Abstract
Lymphocytes from lymphotoxin (LT) α–deficient mice, which lack segregation of their B- and T-cell areas, acquire normal organization following adoptive transfer into RAG-deficient recipients, identifying a non-B non-T cell in the segregation process. Here we show that a CD4+CD3− accessory cell is tightly associated with discrete VCAM-1–expressing stromal cells in B- and T-cell areas of the mouse spleen. CD4+CD3− cells express high levels of LTα, LTβ, and tumor necrosis factor (TNF) α, which are the ligands for the LTβ receptor and TNFR1 expressed by stromal cells. The expression of these ligands is functional, as transferring CD4+CD3− cells derived from either embryonic or adult tissues into LTα-deficient mice organizes B/T segregation and up-regulates CCL21 protein expression in areas where T cells are segregated from B cells. We propose that the function of CD4+CD3− cells is to form a link between primed CD4 T cells and the underlying stromal elements, creating distinct microenvironments in which they enable effector responses.
Introduction
The development of segregated B-cell and T-cell areas within secondary lymphoid organs is the platform for the development of both high-affinity class-switched antibodies and memory antibody responses; neither of these functions develops in lymphotoxin (LT) α−/− mice, in which there is no B/T segregation.1 The absence of segregation is due to impaired organization rather than intrinsic defects in the lymphocytes themselves, as LTα−/− lymphocytes both segregate and function normally following transfer into irradiated normal2 or RAG-deficient1 hosts, which lack B and T cells. A cellular source other than mature B or T cells is therefore implicated in the process of organization.
LTβR signals and perhaps TNFR1 signals mediate lymphoid B/T segregation by activating subpopulations of stromal cells, which then switch on the expression of chemokine genes.3 The expression of CCR7 ligand attracts dendritic cells (DCs) and T cells to form the T-cell area4 ; the expression of CXCR5 ligand brings B cells together to form follicles.5 The genes for these receptors, TNFR1 and LTβR, are tightly linked on chromosome 12 in humans and chromosome 6 in mice, implying that they arose by local gene duplication prior to speciation of human and mouse.
The expression of the T-zone chemokines in lymph nodes is normal in RAG−/− mice, although the expression of the B-zone chemokine, CXCL13, is reduced to approximately 20%, and normal expression depends on B cells.6 Along with the LTα−/− lymphocyte transfer experiments, these data suggest that there is a non-B non-T cell capable of inducing normal (T zone) and partial (B zone) chemokine expression in stroma.
In this paper, we extend previous observations demonstrating a role for a non-B non-T cell in B/T segregation,2 and identify CD4+CD3− cells that we have previously implicated in T-cell memory for antibody responses in adult mice7,8 as playing a role in the lymphoid stromal organization within secondary lymphoid tissues. We report that adult CD4+CD3− cells express high levels of mRNA for LTα, LTβ, tumor necrosis factor (TNF) α, and LIGHT, which are the ligands for TNFR1 and the LTβR. Levels of expression are comparable with those expressed in embryonic and neonatal CD4+CD3− cells, and the expression of LTβ is at least an order of magnitude greater than in CD11c+ DCs or plasmacytoid DCs (pDCs). Furthermore, using adoptive cell-transfer experiments, we demonstrate that the expression of these genes is functional: fetal CD4+CD3− cells derived from embryonic day (E) 15 spleen and adult CD4+CD3− cells, but not lymphocytes, pDCs, and DCs, are able to restore a significant degree of B/T segregation in the spleens of LTα−/− mice, and up-regulate VCAM-1 and CCL21 protein expression on the stroma.
Using confocal microscopy, we demonstrate that this CD4+CD3− cell associates closely with VCAM-1+ follicular dendritic cells (FDCs) in B-cell areas as well as with the VCAM-1+ stromal population within the T zone.
Materials and methods
Mice
All experiments were performed in accordance with the United Kingdom laws and with the approval of the University of Birmingham ethics committee. Normal, RAG1−/−, LTα−/−, and T-cell–deficient9 mice were bred and maintained in our animal facility. Adult CD4+CD3− cells were isolated from RAG1−/− mouse spleens from mice older than 6 weeks, neonatal CD4+CD3− cells were from 2-day-old normal C57Bl/6 or Balb/c mouse spleens, and fetal (E15) CD4+CD3− cells were taken from normal C57Bl/6 or Balb/c embryo spleens from normal pregnant mice at gestation day 15 and cultured with interleukin-7 (IL-7) for 5 to 7 days.
Preparation of CD4+CD3− cells, pDCs, DCs, and other cells
Cell suspensions for isolation of CD4+CD3− cells, DCs, and pDCs were made from the spleens of adult RAG−/− mice as described previously.7,10 Briefly, CD11c+ cells were positively enriched by using CD11c-coated magnetic beads (Miltenyi Biotec, Bergisch Gladbach, Germany), and then fluorescence-activated cell-sorter (FACS) sorted into CD8+ and CD8− populations. CD4+ cells were enriched from CD11c+-depleted populations using CD4-coated magnetic beads, and the resulting CD4+-enriched populations sorted into CD4+CD3−B220−CD11c− (CD4+CD3−) and CD4+CD3−B220+CD11clow (pDC) populations. For Figure 6, CD4 enriched populations were prepared without CD11c+ cell depletion from T-cell–deficient mice.
