immunopurified IgG (β2GP1-IgG) obtained from a sixth patient. MCP-1, ICAM-1, and IL-6 mRNAs were quantified by qRT-PCR and expressed as relative mRNA levels, which correspond to the ratio of mRNA in cells incubated with APLAs over that in cells incubated without APLAs. Values are expressed as mean ± SE of at least 5 independent experiments per patient IgG.
A weak response to incubation with 500 μg/mL of the APLA or 25 μg/mL of β2GP1-IgG was observed in the TLR1−/−EFs and an intermediate response was found in the TLR6−/−EFs (Figure 2; Table 2). The response of the TLR4−/−EFs was on average 1.9 times higher than that of the WT-EFs (Figure 2; Table 2). No change in relative mRNA levels was observed with control IgG (Figure 2; Table 2). Statistical analysis of the responses of the 5 fibroblast lineages to the 6 APLAs indicated a significantly lower response (P < .001) for TLR2−/−EFs, as compared to TLR4−/−EFs and WT-EFs, for the 3 markers.
To test whether other autoantibodies than APLAs might stimulate the mouse fibroblasts, we purified IgG from 2 patients with systemic lupus erythematosus without APLAs and from 2 patients with scleroderma. None of these IgG fractions were able to activate the fibroblasts (Table 2).
Response of human TLR2-complemented TLR2−/−EFs to TLR2 agonists
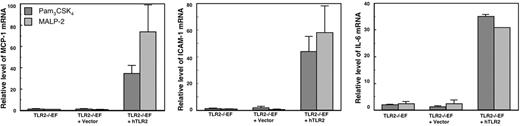
Because TLR2−/−EFs did not respond to APLAs, we expressed human TLR2 in the TLR2−/−EFs to see whether the response to APLAs could be restored. We used lentivirus-mediated gene transfer for stable expression of hTLR2 in the TLR2−/−EFs. As a control, we infected TLR2−/−EFs with a control lentiviral vector. The TLR2 lentiviral vectors express EGFP from an IRES situated downstream of the hTLR2-encoding sequence, whereas the control vector has only IRES-EGFP. After 3 rounds of cell sorting using a fluorescence- activated cell sorter, both cell populations were more than 95% positive for EGFP. In the cells transduced with TLR2-IRES-EGFP both proteins are expressed from the same mRNA. Therefore, 95% of the transduced cells should also express hTLR2. Nevertheless, we verified expression of hTLR2 by qRT-PCR and flow cytometry and its functionality by studying the cellular response to TLR2-specific agonists. By qRT-PCR, hTLR2 mRNA was detectable in the hTLR2-transduced cells, but not in the cells transduced with the control lentiviral vector (data not shown). Flow cytometry analysis revealed the expression of hTLR2 at the surface of the hTLR2-transduced TLR2−/−EFs, whereas no hTLR2 was detectable on the control transduced EFs (data not shown).
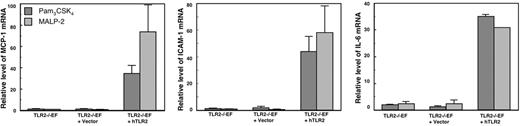
To verify that the hTLR2 expressed by the hTLR2-transduced TLR2−/−EFs was functional, we stimulated the hTLR2-transduced TLR2−/−EFs with Pam3CSK4 and MALP-2, specific agonists of the TLR2 heterodimers, compared the responses to those of control lentiviral vector-transduced TLR2−/−EFs and of TLR2−/−EFs. Transduction of hTLR2 in TLR2−/−EFs restored the response to Pam3CSK4 and MALP-2, whereas TLR2−/−EFs transduced with lentivector alone as well as TLR2−/−EFs did not respond (Figure 3). Thus, hTLR2 is functional in the hTLR2-transduced TLR2−/−EFs. All 3 cell lines responded equally well to mTNF-α and LPS (data not shown), which implies that lentiviral vector gene transfer had not affected downstream signaling pathways, which, to a large extent, are shared by TLR2, TLR4 (activated by LPS), and TNF.
