Abstract
Unmethylated CpG DNA activation of naive CD27− B cells has been reported to require B-cell–receptor (BCR) cross-linking. We describe a culture system using CpG DNA with sequential steps for T-cell–independent activation of naive CD19+CD27− human peripheral blood B cells that induces efficient CD138+ plasma-cell differentiation. CD27+ and CD27− B cells were cultured in a 3-step system: (1) days 0 to 4: CpG, IL-2/10/15; (2) days 4 to 7: IL-2/6/10/15 and anti-CD40L; (3) days 7 to 10: IL-6/15, IFN-α, hepatocyte growth factor, and hyaluronic acid. Both CD27+ and CD27− B cells up-regulated intracytoplasmic TLR-9 following CpG DNA activation. CD27− B-cell activation required cell-cell contact. Both naive and memory B cells progressed to a plasma-cell phenotype: CD19lowCD20lowCD27+CD38+HLA-DRlow. Seventy percent of the CD27−-derived CD138+ cells demonstrated productive V chain rearrangements without somatic mutations, confirming their origin from naive precursors. Plasma cells derived from CD27+ B cells were primarily IgG+, while those from CD27− B cells were IgM+. Our results indicate that under certain conditions, naive B cells increase TLR-9 expression and proliferate to CpG DNA stimulation without BCR signaling. In addition to its immunologic significance, this system should be a valuable method to interrogate the antigenic specificity of naive B cells.
Introduction
Antigen-independent activation of naive B cells is thought to be one mechanism responsible for antibody-mediated autoimmunity as seen in systemic lupus erythematosus (SLE) and other autoimmune diseases. However, normal CD27− naive B cells are reported to have several absolute requirements for activation, including B-cell–receptor (BCR) cross-linking in the presence of costimulation via CD40 ligand (CD40L) and the presence of promitotic cytokines such as IL-2 and IL-15. Some investigators have hypothesized that an absolute requirement for BCR stimulation to activate naive B cells would allow proper self/non–self-discrimination at this developmental stage and prevent the emergence of autoreactive plasma cells via polyclonal antigen-independent B-cell activation. In contrast, intermittent TLR-9 activation of CD27+ memory B cells by unmethylated CpG DNA may be a homeostatic mechanism that maintains adaptive long-term B-cell memory in the absence of antigen.1 Antigen-independent activation of memory B cells via TLR-9 signaling has been reported to support plasma-cell differentiation by CD27+ memory but not CD27− naive B cells. These conclusions have been based on data derived from in vitro analyses of human B cells. In this paper, we demonstrate that TLR-9 mediated activation of human CD27− B cells, and their subsequent plasma-cell differentiation is a function of the in vitro culture conditions rather than an intrinsic biologic limitation.
Several in vitro culture systems have been developed in which human B cells can be activated and driven to a plasma-cell phenotype. These have included the following: a 2-step culture of germinal center B cells, first with CD40L in the presence of IL-2 and IL-10 followed by removal of CD40L to produce plasma cells2 ; activation of peripheral blood CD27+ memory B cells with unmethylated CpG DNA in the presence of IL-151 ; CD40 ligation in the presence of IL-4 followed by incubation with IL-2, IL-3, and IL-103 ; BCR cross-linking plus CD27 ligation, IL-2, IL-4, and IL-104,5 ; CpG-stimulated plasmacytoid dendritic cells (PDCs) with BCR cross-linking6,7 ; and, recently, BCR cross-linking in the presence of IL-21.7 In all of these reports, plasma cells were defined by the CD20−CD38+ isotype-switched phenotype,3-9 but not the characteristic expression of CD138 as found on human bone marrow–resident plasma cells.
CD138 (syndecan-1) expression is a specific marker of terminally differentiated, long-lived bone marrow–resident plasma cells.10-13 While many in vitro systems produced CD20−CD38+++ plasmablasts, it has been difficult to consistently drive large numbers of peripheral blood human B cells to the terminally differentiated CD138+ phenotype.1-9 For example, after BCR-activated IgD+ B cells are incubated with interferon-α (IFN-α), activated dendritic cells, IL-15, and CD40-Ig, up-regulation of CD138 occurs in only 8% of the plasmablasts and is modest at best.14 Human plasmablast expression of CD138 is modulated by the local bone marrow environment, including the presence of IL-6,15-18 IL-21,13,16,19 and interferon-α.16,17,20 Signaling via CD138 by insulin-like growth factor and human hepatocyte growth factor (hHGF) appears to rescue normal plasmablasts from apoptosis.16,19,20 We were therefore interested in determining if a large percentage of activated B cells could be driven to express CD138 given the appropriate in vitro culture conditions.