Adoptive cell transfer
Splenocytes (3 × 107) from either LTα−/− or normal mice were transferred intraperitoneally into RAG−/− hosts. Ten days after transfer the spleens of the injected mice were taken and stained for confocal microscope analysis.
Adult CD4+CD3− cells (1.6 × 105) or 1.7 × 105 E15 CD4+CD3− cells or 1 × 106 CD11c+ DCs and pDCs from RAG−/− mice or 4 × 106 splenocytes from normal mice were transferred intravenously into LTα−/− recipient mice. Each cell population was transferred into 2 mice per experiment, and at least 2 experiments were performed. Ten days after transfer the spleens of the injected mice were taken and stained for confocal microscope analysis.
TaqMan low-density array analysis
TaqMan primer sets (Applied Biosystems, Warrington, United Kingdom) are designed to work with an efficiency approaching 100%, enabling the quantitative comparison of mRNA expression for different genes. Housekeeping genes (β-actin in these experiments) were used to correct for total mRNA (the level to which the β-actin signal was corrected in all mRNA samples). For Figure 3, TNFα, LTβ, LIGHT, and β-actin from the sets were analyzed.
FACS staining
CD4-enriched cells were stained for anti-CD11c FITC, anti-CD4 PE, and anti-B220 allophycocyanin monoclonal antibodies (mAbs) with biotinylated mAbs against OX40L and CD30L (BD Biosciences, Palo Alto, CA) in conjunction with streptavidin cychrome (BD Biosciences) as the second-step staining reagent. To stain with LTβR–immunoglobulin (Ig) or control-Ig fusion protein, CD4+CD3− cells cultured in the presence of 100 ng/mL IL-7 for 7 days prior to staining with LTβR-Ig (a kind gift of Dr Jeff Browning, Biogen, Cambridge, MA). Second-step reagent was goat anti–human IgG FITC (Jackson Immunoresearch Laboratories, West Grove, PA).
Immunohistology for confocal microscope
Confocal images were acquired using a Zeiss Axiovert 100M microscope (Zeiss, Welwyn Garden City, United Kingdom) equipped with a c-apochromat 10×/0.45 numerical aperture (NA) or a c-apochromat 63×/1.2 NA water objective. Comparison of lymphocyte architecture in spleens from wild-type and variously treated or gene-modified mice was performed using Zeiss LSM510 (laser scanning microscopy) software on confocal micrographs taken from sections stained for IgM and CD3 as described previously,7 in conjunction with staining with fluoresceinated anti–VCAM-1 mAb (BD Biosciences) at a previously optimized dilution. The intense staining of VCAM-1+ cells in splenic red pulp contrasted with less intense and patchier appearance of VCAM-1 staining in white pulp areas. We therefore used the limits of the VCAM-1hi staining as a lymphocyte-independent indicator of the extent of the white pulp areas. Intuitively, if B cells are stained with 1 fluorochrome and T cells with another, when there is increased B/T segregation, then there will be less overlapping of colors. This is what the following objective algorithm was designed to test.
Regions of white pulp delineated by red pulp VCAM-1 expression were extracted and the area (square micrometers) and total pixels determined by Zeiss confocal software were recorded. Within the extracted region we enumerated the pixels registering intensity for IgM (B cells) and CD3 (T cells). Where B cells and T cells are within 0.25 μm (the pixel dimension using the × 25 objective) of one another, the pixels are recorded as double positive. We quantified double positivity by multiplying the IgM and CD3 matrices of the micrographs together to produce a separate array (IgM+CD3+) that we used as a measure of the degree of contact between B and T cells. Numbers of singly positive pixels (IgM or CD3) were determined by subtracting the numbers of IgM+CD3+ pixels from those in the respective original arrays. Pixel number (for each magnification on the confocal microscope there is a fixed relationship between pixel number and area in square micrometers) was then used to provide an estimate of the areas within each white pulp region taken up by B-cell or T-cell membrane, or both (IgM+, CD3+, and IgM+CD3+, respectively).
To quantify the VCAM-1 expression in T zones (shown in Figure 4B), T-cell areas were delineated and the number of VCAM-1–positive pixels were enumerated, both using Zeiss confocal software.
Spleen sections were examined systematically for all identifiable areas of white pulp; routinely, 10 different areas were photographed per spleen. Following statistical evaluation, median values with ranges for each treatment were selected for display purposes.
For detection of chemokine expression, sections were first blocked with 10% horse serum for 10 minutes, then stained with polyclonal IgG goat sera directed against mouse CCL19, mouse CCL21, or mouse CXCL13 (R&D Systems, Minneapolis, MN) for 50 minutes at room temperature, at concentrations determined by titration. The Abs were detected using donkey anti–goat IgG Cy2 (Jackson Immunoresearch Laboratories) preabsorbed with 10% horse serum.