Response to TLR2-specific agonists of TLR2−/−EFs transfected with hTLR2. Responses were compared for TLR2−/−EFs or TLR2−/−EFs transfected with hTLR2 (TLR2−/−EF + hTLR2) or with the empty vector (TLR2−/−EF + vector). The cells were stimulated for 4 hours with 100 ng/mL Pam3CSK4 or 100 ng/mL MALP-2. The results show mRNA levels of MCP-1, ICAM-1, and IL-6 quantified by qRT-PCR. Data are mean value ± SE of 3 independent experiments.
Response to TLR2-specific agonists of TLR2−/−EFs transfected with hTLR2. Responses were compared for TLR2−/−EFs or TLR2−/−EFs transfected with hTLR2 (TLR2−/−EF + hTLR2) or with the empty vector (TLR2−/−EF + vector). The cells were stimulated for 4 hours with 100 ng/mL Pam3CSK4 or 100 ng/mL MALP-2. The results show mRNA levels of MCP-1, ICAM-1, and IL-6 quantified by qRT-PCR. Data are mean value ± SE of 3 independent experiments.
Response of human TLR2-transduced TLR2−/−EFs to APLAs
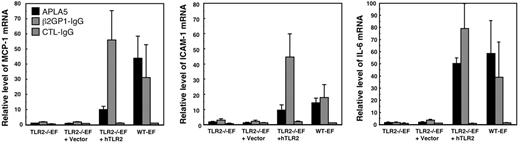
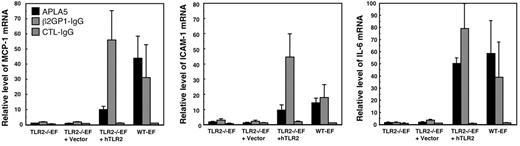
We investigated whether expression of hTLR2 in TLR2−/−EFs enable them to respond to APLAs. For this experiment we used the IgG fraction from patient 5 and the β2GP1-IgG. Cell activation was detected by quantifying the mRNA level of MCP-1, ICAM-1, and IL-6 by qRT-PCR. As shown in Figure 4, both APLAs from patient 5 and β2GP1-IgG stimulated hTLR2-transduced TLR2−/− EFs to the same extent as WT-EFs, whereas the TLR2−/−EFs or the TLR2−/−EFs transduced with vector alone did not respond to the 2 patient IgG preparations. Control IgG induced no response in any of the fibroblasts.
Response of the hTLR2-transfected TLR2−/−EFs to APLA5 and β2GP1-IgG. The cells were stimulated for 8 hours with 500 μg/mL APLA from patient 5 or control IgG, or with 25 μg/mL β2GP1-IgG from patient 6. The results show mRNA levels of MCP-1, ICAM-1, and IL-6 quantified by qRT-PCR. Data are mean value ± SE of 5 (APLA5) to 9 (β2GP1-IgG) independent experiments.
Response of the hTLR2-transfected TLR2−/−EFs to APLA5 and β2GP1-IgG. The cells were stimulated for 8 hours with 500 μg/mL APLA from patient 5 or control IgG, or with 25 μg/mL β2GP1-IgG from patient 6. The results show mRNA levels of MCP-1, ICAM-1, and IL-6 quantified by qRT-PCR. Data are mean value ± SE of 5 (APLA5) to 9 (β2GP1-IgG) independent experiments.