In this report, we describe a 3-step culture system that promotes differentiation and survival of CpG DNA–activated CD27+ and CD27− human B cells into CD138+ plasma cells. Using this system, we found that both CD27+ and CD27− peripheral blood B cells proliferated and could be driven to a terminal plasma-cell phenotype expressing high levels of surface CD138. Seventy percent of the CD27−-derived CD138+ cells demonstrated a germ-line configuration without somatic mutations, consistent with their origin from naive precursors.
Materials and methods
Human subject protection
This study was approved by the Research Subjects Review Board at the University of Rochester Medical Center. Informed consent was obtained from all participants, in accordance with the Declaration of Helsinki. Research data were coded such that subjects could not be identified, directly or through linked identifiers, in compliance with the Department of Health and Human Services Regulations for the Protection of Human Subjects.21
Peripheral blood B-cell isolation
Peripheral blood mononuclear cells (PBMCs) were isolated from peripheral blood of healthy human volunteers using a modification of our method previously described.22 After Ficoll density gradient centrifugation, CD19+ B cells were positively selected by immunomagnetic bead isolation, followed by detachment of the anti-CD19+ beads per the manufacturer's instructions (Miltenyi, Auburn, CA). To insure that no contamination with CD3+ cells was present, the CD19+ fraction was then incubated with anti-CD3 immunomagnetic beads and passed over a magnetic column. The flow-through fraction was then incubated with anti-CD27 immunomagnetic beads and again passed over a magnetic column to separate CD19+ B cells into CD27+ (retained on the column) and CD27− (flow-through fraction) populations. Cell purity for all isolations was more than 96% by fluorescence-activated cell sorter (FACS) staining.
Three-phase culture system
CD27+ and CD27− B-cell isolates were cultured separately at a density of 1 × 105 cells/200-μL well in 96-well U-bottom plates. All cells were cultured in a base medium consisting of Iscoves MDM (Invitrogen, Carlsbad, CA) with 10% heat-inactivated fetal bovine serum (Sigma, St Louis, MO), 50 μg/mL human transferrin, 5 μg/mL human insulin, 15 μg/mL gentamicin, and 100 U/mL penicillin-streptomycin. Recombinant human lacromin (Seracare, West Bridgewater, MA) was substituted for transferrin in some experiments. A 3-phase culture system was used with different media supplements (Figure 1A). All cytokines and growth factors are from BD Pharmingen (San Diego, CA), unless otherwise noted. In phase I, CpG oligodeoxynucleotide 2006 (Oligos Etc; Wilsonville, OR) was used at 10 μg/mL with human recombinant IL-2 (20 U/mL), IL-10 (50 ng/mL), and IL-15 (10 ng/mL) and the cells were cultured for 5 days. In phase II, human recombinant IL-2 (20 U/mL), IL-6 (50 ng/mL), IL-10 (50 ng/mL), IL-15 (10 ng/mL), and mouse anti–CD40L IgG (1 μg/mL; clone TRAP1) were used, and the cells were cultured for 3 days. In phase III, human recombinant IL-6 (50 ng/mL) and IL-15 (10 ng/mL) plus IFN-α (500 U/mL) (R&D Systems, Minneapolis, MN), human recombinant hepatocyte growth factor (hHGF) 20 ng/mL (Cell Sciences, Canton, MA), and hyaluronic acid (HA; 100 μg/mL; Sigma) were used, and the cells were cultured for 3 days. Cell viability was assessed at each culture stage by trypan blue exclusion.
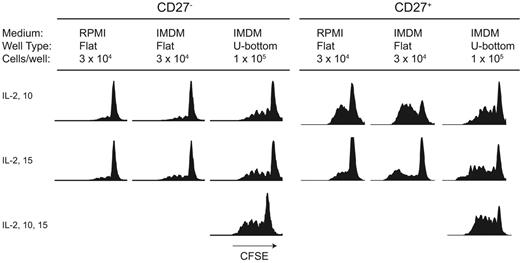
Activation of CD27− B cells with CpG DNA is a function of cell-cell contact conditions. CD27− and CD27+ B cells were labeled with CFSE and activated with CpG DNA2006. After 4 days, proliferation was analyzed by flow cytometry. Activation and proliferation of CD27− naive B cells required an increase in cell density to 1 × 105 cells per well for maximal proliferation to occur. In contrast, CD27+ B cells proliferated following CpG DNA at all cell concentrations and densities, although higher cell densities led to an increase in proliferation. IL-10 improved proliferation of both CD27+ and CD27− cells, while the combination of IL-2, IL-10, and IL-15 and cell-cell contact gave maximal proliferation. Results are representative of 15 independent experiments from 9 different blood donors.
Activation of CD27− B cells with CpG DNA is a function of cell-cell contact conditions. CD27− and CD27+ B cells were labeled with CFSE and activated with CpG DNA2006. After 4 days, proliferation was analyzed by flow cytometry. Activation and proliferation of CD27− naive B cells required an increase in cell density to 1 × 105 cells per well for maximal proliferation to occur. In contrast, CD27+ B cells proliferated following CpG DNA at all cell concentrations and densities, although higher cell densities led to an increase in proliferation. IL-10 improved proliferation of both CD27+ and CD27− cells, while the combination of IL-2, IL-10, and IL-15 and cell-cell contact gave maximal proliferation. Results are representative of 15 independent experiments from 9 different blood donors.