Results
Evidence that TNFR and LTβR signals from a non-B non-T cell play a critical role in B/T segregation
Many groups have demonstrated that continuous LTβR signals are required to maintain B/T segregation and B follicles.3,11 We confirmed these findings by acutely blocking LTβR signals (data not shown). Injection of LTβR-Ig was associated with rapid loss of discrete B follicles and VCAM-1+ FDCs, as reported by others.12 LTα−/− mice, which also have low levels of TNFα13 and are therefore deficient in both TNFR1 and LTβR signals, show an even greater degree of disorganization of lymphocytes (Figure 1), confirming that these 2 signaling pathways have independent effects on lymphoid organization.14,15
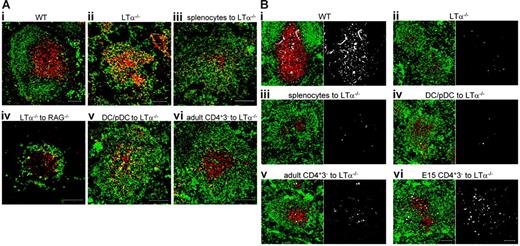
Regulation of B/T segregation. (A) Low-magnification confocal images of spleen sections. CD3 is shown in red and IgM in green. Yellow shows colocalization of B and T cells. (i) Wild-type (WT) mice. (ii) LTα−/− mice. (iii) Organization of the spleens of LTα−/− mice 10 days after transfer of normal splenocytes. (iv) Organization of the spleens of RAG−/− mice 10 days after transfer of LTα−/− splenocytes. (v) Organization of the spleens of LTα−/− mice 10 days after transfer of CD11c+-enriched population (DCs and pDCs). (vi) Organization of the spleens of LTα−/− mice 10 days after transfer of CD4+CD3− cells. (B) CCL21 expression. CD3 is shown in red, IgM in green, and CCL21 in white. Single staining for CCL21 is shown in the adjacent box. Scale bars represent 100 μm. Results representative of at least 2 separate experiments.
Regulation of B/T segregation. (A) Low-magnification confocal images of spleen sections. CD3 is shown in red and IgM in green. Yellow shows colocalization of B and T cells. (i) Wild-type (WT) mice. (ii) LTα−/− mice. (iii) Organization of the spleens of LTα−/− mice 10 days after transfer of normal splenocytes. (iv) Organization of the spleens of RAG−/− mice 10 days after transfer of LTα−/− splenocytes. (v) Organization of the spleens of LTα−/− mice 10 days after transfer of CD11c+-enriched population (DCs and pDCs). (vi) Organization of the spleens of LTα−/− mice 10 days after transfer of CD4+CD3− cells. (B) CCL21 expression. CD3 is shown in red, IgM in green, and CCL21 in white. Single staining for CCL21 is shown in the adjacent box. Scale bars represent 100 μm. Results representative of at least 2 separate experiments.
Although lymphocytes, particularly B cells, express both TNFR1 and LTβR ligands (Figure 2),1 injection of normal splenocytes into LTα−/− mice failed to restore a B/T-segregated architecture (Figure 1Aiii) as reported previously.2 RAG−/− mice lack B and T cells, but other cell types are present. To test whether the environment within RAG−/− mice was capable of segregating B and T cells, splenocytes from LTα−/− mice were transferred into RAG−/− mice. Ten days after cell transfer, the reconstituted spleens were examined for lymphocyte architecture. Whether reconstituted with LTα−/− (Figure 1Aiv) or normal (data not shown) lymphocytes, RAG−/− spleens demonstrated clear B/T segregation by comparison with spleens from LTα−/− mice (Figure 1Aiv).
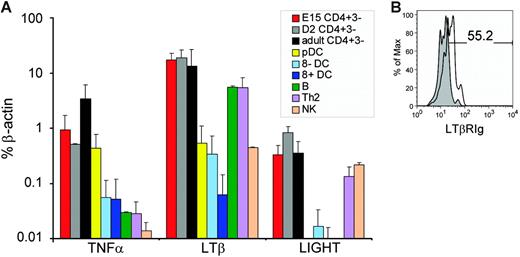
mRNA expression of TNFR1 and LTβR ligands on CD4+CD3− and other cell populations. (A) mRNA for the genes indicated were assayed by quantitative PCR and corrected for β-actin expression as described in “Materials and methods.” Bars show standard deviations for 4 separate experiments. (B) Staining of CD4+CD3− cells with LTβR-Ig. Isolated CD4+CD3− cells were cultured in the presence of 100 ng/mL IL-7 prior to staining with LTβR-Ig (□) or control-Ig fusion protein (⊡). Of live gated CD4+CD3− cells, 55.2% expressed LTβR ligands.
mRNA expression of TNFR1 and LTβR ligands on CD4+CD3− and other cell populations. (A) mRNA for the genes indicated were assayed by quantitative PCR and corrected for β-actin expression as described in “Materials and methods.” Bars show standard deviations for 4 separate experiments. (B) Staining of CD4+CD3− cells with LTβR-Ig. Isolated CD4+CD3− cells were cultured in the presence of 100 ng/mL IL-7 prior to staining with LTβR-Ig (□) or control-Ig fusion protein (⊡). Of live gated CD4+CD3− cells, 55.2% expressed LTβR ligands.