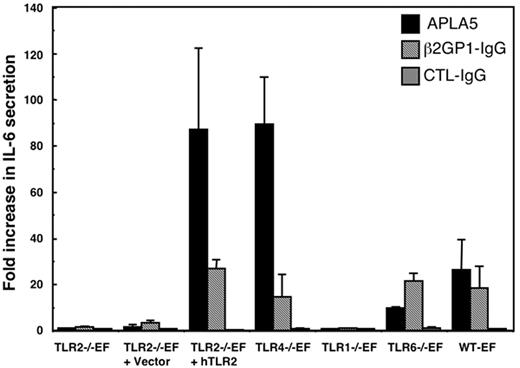
Quantification of IL-6 secretion by the fibroblasts after stimulation with APLAs
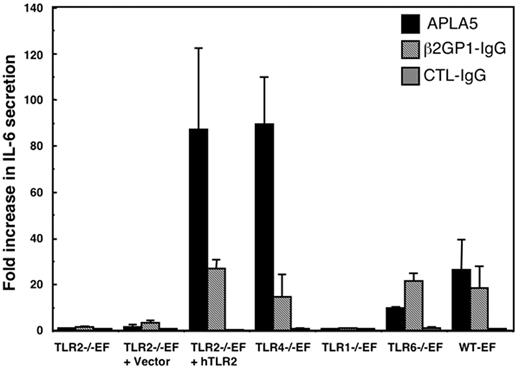
The role of TLR2 in cellular activation by APLAs was also studied at the protein expression level, by measuring IL-6 in cell supernatants. The various TLR-deficient EFs, as well as hTLR2-transduced TLR2−/−EFs and WT-EFs, were treated with APLA from patient 5, with β2GP1-IgG, and with control IgG for 8 hours, to allow accumulation of IL-6 in the cell supernatant. APLA from patient 5 induced an 80-fold increase of IL-6 secretion by the hTLR2-transduced TLR2−/−EFs and by the TLR4−/−EFs, and about a 20-fold increase by the TLR6−/−EFs and WT-EFs, as compared to nonstimulated cells (Figure 5). β2GP1-IgG induced about a 20-fold increase of IL-6 secretion by hTLR2-tranduced TLR2−/−EFs, TLR4−/−EFs, TLR6−/−EFs, and WT-EFs. No increase of IL-6 secretion was detected in the supernatants of TLR1−/−EFs, TLR2−/− EFs, or vector-transduced TLR2−/−EFs after stimulation with both APLA preparations (Figure 5). Control IgG had no effect on IL-6 secretion in any of the cells tested.
Quantification of IL-6 secretion induced by APLAs. TLR2−/−EFs, TLR2−/−EFs transduced with control vector or vector for hTLR2, EFs deficient in TLR1, TLR4, or TLR6, or WT-EFs were treated for 8 hours with 500 μg/mL APLA5 or control IgG, or with 25 μg/mL β2GP1-IgG. IL-6 concentrations in the culture medium of the cells were quantified by ELISA. Data are expressed as fold increase of IL-6 quantified in the supernatant of stimulated cells versus IL-6 quantified in the supernatant of unstimulated cells. Data are mean ± SE of at least 5 independent experiments.
Quantification of IL-6 secretion induced by APLAs. TLR2−/−EFs, TLR2−/−EFs transduced with control vector or vector for hTLR2, EFs deficient in TLR1, TLR4, or TLR6, or WT-EFs were treated for 8 hours with 500 μg/mL APLA5 or control IgG, or with 25 μg/mL β2GP1-IgG. IL-6 concentrations in the culture medium of the cells were quantified by ELISA. Data are expressed as fold increase of IL-6 quantified in the supernatant of stimulated cells versus IL-6 quantified in the supernatant of unstimulated cells. Data are mean ± SE of at least 5 independent experiments.
To further investigate the role of TLR2 or TLR4, we preincubated WT-EFs with activity-blocking TLR2 or TLR4 antibodies. In cells treated 8 hours with 500 μg/mL APLA from patient 5, release of IL-6 in the medium was over 99% reduced by anti–murine TLR2 antibodies as compared to isotype-matched control antibodies or in conditions without blocking antibodies (16.3 ± 0.7 pg/mL versus 2500 ± 50 pg/mL and 2550 ± 50 pg/mL, respectively). Untreated cells present basal levels of IL-6 of about 15.09 ± 9 pg/mL. Antimurine TLR4 had no effect on IL-6 release (2530 ± 65 pg/mL).