Antibody reagents, CFSE labeling, and flow cytometry
The following antibodies were used for staining (murine monoclonal IgG was from BD Pharmingen, unless otherwise noted): CyChrome anti-CD19, CyChrome anti-CD20 (clone 2H7), CyChrome anti-CD38 (HIT2), PE anti-CD27, PE anti-CD138, PE anti–HLA-DR, PE anti-CD40 (5C3), PE anti-CD154 (TRAP1), and FITC anti-IgD (IA6-2). Intracellular staining for TLR-9 and Ig was performed after fixation and permeabilization (Fix & Perm kit; Caltag, Burlingame, CA). For intracellular staining, the following antibodies were used: FITC-mouse monoclonal antibody to TLR-9 (26C593; Imgenex, San Diego, CA), FITC anti-IgG (G18-145), anti-IgD (IA6-2), or anti-IgM (JDC-15; BD Pharmingen). For studies of cell division, isolated B cells were labeled with 10 nM CFSE (Molecular Probes, Eugene, OR) as previously described and cultured according to phase I. Cells were analyzed at 96 hours for both CD27+ and CD27− populations. All flow cytometric analyses were performed with a FACS Calibur dual laser cytometer (BD Biosciences, San Jose, CA) using CellQuest (BD Biosciences) acquisition and Flowjo (Treestar, Ashland, OR) offline analysis software.
ELISA for immunoglobulin production
Supernatants from the 3-phase culture system were analyzed by enzyme-linked immunosorbent assay (ELISA) for IgM, IgG, and IgA production. Ninety-six–well plates were coated with 10 μg/mL capture antibody against the human immunoglobulin of interest: polyclonal F(ab)2 goat anti–human IgG heavy chain (Biosource, Camarillo, CA), polyclonal F(ab)2 goat anti–human IgM (Biosource), or polyclonal F(ab)2 goat anti–human IgA (Southern Biotechnology, Birmingham, AL) at 4°C overnight. Plates were washed 3 times with PBS with 0.1% Tween-20 and blocked with PBS containing 2% BSA. Supernatants from each culture phase, a negative control consisting of media alone, and Ig standards were diluted 1:2000 to 1:10 000 and 100 μL incubated in each well for 1 hour at 37°C. Plates were again washed with PBS-Tween and a biotinylated detection antibody was added at 1:2000 to 1:8000 and incubated again for 1 hour at 37°C. Plates were again washed and incubated with horseradish peroxidase–conjugated streptavidin (Southern Biotechnology) for 30 minutes at room temperature, washed 6 times in PBS-Tween buffer, and developed with ABTS substrate (Southern Biotechnology), and optical densities were read. Total amounts of Ig were derived from the standard curve. Ig secretion rates were calculated by dividing the total Ig measured by the number of live cells in culture at the time of supernatant harvest and normalizing to 24 hours (ng Ig · cell−1 · 24 hours−1).
Germ-line sequence examination of CD138+ cells
Magnetic bead immunoaffinity columns were used to remove dead cells and enrich CD138+ cells following phase III of the culture system for cells derived from CD27+ and CD27− precursors. Cells were lysed and genomic DNA was extracted by protease digestion (Qiagen, Valencia, CA). Rearranged VH3 gene segments were amplified from approximately 2000 cells with AccuPrime Pfx DNA polymerase (Invitrogen). For the amplification, a VH3 primer mix (5′-GAGGTGCAGCTGGTGGAGTCTGG-3′ and 5′-GAGGTGCAGCTGTTGGAGTCTGG-3′ at a 7:3 ratio) and a JH primer mix were used (30 seconds at 95°C and 60 seconds at 68°C for 35 cycles). Gel-purified polymerase chain reaction (PCR) products were cloned using the Zero Blunt TOPO PCR Cloning Kit (Invitrogen), and positive colonies were sequenced. The obtained sequences were compared with the germ-line V gene sequences from Glu1 to Tyr91.