Adult CD4+CD3− and embryonic/neonatal CD4+CD3− cells, but not pDCs or DCs, express high levels of LTβ and TNFα
To investigate which non-B non-T cells in adult tissue expressed LTα, LTβ, and TNFα, we used TaqMan polymerase chain reaction (PCR) analysis on purified subpopulations: CD8+CD11c+B220− and CD8−CD11c+B220− DCs, pDCs (CD4+CD11clowB220+) and the CD4+CD3− accessory cells that we have recently characterized (CD4+CD3−CD11c−B220−) from adult RAG−/− mice,7,8 as well as CD4+CD3−CD11c−B220−cells from embryonic (E15) and neonatal (day [D] 2) spleens. Results are shown in Figure 2A: levels of TNFα, LTβ, and LIGHT (also a LTβR ligand) were comparable in all 3 populations of CD4+CD3−CD11c−B220− cells, as was the expression of LTα (determined by conventional semiquantitative PCR; data not shown). In contrast, the expression of TNFα, LTβ, and LIGHT on pDC, natural killer (NK) cell, or DC subpopulations was at least an order of magnitude less. T and B cells expressed high levels of LTβR ligands, but much lower levels of TNFα than either embryonic/neonatal or adult CD4+CD3− cell populations.
To confirm that CD4+CD3− cells expressed LTβR ligands at the protein level, we stained CD4+CD3− cells with a LTβR-Ig fusion protein. Although this reagent stains freshly isolated CD4+CD3− cells, following in vitro culture in the presence of IL-7, LTβR ligands were clearly up-regulated on CD4+CD3− cells (Figure 2B).
CD4+CD3− cells but not splenocytes are capable of organizing lymphocytes in LTα−/− mice
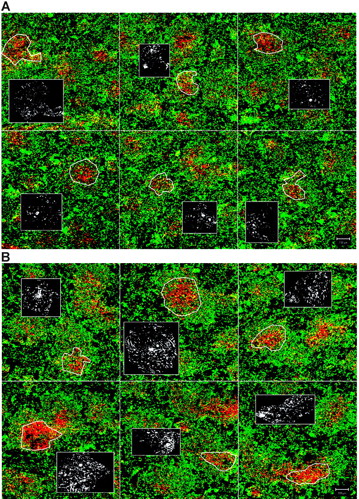
The strong expression of both TNFR1 and LTβR ligands on both embryonic/neonatal and adult CD4+CD3− cells suggested that they might be capable of organizing LTα−/− lymphocytes. To test this directly, CD4+CD3− cells from RAG−/− mice were transferred intravenously (or in some experiments intraperitoneally) into LTα−/− recipient mice. Ten days after transfer there was evidence of B/T segregation in LTα−/− mice (Figure 1Avi). A montage of 6 confocal images taken of LTα−/− spleen sections with a × 10 objective is shown without (Figure 3A) and following (Figure 3B) reconstitution with CD4+CD3− cells. In contrast, mice that received CD11c-enriched fractions (CD11c+ DCs and CD11clow pDCs; Figure 1Av) or splenocytes (Figure 1Aiii) showed little evidence of B/T segregation.
Montage of low magnification confocal images of spleen sections of LTα−/− mice. (A) LTα−/− mice. (B) Organization of the spleens of LTα−/− mice 10 days after transfer of CD4+CD3− cells. CD3 is shown in red and IgM in green. Yellow shows colocalization of B and T cells. Inset boxes show single staining for VCAM-1 (in white) in the T zones as outlined by white figures in main pictures. Scale bar represents 100 μm. Results representative of at least 2 separate experiments.
Montage of low magnification confocal images of spleen sections of LTα−/− mice. (A) LTα−/− mice. (B) Organization of the spleens of LTα−/− mice 10 days after transfer of CD4+CD3− cells. CD3 is shown in red and IgM in green. Yellow shows colocalization of B and T cells. Inset boxes show single staining for VCAM-1 (in white) in the T zones as outlined by white figures in main pictures. Scale bar represents 100 μm. Results representative of at least 2 separate experiments.
To detect whether chemokine expression was up-regulated in LTα−/− spleens after cell transfer, CCL19 and CCL21 (T-zone chemokines) and CXCL13 (B-zone chemokine) were stained as described.16 Wild-type spleens showed strong CCL21 expression in T zones (Figure 1Bi) compared with LTα−/− spleens (Figure 1Bii). Following reconstitution with adult CD4+CD3− cells or E15 CD4+CD3− cells (Figure 1Bv-Bvi), CCL21 expression in LTα−/− spleens was clearly up-regulated in areas where T cells are segregated from B cells. In contrast, LTα−/− spleens that received either splenocytes (Figure 1Biii) or CD11c-enriched cells (Figure 1Biv) did not show increased CCL21 expression. CCL19 expression was much weaker than CCL21 expression in spleens from normal mice, and was not detected in LTα−/− spleens before or after cell transfer (data not shown). Although CXCL13 was strongly expressed in normal B follicles, its expression was not detected in LTα−/− spleens before or after cell transfer (data not shown). Furthermore, FDC markers were not induced (data not shown), consistent with previous reports that B-cell expression of LTβR ligands is critical for CXCL13 expression.3
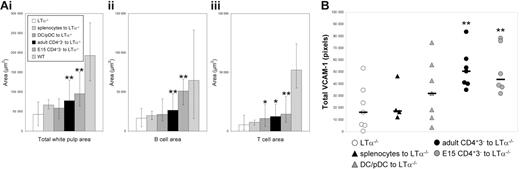
As described in “Materials and methods” for each experiment, areas of splenic white pulp from reconstituted LTα−/− mice were analyzed to identify the degree of B/T segregation: discrete red (T cell) and green (B cell) areas versus yellow areas (mixed). Compared with control LTα−/− spleen sections, white pulp areas containing B and T lymphocytes were significantly larger in mice reconstituted with adult CD4+CD3− cells (P = .005) and E15 CD4+CD3− cells (P = .001), but not CD11c+ populations (P = .07) or splenocytes (P = .28) (Figure 4Ai). This was due to significantly increased T-cell–free B-cell areas for adult CD4+CD3− cells (green; P = .005) and E15 CD4+CD3− cells (P = .002), but not CD11c+ cells (P = .6) or splenocytes (P = .28) (Figure 4Aii). The B-cell–free T-cell areas (red) were also significantly bigger (almost twice as large) in the spleens transferred with adult CD4+CD3− cells (P = .008) and E15 CD4+CD3− cells (P = .004) and CD11c+ cells (P = .04), but not splenocytes (P = .39) (Figure 4Aiii).