Discussion
APLAs are heterogeneous antibodies that promote arterial and venous thrombosis in patients with APS. Endothelial cell activation by APLAs leading to the expression of an inflammatory and procoagulant state has been proposed as a factor contributing to the pathogenic effects of these autoantibodies. The precise mechanisms involved in this process are still not completely elucidated. It is known that APLAs induce the nuclear translocation of NF-κB15,16 and the activation of p38 MAP kinase17,18 and that the intracellular signaling molecules MyD88 and TRAF6 are required.19 These observations made TLRs good candidates for mediating cell activation by APLAs. To establish which TLR mediates cell activation we used murine fibroblasts. The choice of murine model cells is justified because injection of human APLAs into mice leads to complications such as thrombophilia or pregnancy loss, which are typical of human APS.4,37 In preliminary studies we observed that WT-EFs respond to APLAs by an increased expression of inflammatory marker proteins. This implies that all proteins necessary for cell activation by APLAs are present in the murine fibroblast cell system. The availability of mouse EFs deficient in TLR1, TLR2, TLR4, or TLR6 allowed us to directly study the consequences on cell activation of the absence of only one TLR. A role for fibroblasts in the APS has not been established at present, but our finding that they respond to APLAs implies that they might contribute to the pathology of this disease. Indeed, fibroblasts, like ECs, participate in procoagulant and inflammatory reactions,38 and are directly involved in some autoimmune diseases.39,40
Here, we compared effects of patient APLA or control IgG on the expression of inflammatory marker proteins in wild-type mouse EFs and TLR-deficient EFs. For our studies we used the IgG fraction from 5 patients with APS and an IgG fraction that was immunopurified on immobilized human β2GP1. The patients selected had to have APS, to be positive for anti-β2GP1 antibodies, and to have antibodies that activate human ECs. We analyzed the effect of APLAs on the expression of 3 inflammatory marker proteins representing different aspects of the inflammatory response. MCP-1, a chemokine, and ICAM-1, a leukocyte adhesion molecule, were measured at the mRNA level and IL-6, an inflammatory cytokine, at the mRNA and protein levels. The various TLR-deficient fibroblasts had the correct genotype and responded to agonists in the appropriate manner. Thus, the TLR2−/−EFs did not respond to the TLR2-specific agonists Pam3CSK4 or MALP-2 and had a normal response to LPS, whereas the TLR4−/−EFs did not respond to LPS, but normally to the TLR2 agonists. The lack of response to MALP-2 in TLR6-deficient fibroblasts and the observation that TLR1−/−EFs are able to respond to Pam3CSK4 had been reported previously.23,30,36,41
Our results show that IgG fractions purified from 5 APS patient plasmas activated WT-EFs, whereas IgG from healthy controls or from patients with autoimmune disease unrelated to APS were unable to do so. These IgG fractions from the APS patients were previously shown to be able to activate human ECs.32 In addition, IgG from a sixth APS patient, immunopurified on immobilized β2GP1 also activated WT-EFs. Cell activation was evident by 5- to 40-fold increases in mRNA levels of MCP-1, ICAM-1, and IL-6 and by a greater than 10-fold increase in secreted IL-6. Taken together, our results imply that WT-EFs express the receptors required for their activation by APLAs. The observation that antibodies immunopurified on β2GP1 strongly activated WT-EFs makes it very likely that β2GP1 can function as a serum cofactor in cell activation.
All TLR-deficient fibroblasts, except TLR2−/−EFs, responded to the APLAs and the β2GP1-immunopurified IgG. The unresponsiveness of TLR2−/−EFs was not due to suboptimal concentrations of APLAs or to a time delay in the response. Expression of human TLR2 in the TLR2−/−EFs restored the response of these cells to the APLAs and β2GP1-immunopurified IgG. Because the TLR2−/− EFs transduced with control vector did not respond to APLAs nor to the TLR2-specific agonists, we can exclude a nonspecific effect of vector-mediated gene transfer in the activation of the cells. The response of the hTLR2 reconstituted TLR2−/−EFs was similar to that of WT-EFs. This implies that human and mouse TLR2 are equally efficient in mediating the response to APLAs. This is in agreement with findings that injection of human APLAs into mice leads to pathologic effects—thrombosis and pregnancy loss—that are similar to those encountered in patients with APS.4,37 Further support for a role of TLR2 in cell activation by APLAs is given by our finding that the presence of activity-blocking anti-TLR2 antibodies completely abolished cell activation, whereas blocking anti-TLR4 antibodies had no effect.