Western blot detection of bcl-2
CD27+ and CD27− cells at each stage of the culture system were analyzed for bcl-2 expression by Western blot as previously described.23 Frozen cell preparations were lysed in Nonidet P-40 lysis buffer containing a protease inhibitor cocktail (4-(2-aminoethyl)-benzenesulfonyl fluoride, pepstatin A, transepoxysuccinyl-l-leucylamido (4-guanidino) butane, bestatin, leupeptin, and aprotinin; Sigma), and total protein was quantified using the bicinchoninic acid protein assay (BCA assay kit; Pierce, Rockford, IL). Total protein (1-5 μg) was electrophoresed on a 10% denaturing polyacrylamide-stacking gel and transferred to a nitrocellulose membrane (Amersham). The membranes were blocked for 2 hours with 10% Blotto (PBS/0.1% Tween 20 and 10% milk) and incubated for 1 hour with murine monoclonal antibody against bcl-2 (clone sc-7382; Santa Cruz Biotechnology, Santa Cruz, CA) or β-actin (clone JLA20; Calbiochem, San Diego, CA) at a dilution of 1:5000. The membrane was washed with PBS/0.1% Tween and incubated with a goat anti–rabbit HRP secondary antibody (Santa Cruz Biotechnology) at a 1:2000 dilution. After washing in PBS/0.1% Tween, the membrane was developed by chemiluminescence using an enhanced chemiluminescence (ECL) kit (Pierce). Mean band density was measured using NIH Image software (Bethesda, MD), and the ratio of bcl-2/β-actin was plotted for each phase of the culture system.
Results
Culture conditions alter B-cell proliferation
We first investigated whether there were any differences in the degree of proliferation elicited by CpG DNA in CD27+ memory and CD27− naive B cells in phase I. Other groups have reported that some CpG DNA sequences will cause memory B cells to activate and proliferate without the need for BCR triggering.24 In contrast, CpG DNA–stimulated naive B cells will up-regulate CD69 and CD86 but do not divide without BCR cross-linking. In our system, we found that activation and proliferation of CpG DNA–activated CD27− B cells was dependent on cell density and cell-cell contact (Figure 1).
When CD27− naive B cells were cultured in the presence of CpG DNA, IL-2, IL-10, and IL-15 under conditions that permitted high-density cell-cell contact (1 × 105 cells/200-μL well in U-bottom plates), they underwent robust proliferation in the absence of BCR cross-linking (Figure 1). We next compared the responses of CD27− B cells cultured under high-density and contact conditions with previously reported culture conditions.1,24 Other investigators have cultured CD27− B cells at low density (3 × 104 cells/well) in flat-bottom 200-μL wells in the presence of CpG DNA, IL-2, and IL-10,24 or alternatively with IL-2 and IL-15.1 Consistent with published results,24 under these conditions CD27− B cells did not proliferate (Figure 1). In addition, CD27+ B cells cultured under high-density and contact conditions gave rise to a much greater percentage of CD27+ precursors proliferating for more cycles than previously reported systems. Cells cultured with control nonstimulatory CpG DNA sequences (sequence 2005) or in the absence of CpG DNA did not proliferate (data not shown).
B-cell proliferation occurs in conjunction with up-regulation of intracytoplasmic TLR-9 following CpG DNA stimulation
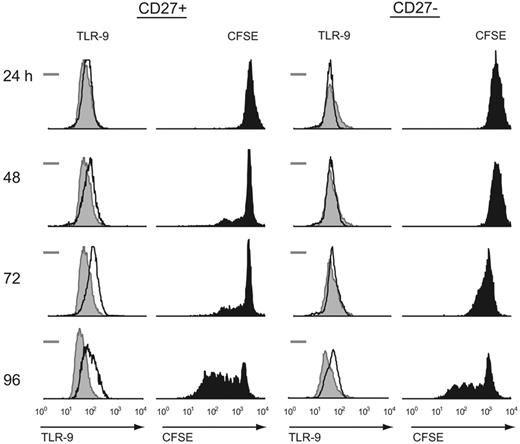
Given that CpG DNA induced proliferation of CD27− B cells under high-density culture conditions, we next examined the intracellular expression of TLR-9. PCR studies have shown that TLR-9 mRNA is expressed at low levels in CD27− naive B cells and at high levels in CD27+ memory B cells.24 BCR triggering rapidly up-regulates TLR-9 mRNA in naive B cells, as does IL-10 in memory B cells.25 However, less is known about cytoplasmic stores or persistence of the TLR-9 protein in the absence of active transcription. For CD27− B cells to proliferate in the presence of CpG DNA, we hypothesized that intracellular expression of the TLR-9 protein must occur at some level and be inducible by a positive-feedback mechanism. We therefore measured intracytoplasmic TLR-9 expression by flow cytometry and correlated this with simultaneous CFSE measurement during culture with CpG DNA (Figure 2). We found that intracellular staining for TLR-9 increases during activation-induced B-cell division in both the naive and memory populations, although the increased intracellular staining for TLR-9 in CD27− naive B cells lags behind that of memory B cells by 24 to 48 hours. This observation suggests a possible mechanism for TLR-9–triggered proliferation of CD27− B cells in the absence of BCR signaling.