Splenic areas and VCAM-1 expression after adoptive transfer. (A) Areas of splenic white pulps, B cells, and T cells. White pulp area (i), B-cell area (ii), and T-cell area (iii) in LTα−/− mice that were adoptively transferred with normal splenocytes, CD11c+ DCs and pDCs, adult CD4+CD3− cells, or E15 CD4+CD3− cells compared with normal white pulp areas. Bar shows mean values with high and low values calculated from 10 different white pulp areas. (B) VCAM-1 expression after adoptive transfer. Statistical differences were calculated using a nonparametric Mann-Whitney test. *Statistical significance by Mann-Whitney P ≤ .05; **P ≤ .005. Results representative of at least 2 experiments
Splenic areas and VCAM-1 expression after adoptive transfer. (A) Areas of splenic white pulps, B cells, and T cells. White pulp area (i), B-cell area (ii), and T-cell area (iii) in LTα−/− mice that were adoptively transferred with normal splenocytes, CD11c+ DCs and pDCs, adult CD4+CD3− cells, or E15 CD4+CD3− cells compared with normal white pulp areas. Bar shows mean values with high and low values calculated from 10 different white pulp areas. (B) VCAM-1 expression after adoptive transfer. Statistical differences were calculated using a nonparametric Mann-Whitney test. *Statistical significance by Mann-Whitney P ≤ .05; **P ≤ .005. Results representative of at least 2 experiments
The hypothesis that CD4+CD3− cells caused B/T segregation was supported by evidence demonstrating significant up-regulation of VCAM-1 in T-cell stroma in LTα−/− mice injected with both embryonic and adult CD4+CD3− cells (Figures 3, 4B). Analysis showed adult CD4+CD3− cells (P = .001) and E15 CD4+CD3− cells (P = .004) but not CD11c+ cells (P = .25) or splenocytes (P = .68) (Figure 4B) induced significantly more VCAM-1 expression in T-cell areas.
Although by confocal analysis we detected increased B/T segregation in CD4+CD3−-cell–injected mice, we did not detect up-regulation of mRNA for the homeostatic chemokines CCL19, CCL21, and CXCL13 (data not shown). We propose this is because the small numbers of donor CD4+CD3− cells that reach the recipient spleen are unable to raise the total homeostatic chemokines levels above background, but that focal increases in chemokine expression account for the observed increased degree of B/T segregation observed.
Although VCAM-1 expression was up-regulated by CD4+CD3− cells, we did not observe up-regulation of the T-zone stromal marker, gp38,17 or expression of MadCAM-1,18 which stains the marginal sinus of normal mice but not LTα−/− splenic tissue. We also stained for CR1 (CD35) and the FDC markers FDC-M1 and FDC-M2. Although there was up-regulation of CD35 by all populations transferred into LTα−/− mice, no population (including B and T splenocytes or CD4+CD3− cells) up-regulated either FDC-M1 or FDC-M2 (data not shown).
CD4+CD3− cells are closely associated with VCAM-1+ stromal cells in B- and T-cell areas
In the developing embryo, lymphoid tissue inducer (LTi) cells interact with stromal cells in lymph node anlagen to up-regulate the expression of the chemokines that recruit lymphocytes to form lymph nodes,19 and there is evidence that LTα and LTβ expressing LTi cells are responsible for B/T segregation in the neonatal lymph node.20 Effective delivery of LTβR signals to the stromal cells involves interactions between the LTi-cell integrin, α4β1, and its ligand, VCAM-1, which is expressed on stroma.21 We reasoned that adult CD4+CD3− cells would function in a similar way. To examine their relationship with VCAM-1+ stromal cells we first identified VCAM-1+ populations in normal adult mice. The red pulp of the spleen exhibits strong staining for VCAM-1+ cells, but there is also generally less intense staining in white pulp areas, with discrete staining in both B follicles (including FDC populations) and T-cell areas (Figure 5A). Comparable with normal mice, LTα−/− mice show strong staining for VCAM-1 in the red pulp of the spleen, but VCAM-1 staining is largely missing from white pulp areas, which, although lymphocyte rich, show no segregation of B and T cells (Figure 5B). In mice injected with LTβR-Ig, where LTβR but not TNFR1 signals are blocked, VCAM-1 expression is maintained in both T zone and red pulp areas, but there is selective loss of VCAM-1 expression within B follicles consistent with rapid loss of VCAM-1+–expressing FDCs (data not shown).12
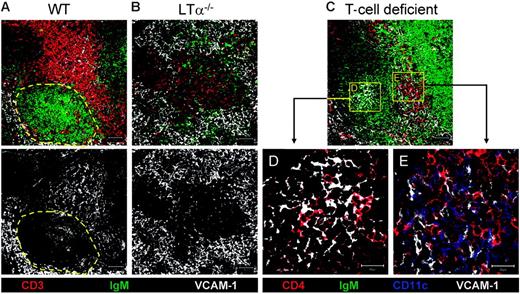
Evidence that CD4+CD3− cells are associated with VCAM-1+ stromal cells. Confocal images of spleen sections showing (A) wild-type (WT) and (B) LTα−/− mice. Dotted yellow area of panel A identifies B follicles in WT mice. To show association of VCAM-1+ cells with CD4+CD3− cells, confocal images from T-cell–deficient mice were analyzed at low magnification (10×) (C), high magnification (63×) of B-cell area (D), and high magnification of T-cell area (63×) from panel C (E). Scale bar represents 50 μm for low magnification, 20 μm for high magnification.