TLR2 is known to be functionally active only when it is associated as a heterodimer with TLR1 or TLR6.20 For most patient APLAs the response in TLR1−/−EFs was lower than in TLR6−/− EFs, which in turn was lower than in WT-EFs. This suggests that the cellular response to APLA is mediated preferentially by heterodimers of TLR2 with TLR1, whereas TLR6 might partially compensate for the lack of TLR1.
The response of TLR4−/−EFs to APLAs was higher (1.9-fold) than the response of WT-EFs, and blocking anti-TLR4 antibodies had no effect on WT-EF stimulation by APLAs. These results rule out a direct role for TLR4 in fibroblast activation by APLAs. However, in vivo, TLR4 might play a role in APS. It is known that TLR2 expression by ECs is very low42,43 and that cell activation by the TLR4 agonist LPS or by inflammatory cytokines directly increases the expression of TLR2 by ECs or monocytes.44,45 Injection of LPS in wild-type mice, but not in TLR4−/− mice, leads to increased TLR2 expression by ECs.46 The increased expression of TLR2 induced by TLR4 agonists may have clinical consequences because this may lead to an increase in the TLR2-mediated cellular response to APLAs. Further in vivo studies are required to fully unravel to what extent changes in TLR2 expression on ECs due to the presence of TLR4 ligands such as LPS contribute to the pathologic consequences of APLAs.
In recent years the understanding of cell activation by TLR4 or TLR2 agonists has greatly improved. A common theme is that a ligand first binds to an adaptor molecule before being transferred to the TLR. As an example, CD14 binds the TLR4 agonist LPS or the TLR2 agonist Pam3CSK4. The formation of the ligand-CD14 complex is a prerequisite for binding to TLR4/MD2 or TLR2/TLR1, respectively, and for inducing cell activation.47,48 Cell activation by APLAs may use a similar mechanism. In support for this hypothesis, a recent study has shown that dimerization of β2GP1 by anti-β2GP1 antibodies increases binding to purified extracellular domains of receptors of the LDLR family such as LRP, megalin, VLDLR, and apoE2R′.49 These receptors are expressed in cell type-specific patterns on many cells, including ECs, fibroblasts, and monocytes. Furthermore, binding of β2GP1 to ECs is mediated by annexin II50 and cross-linking of annexin II by antibodies induced EC activation.51 Because annexin II is not a transmembrane protein, a separate receptor appears to be required for cell activation. By analogy with the role for CD14 in cell activation by lipopeptides or LPS, we may hypothesize a model of cell activation in which multiprotein complexes are formed at the cell surface. Members of the LDLR family or annexin II may form part of these complexes and function as adaptor molecules for TLR-mediated cell activation by APLAs.
In conclusion, our results indicate that TLR2 plays a critical role in cell activation by APLAs in fibroblasts. Further studies need to be done to determine the molecular interactions between TLR2 and β2GP1-anti-β2GP1 complexes, as well as the role of LDLR family proteins and annexin II in this context.
Authorship
Contribution: N.S., E.K., and P.dM. designed the research; N.S., S.D.G., R.F., and G.R. performed research; F.B. and P.dM. contributed patients' antibodies; N.S. and E.K. analyzed data; and N.S., E.K., and P.dM. wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Philippe de Moerloose, Division of Angiology and Hemostasis, University Hospital of Geneva, 24 Rue Micheli-du-Crest, 1211 Geneva 14, Switzerland; e-mail: philippe.demoerloose@hcuge.ch.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
This work was supported by the Swiss National Science Foundation, grants 3200-067746 and 3100-105844, as well as by the Swiss Cardiology Foundation.
We would like to extend special thanks to Dr Bernadette Mermillod for her assistance in performing biostatistical analysis of the data and to Sabrina Soldini for her helpful technical support.