Intracellular TLR-9 correlates with proliferation following CpG DNA stimulation for both naive (CD27−) and memory (CD27+) B cells. Intracytoplasmic TLR-9 was measured by flow cytometry immediately after isolating naive and memory B cells (shaded histogram) for both populations. The bar represents the isotype control measurement. Simultaneous intracytoplasmic TLR-9 and CFSE measurements were obtained following CpG DNA stimulation in the presence of IL-2, IL-10, and IL-15 at the time intervals listed for each population. Both cell types expressed a basal level of intracytoplasmic TLR-9, while memory B cells had a faster increase in intracytoplasmic levels than their CD27− counterparts. Results are representative of 7 independent experiments from different blood donors.
Intracellular TLR-9 correlates with proliferation following CpG DNA stimulation for both naive (CD27−) and memory (CD27+) B cells. Intracytoplasmic TLR-9 was measured by flow cytometry immediately after isolating naive and memory B cells (shaded histogram) for both populations. The bar represents the isotype control measurement. Simultaneous intracytoplasmic TLR-9 and CFSE measurements were obtained following CpG DNA stimulation in the presence of IL-2, IL-10, and IL-15 at the time intervals listed for each population. Both cell types expressed a basal level of intracytoplasmic TLR-9, while memory B cells had a faster increase in intracytoplasmic levels than their CD27− counterparts. Results are representative of 7 independent experiments from different blood donors.
Rationale for cytokines and growth factors used in culture system
After CpG activation, we used changes in the cytokine milieu, coupled with blocking CD40 signaling, to induce plasma-cell differentiation in both naive and memory B cells. We developed a 3-phase culture system designed to optimize the conditions for B-cell activation (phase I), plasmablast differentiation and proliferation (phase II), and plasma-cell differentiation (phase III). A graphic representation of each phase is shown in Figure 3. During each phase, cell-signaling ligands and cytokines specific for the B-cell activity to be optimized were added to the culture medium. During activation (phase I), CpG DNA was used to trigger TLR-9–mediated proliferation and differentiation.1 IL-10 was added during phases I and II to increase mRNA expression of TLR-925 and to support B-cell blast Ig production.26-28 IL-2 (phases I and II) and IL-15 (phases I-III) were also added to sustain B-cell proliferation. CD40 signaling is important for activated B-cell proliferation, but inhibits terminal plasma-cell differentiation. CD40 ligand (CD154) is also expressed by activated B cells, and thus a blocking anti-CD40 antibody was added after phase I to promote plasma-cell differentiation. IL-6 is a trophic and growth factor for activated B cells, which express the IL-6 receptor CD126, and it is required for plasma-cell differentiation and immunoglobulin production.9,15,17,29 We therefore added IL-6 in the second and third culture phases to promote plasma-cell differentiation. However, IL-6 alone is not sufficient for the induction of syndecan-1 (CD138) expression, the hallmark of terminally differentiated human plasma cells. For this reason, we added 3 factors known to promote CD138 expression in phase III: interferon α to increase CD138 expression and promote terminal plasma-cell differentiation,17 as well as hepatocyte growth factor and hyaluronic acid, which bind to and promote plasma-cell survival.19,30
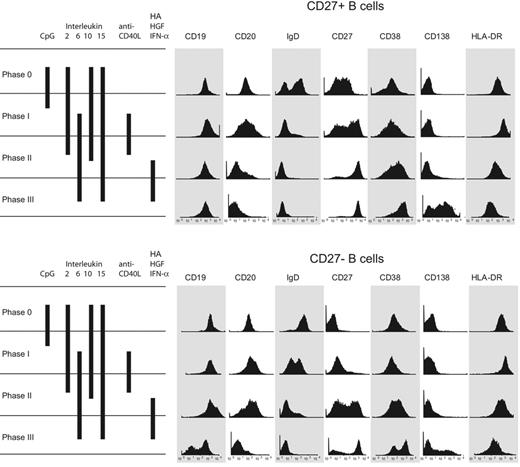
Surface phenotype of CpG DNA2006 activated CD27+ and CD27− B cells with CD138 induction. Surface phenotype of the CD27+ and CD27− peripheral blood B cells beginning with the initial isolation and following each culture phase. Both CD27+ and CD27− B cells were activated by CpG DNA and underwent plasmablast differentiation, ultimately resulting in a surface phenotype characteristic of human bone marrow–resident plasma cells: CD20−, CD38+++, CD138++, and HLA-DR−. Results are representative of 15 independent experiments from 12 different blood donors.
Surface phenotype of CpG DNA2006 activated CD27+ and CD27− B cells with CD138 induction. Surface phenotype of the CD27+ and CD27− peripheral blood B cells beginning with the initial isolation and following each culture phase. Both CD27+ and CD27− B cells were activated by CpG DNA and underwent plasmablast differentiation, ultimately resulting in a surface phenotype characteristic of human bone marrow–resident plasma cells: CD20−, CD38+++, CD138++, and HLA-DR−. Results are representative of 15 independent experiments from 12 different blood donors.