Evidence that CD4+CD3− cells are associated with VCAM-1+ stromal cells. Confocal images of spleen sections showing (A) wild-type (WT) and (B) LTα−/− mice. Dotted yellow area of panel A identifies B follicles in WT mice. To show association of VCAM-1+ cells with CD4+CD3− cells, confocal images from T-cell–deficient mice were analyzed at low magnification (10×) (C), high magnification (63×) of B-cell area (D), and high magnification of T-cell area (63×) from panel C (E). Scale bar represents 50 μm for low magnification, 20 μm for high magnification.
To examine the relationship between CD4+CD3− cells and the VCAM-1+ stromal cells, we examined sections of splenic tissue from T- and NK-cell–deficient mice,9 where the absence of CD4+CD3+ T cells in the DC-rich areas makes it straightforward to identify other CD4+ cells (Figure 5C). As reported previously,7 we found CD4+CD3− cells in B follicles, and these were closely associated with VCAM-1+ cells (Figure 5C [low magnification]–D [high magnification]). However, in the T- and NK-cell–deficient mice we were also able to identify a similar association between a CD4+CD3− population and the local VCAM-1+ population among the CD11c+ DCs in the area populated by T cells in normal mice (Figure 5C [low magnification], E [high magnification]).
CD4+CD3− cells interact with CD11c+ DCs
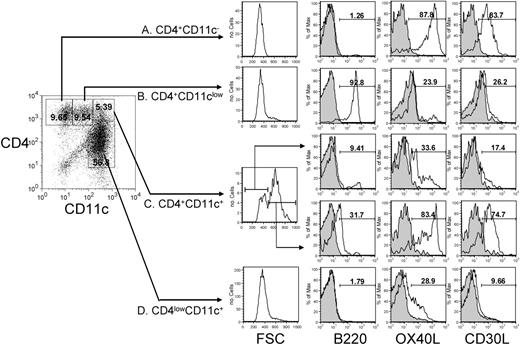
To ensure that the CD4+CD3− cell population that we identified in the T zone was not CD4+ DCs or pDCs, we carefully characterized all the CD4+ populations from T-cell– and NK-cell–deficient mice.9 Four CD4+ populations could be identified from these mice. The first is the CD4+CD3− cell population which we have characterized previously,7 which lacks expression of B220 and CD11c but which expresses high levels of OX40L and CD30L (Figure 6A). The second is the pDC population, which expresses B220 and low levels of CD11c but lacks expression of OX40L and CD30L (Figure 6B), and the third is the CD4lowCD11c+ myeloid DC population, which expresses low levels of OX40L but not CD30L (Figure 6D). Whereas the first 3 populations are homogeneous with respect to size, the fourth population (CD4+CD11c+) is heterogeneous, being divisible by differential forward scatter into 2 subpopulations (Figure 6C). The population of smaller cells closely resembles classic myeloid DC phenotype cells, whereas the population of larger cells has the appearance of clusters of CD4+CD3− cells and myeloid DCs: its phenotype is mixture of the phenotypes of these 2 cell types. Although the clusters had apparently slightly higher levels of B220, we think this is a result of the increased fluorescence of larger objects. These data support the idea that there is some association between CD4+CD3− populations and DCs, confirming the impressions formed from our confocal microscopy.
CD4+ populations in the spleen. CD4+ populations were isolated from T- and NK-cell–deficient mice and characterized for expression of CD11c, B220, and OX40L and CD30L. Shaded histograms show control staining. Results representative of 4 experiments.
CD4+ populations in the spleen. CD4+ populations were isolated from T- and NK-cell–deficient mice and characterized for expression of CD11c, B220, and OX40L and CD30L. Shaded histograms show control staining. Results representative of 4 experiments.