Phenotypic differentiation of CpG DNA–activated CD27+ and CD27− B cells
Figure 3 displays surface marker analysis of cultured cells beginning with the initial CD27+ and CD27− B-cell populations and following each culture phase. As one would expect, CD38, a marker of B-cell activation and plasma-cell differentiation, is increasingly expressed on the cell surface following each step of the culture system. Consistent with the known finding that ligation of TLR-9 increases surface HLA-DR expression,26 HLA-DR surface staining increased after phase I in our culture system in both naive and memory B cells. The initial CD27+ B cells were IgD+ and IgD−, reflecting the known presence of switched and nonswitched populations. However, IgD expression was lost during the subsequent phases either from preferential death or differentiation of the nonswitched cells. As the CD27+ B cells traverse the 3-phase culture system, there is down-regulation of CD19, CD20, and HLA-DR, consistent with plasma-cell development. It is not until completion of phase III of the culture system that there is robust expression of syndecan-1 (CD138) irrespective of the phenotype (naive or memory) of cells in the initial culture.
The initial CD27− B-cell population was primarily IgD+, as expected of predominately mature naive B cells (Figure 3), but following CpG DNA stimulation there are IgDhigh and IgDlow B cells. By the end of the 3-phase culture system, surface IgD expression was lost. There was progressive up-regulation of CD27 and CD38 throughout the duration of culture. As with the CD27+ B cells, HLA-DR surface expression was initially up-regulated following CpG DNA ligation of TLR-9, and then gradually lost following phase II. Similar to the CD27+ B cells, CD20 expression was low after phase III. Unique to the initial CD27− B-cell population, there are CD19high and CD19low populations among those cells that are alive at the end of the culture system. Approximately 50% of the final cells express CD138.
Cell death during culture
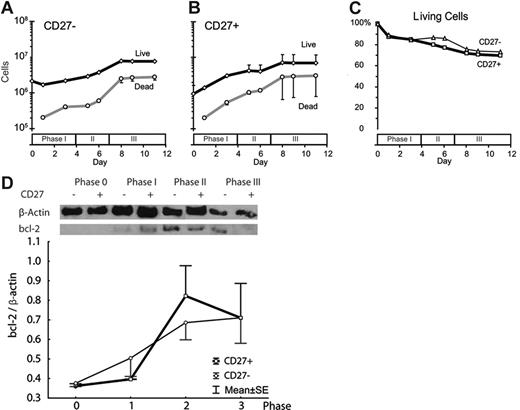
We next examined the degree of cell death in the in vitro culture system. A major limitation for the generation of mature plasma cells in vitro has been their high level of spontaneous cell death in some systems upon acquiring a fully differentiated phenotype.4,30 In addition, proliferation of naive B cells could be associated with cell death, especially in conditions where the stimulation signal is not optimal. To address these issues, we assessed the viable fraction of cells in each culture phase derived from the CD27− and CD27+ isolates. Figure 4 shows the absolute numbers of live and dead cells during each phase of culture, as well as the fraction of living cells. Cultures of starter cells derived from either CD27− (Figure 4A) or CD27+ (Figure 4B) B cells had statistically indistinguishable proportions of living cells through the culture process. For cultures started with CD27− cells, these results make it unlikely that the resulting plasma cells came from contaminating CD27+ cells in the setting of a high proportion of cell death in the CD27−-derived cells. Consistent with this finding, analysis of bcl-2 expression by Western blotting showed a relative increase in bcl-2 expression through the various culture phases, with a decrease in phase III for CD27+ cells (Figure 4D).
Death of CD27+- and CD27−-derived plasma cells during differentiation in vitro. (A-B) At the indicated time points, cells were stained with trypan blue, and the absolute number of live (open diamonds with black lines) and dead (open circles with gray lines) cells were counted. (C) The percentage of living cells at each culture day for CD27− (open triangles with thin line) and CD27+ (open squares with thick line) cells. (D) Western blot analysis of bcl-2 protein expression during in vitro plasma-cell differentiation from naive (thin line) and memory (solid line) B-cell precursors. (A-D) Error bars are mean ± standard error for all plots (n = 2 experiments from 2 subjects).
Death of CD27+- and CD27−-derived plasma cells during differentiation in vitro. (A-B) At the indicated time points, cells were stained with trypan blue, and the absolute number of live (open diamonds with black lines) and dead (open circles with gray lines) cells were counted. (C) The percentage of living cells at each culture day for CD27− (open triangles with thin line) and CD27+ (open squares with thick line) cells. (D) Western blot analysis of bcl-2 protein expression during in vitro plasma-cell differentiation from naive (thin line) and memory (solid line) B-cell precursors. (A-D) Error bars are mean ± standard error for all plots (n = 2 experiments from 2 subjects).
Morphologic progression to plasma cells
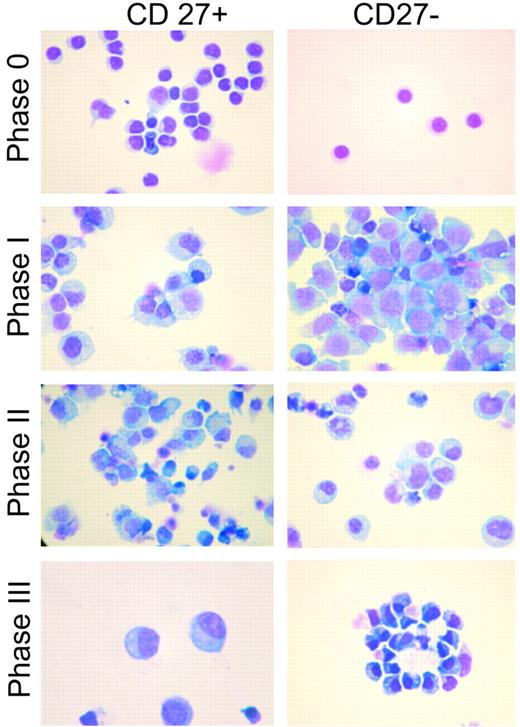
The morphology of the CD27+ and CD27− populations was analyzed by Wright-Giemsa staining following each phase of the culture system (Figure 5). Initially, the CD27+ B cells are larger with more abundant cytoplasm than the CD27− B cells. Following CpG DNA stimulation, however, both populations increased in size and developed a blastic appearance. Those cells obtained at the completion of phase III are oval cells with compact, dense, eccentric nuclei and basophilic cytoplasm with pale Golgi zones, and are morphologically indistinguishable from normal human bone marrow–resident plasma cells.
Morphology of CpG DNA2006–activated CD27+ and CD27− B cells. Wright-Giemsa staining was used to demonstrate morphologic differences between the initial CD27+ and CD27− peripheral blood B cells as well as differentiation changes following each phase of the culture system for both populations. By the end of phase III, CD27+ and CD27− B cells had an identical plasmablast morphology. Results are representative of 4 independent experiments, each from a different blood donor. Images were photographed using a Nikon Labophot microscope (100×/1.17 numerical aperture oil objective) with a Nikon Coolpix digital camera using the manufacturer's software (Nikon, Melville, NY). JPEG images were viewed using Adobe Photoshop CS2 (Adobe Systems, San Jose, CA), and contrast adjustments were made.
Morphology of CpG DNA2006–activated CD27+ and CD27− B cells. Wright-Giemsa staining was used to demonstrate morphologic differences between the initial CD27+ and CD27− peripheral blood B cells as well as differentiation changes following each phase of the culture system for both populations. By the end of phase III, CD27+ and CD27− B cells had an identical plasmablast morphology. Results are representative of 4 independent experiments, each from a different blood donor. Images were photographed using a Nikon Labophot microscope (100×/1.17 numerical aperture oil objective) with a Nikon Coolpix digital camera using the manufacturer's software (Nikon, Melville, NY). JPEG images were viewed using Adobe Photoshop CS2 (Adobe Systems, San Jose, CA), and contrast adjustments were made.
Intracytoplasmic and secreted IgM and IgG
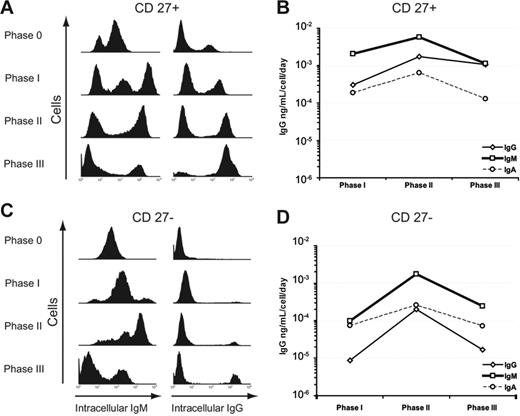
Resting CD27+ memory B cells had low levels of intracytoplasmic IgG that increased steadily after activation and through each culture phase (Figure 6A,C). After CpG DNA stimulation, the CD27+ cells had 3 IgM populations: high, intermediate, and low expression. The proportion of cells staining positive for intracellular IgM decreased throughout the culture phases, and the resultant CD38+++ CD138+ plasma cells are primarily IgG producers (94% ± 6%, n = 3 independent experiments). In contrast, CD27− naive B cells initially had low levels of intracellular IgM and no IgG. After CpG DNA stimulation, we observed a steady increase in the level of intracytoplasmic IgM following each phase resulting in predominantly IgM-producing plasma cells by the end of phase II (98% ± 8%). Of interest, intracellular IgM staining decreased in both CD27+ and CD27− populations in phase III. ELISA results for total IgG, IgA, and IgM secretion confirmed the secretion of each immunoglobulin (Figure 6B,D).