Many CD4+CD3− cells identified in T-cell areas are not pDCs or DCs, and are also found adjacent to central arterioles in spleen and PNAd+ HEVs in the lymph nodes
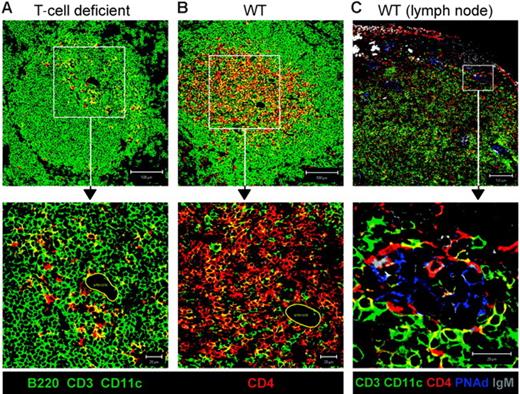
To exclude the possibility that CD4+CD3− cells identified in T-cell areas were pDCs or DCs, we stained sections from mice deficient in T and NK cells,9,22 excluding CD3, CD11c, and B220, using FITC-conjugated antibodies (green) and counterstaining with CD4 (red) (Figure 7A). Although some CD4+ cells expressed either CD11c or B220 (yellow), there were also many red-only cells, which therefore express neither B220 nor CD11c, providing direct confirmation that CD4+CD3− cells are located in the T zone. Furthermore, the red-only cells are often found adjacent to yellow cells, consistent with the possibility that CD4+CD11c+ DCs and CD4+CD3− cells associate. The location of CD4+CD3− cells in the T-cell areas was not an artifact of T-cell–deficient mice, as careful analysis of normal mouse spleens revealed CD4+CD3− cells within the T-cell areas (Figure 7B). These data indicate that CD4+CD3− cells associate with stroma in both B- and T-cell areas and are therefore well positioned to provide the TNFR1 and LTβR signals that induce chemokine expression. Furthermore, like LTi cells, which are found clustered around blood vessels in neonatal spleens,23 CD4+CD3− cells are also found around central arterioles in the spleen (Figures 7A-B) and in the lymph nodes are found associated with peripheral lymph node addressin–positive (PNAd+) high endothelial venules (HEVs; Figure 7C).
CD4+CD3− cells found around the central arteriole in the spleen and associated with PNAd+ HEVs in the lymph node. Confocal images of T-cell–deficient mouse spleens stained with B220 and CD11c in green, and CD4 in red (A), and normal mouse spleens stained with CD3, B220, and CD11c in green and CD4 in red (B). Area indicated by closed yellow line identifies central arteriole in high magnification images of panels A and B. (C) Confocal images of lymph node from a normal mouse stained with CD3, B220, and CD11c in green, CD4 in red, IgM in gray, and PNAd in blue. Scale bar represents 100 μm for low magnification (10×), and 20 μm for high magnification (63×).
CD4+CD3− cells found around the central arteriole in the spleen and associated with PNAd+ HEVs in the lymph node. Confocal images of T-cell–deficient mouse spleens stained with B220 and CD11c in green, and CD4 in red (A), and normal mouse spleens stained with CD3, B220, and CD11c in green and CD4 in red (B). Area indicated by closed yellow line identifies central arteriole in high magnification images of panels A and B. (C) Confocal images of lymph node from a normal mouse stained with CD3, B220, and CD11c in green, CD4 in red, IgM in gray, and PNAd in blue. Scale bar represents 100 μm for low magnification (10×), and 20 μm for high magnification (63×).
Discussion
The adult CD4+CD3− cell in secondary lymphoid tissues constitutively expresses the TNF family members OX40L and CD30L.7,8,24 It provides the signals through OX40 and CD30 on activated CD4 T cells that (1) maintain them as follicular T cells that select B cells in affinity maturation within germinal centers; and (2) support them as the memory T cells that help secondary B-cell responses. Here we demonstrate that adult CD4+CD3− cells, like the embryonic LTi cells with which they share a common phenotype,7,24 express high levels of a second set of TNF family genes, LTα, LTβ, and TNFα, which are linked with the organized B/T segregation observed in lymphoid tissues.1 Several pieces of evidence support the role of these cells in B/T segregation. First, we show that the LTα−/− lymphocytes that are unable to segregate in their parental environment segregate normally in RAG-deficient hosts, which lack mature B and T cells. When we subfractionated RAG-deficient accessory cells and transferred them into LTα−/− hosts, we identified CD4+CD3− cells as cells capable of the segregation of lymphocytes into B- and T-cell areas, whereas splenocytes (including B and T cells) or CD11c+ DC and pDC populations failed to do so to a significant extent.
Evidence that CD4+CD3− cells were in a position to elicit the secretion of homeostatic chemokines from stromal populations was provided by the demonstration of their tight association with VCAM-1+ stromal cells in both T- and B-cell areas. Furthermore, transfer of CD4+CD3− cells into LTα−/− recipients up-regulated VCAM-1 and CCL21 expression on host T-zone stromal cells in areas where T cells were segregated from B cells, a situation similar to that reported for the LTi-cell–induced expression of VCAM-1 in lymph node anlage.19 The conclusion that CD4+CD3− cells are important for organizing lymphoid tissues is further supported by observations that lymph node expression of CCL19 and CCL21 is not dependent on B or T cells.6 Although expression of CXCL13 does appear to substantially depend on B cells,6 we found that normal splenocytes alone were not sufficient to induce either FDC markers or CXCL13 expression in LTα−/− mice. We propose that CD4+CD3− cells might be essential for B follicle formation because they express a critical signal not expressed by B cells, and that the 2 cell types might act synergistically together in B follicle formation. A possible candidate for such a signal is TNFα, which is expressed at much higher levels on CD4+CD3− cells than on B cells, and which is essential for the formation of primary B follicles and FDC networks.25
Therefore, the data reported previously and here link CD4+CD3− cells with 2 functions: T-cell survival via OX40 and CD30 signals,7,8,24 and with the activation of stromal cells that produce homeostatic chemokines. We propose that CD4+CD3− cells help to establish the chemokine gradients that guide the migration of B and T cells to their respective locations by activating stromal cells to up-regulate CCR7 and CXCR5 ligands. In addition, we propose that CD4+CD3− cells enable germinal center (GC) T-cell selection of B cells by excluding CCR7+ T cells from B follicles, while allowing locally primed CXCR5+ T cells into B follicles. Within B-cell GCs, the CD4+CD3− cells are attached both to FDCs and to the CXCR5+ T cells that select GC B cells.7,8 We suggest that they act as a tether between the selecting T cells and the FDCs, thus forming the microenvironment for B-cell selection that is essential for affinity maturation.
In the outer T zone, CD4+CD3− cells interact with both T-zone stroma and primed CD4 T cells. Memory B and T cells depend on the cues established by homeostatic chemokines expressed in B- and T-zone stroma to guide their interactions to the B/T interface. Although T-cell priming does not depend on LTα-dependent B/T segregation, B/T collaboration for memory antibody responses does.26 The observation that “balanced” expression of CXCR5 and CCR7 on antigen-activated B cells27 and CD4 T cells28 locates them at the B/T interface suggests a mechanism whereby B- and T-zone chemokine signals regulate B/T interactions during secondary antibody responses. We propose that the outer T zone therefore forms the microenvironment for memory responses: OX40- and CD30-deficient mice have grossly impaired memory antibody responses because of failure of T cells to survive in this location,7,8 which is where memory T and B cells interact during secondary responses.29
We also found evidence for an association between DCs and CD4+CD3− cells. CD4+CD3− cells express LTβR ligands implicated in homeostatic signaling to the CD4+ myeloid DCs30 associated with the development of T helper 2 (Th2) antibody responses.31 In addition, CD4+CD3− cells constitutively express TRANCE (TNFSF11),7 which can signal survival through its ligand RANK (TNFRSF11A), expressed on DCs.32 TRAF6, a key target for RANK signaling, is essential for CD4+ DCs.33 Our data are therefore consistent with the view that CD4+CD3− cells attach to stroma at the B/T interface and activate their expression of the chemokines that recruit both myeloid DCs and primed T cells. Once recruited, DCs and T cells would then be maintained by combinations of TNF survival signals and homeostatic chemokines, provided by the CD4+CD3−/stromal association.
Our hypothesis that CD4+CD3− cells play a role in segregating B and T cells in normal lymphoid tissue is at odds with the observation that mice deficient in the splice variant of the retinoic acid orphan receptor (RORγt) lack LTi cells. While these mice fail to develop lymph nodes and gut-associated lymphoid tissue, their spleens are normally segregated into B- and T-cell areas.34,35 However, we have examined spleens from RORγt−/− mice and found, at least by confocal microscopy, that CD4+CD3− cells are present, indicating that splenic CD4+CD3− cell populations might not depend on RORγt expression (F.M.M. and P.J.L.L., unpublished observations, December 2004). In support of this proposition, RORγt−/− mice also have nasal-associated lymphoid tissue (NALT),36 and there is evidence that CD4+CD3− cells are required for NALT formation.37 Expression of RORγt has been primarily linked with survival signals to CD4+CD3− cells,34,38 so it might only be required for their survival in lymph node anlage but not in the spleen and NALT.
The function of CD4+CD3− accessory cells in forming microenvironments for adaptive T-cell responses is possibly relevant to the pathogenesis of AIDS in HIV infection. The expression by CD4+CD3− cells of CXCR4,39 which is associated with the development of normal GCs,40 may be the link between the emergence of CXCR4-tropic variants of HIV and progressive disease.41-43 HIV localizes on FDCs,44 with which CD4+CD3− cells are directly associated; therefore, they are potentially susceptible to infection. Destruction of CD4+CD3− cells, either by CXCR4-tropic HIV variants or, more likely, by the host CD8 immune response that invades B follicles,45 predicts the signature of progressive disease: loss of the discrete B follicle structure (follicular fragmentation and lysis), disruption of the FDC network,46 and loss of the functional capacity to make de novo high-affinity antibody responses.47 The impairment of the capacity to mount neutralizing antibodies is associated with rising viral titers and loss of T cells from the T zone. We propose that the decline in T-cell numbers in humans might be due at least in part to loss of T-zone CD4+CD3− cells, with consequent loss of CCL19 and CCL21, a view supported by the loss of these chemokines in a simian model of AIDS.48 Finally, our hypothesis provides an explanation for the loss of HEVs in terminal AIDS.49
Authorship
Author contributions: M.-Y.K., F.M.M., G.A., and P.J.L.L. designed and performed the research, collected and analyzed the data, and wrote the paper; F.M.M., F.M.C.G., A.W., S.H.G., and V.B. performed the research and confocal picture analysis; and L.S.K.W., J.C., and E.J. contributed to performing the research.
Conflict-of-interest statement: The authors declare no competing financial interests.
Correspondence: P. J. L. Lane, MRC Centre for Immune Regulation, Institute for Biomedical Research, Birmingham Medical School, Birmingham B15 2TT, United Kingdom; e-mail: p.j.l.lane@bham.ac.uk.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We would like to thank Jeff Browning (Biogen) for providing LTβR-Ig.
Supported by a Wellcome Programme Grant to P.J.L.L.