Induction of IgG-producing plasma cells from CD27+ B cells and IgM-producing plasma cells from CD27− B cells. (A,C) Intracytoplasmic immunoglobulin staining was used to assess immunoglobulin production with each phase. Intracellular IgM increased in both (A) CD27+ and (C) CD27− B cells by the end of phase II, and then declined after phase III. In contrast, CD27+ cells had a steady increase in intracellular IgG staining through terminal differentiation into CD138+ plasma cells. Figures are representative of 4 independent experiments with cells from 4 different blood donors. (B,D) IgG, IgM, and IgA were measured by ELISA from supernatants taken at the end of each culture phase for (B) CD27+ and (D) CD27− B cells. Error bars are mean ± standard error for all plots. Experimental data are from 5 separate experiments each with different blood donors.
Induction of IgG-producing plasma cells from CD27+ B cells and IgM-producing plasma cells from CD27− B cells. (A,C) Intracytoplasmic immunoglobulin staining was used to assess immunoglobulin production with each phase. Intracellular IgM increased in both (A) CD27+ and (C) CD27− B cells by the end of phase II, and then declined after phase III. In contrast, CD27+ cells had a steady increase in intracellular IgG staining through terminal differentiation into CD138+ plasma cells. Figures are representative of 4 independent experiments with cells from 4 different blood donors. (B,D) IgG, IgM, and IgA were measured by ELISA from supernatants taken at the end of each culture phase for (B) CD27+ and (D) CD27− B cells. Error bars are mean ± standard error for all plots. Experimental data are from 5 separate experiments each with different blood donors.
V gene sequencing of cells expressing CD138
To further evaluate the possibility of CD27+ or CD27− memory B cells contaminating the initial CD27− naive B-cell population, we analyzed heavy chain variable region gene sequences from the cells that were CD138+ from both the initially CD27+ and CD27− populations for the presence of somatic mutations (Table 1). DNA was made from CD138+ cells purified from phase III. Rearranged VH3 gene segments were PCR amplified from approximately 2 × 103 cells; gel-purified products were cloned and DNA sequenced. We found that 70% of productively rearranged variable regions derived from CD27− precursors contained unmutated germ-line sequences, consistent with their origin from naive B-cell precursors. These results also demonstrate that at least the majority of these plasma cells were not derived from memory cells that may represent a very small fraction of peripheral blood CD27− B cells but are universally mutated.31 As expected, all productively rearranged variable region genes from the CD27+-derived cells contained somatic mutations.
Characterization of VH-D-JH transcripts obtained from CD138+ cells derived from naive (CD27−) and memory (CD28+) B cells
| Transcript . | VH . | No. nt mutated/nt sequenced . | Mutation frequency, % . | No. of replacement/silent mutations . | Total R/S . | D . | JH . | Rearrangement . | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| FR1 . | CDR1 . | FR2 . | CDR2 . | FR3 . | ||||||||
| CD27− naive-derived CD138+ plasma cells | ||||||||||||
| R1-1* | VH3-73 | 0/291 | 0 | 0/0 | 0/0 | 0/0 | 0/0 | 0/0 | 0:0 | D6-13 | JH3 | PR |
| R1-2* | VH3-30.5 | 0/285 | 0 | 0/0 | 0/0 | 0/0 | 0/0 | 0/0 | 0:0 | D5-5 | JH4 | PR |
| R1-3 | VH3-30 | 34/285 | 11.9 | 1/1 | 3/0 | 0/0 | 9/2 | 12/1 | 25:4 | D6-25 | JH6 | Non-PR |
| R1-5 | VH3-33 | 8/285 | 2.8 | 2/0 | 0/0 | 1/0 | 2/1 | 1/0 | 6:1 | D6-13 | JH4 | PR |
| R1-6 | p1 | 37/285 | 13.0 | 3/2 | 2/0 | 3/0 | 5/3 | 9/2 | 22:7 | D5-24 | JH4 | PR |
| R1-7* | VH3-15 | 0/291 | 0 | 0/0 | 0/0 | 0/0 | 0/0 | 0/0 | 0:0 | D6-13 | JH6 | PR |
| R1-8 | VH3-53 | 28/282 | 9.9 | 2/1 | 1/0 | 2/0 | 7/1 | 8/2 | 20:4 | D3-3 | JH4 | Non-PR |
| R1-9* | VH3-23 | 1/285 | 0.4 | 0/0 | 0/0 | 0/0 | 0/0 | 1/0 | 1:0 | D6-25 | JH4 | PR |
| R1-11* | VH3-30.5 | 0/285 | 0 | 0/0 | 0/0 | 0/0 | 0/0 | 0/0 | 0:0 | D5-5 | JH4 | PR |
| R1-12* | VH3-21 | 0/285 | 0 | 0/0 | 0/0 | 0/0 | 0/0 | 0/0 | 0:0 | D5-5 | JH4 | PR |
| R1-13 | VH3-30.5 | 3/285 | 1.1 | 0/0 | 1/0 | 0/0 | 1/1 | 0/0 | 2:1 | D3-10 | JH4 | PR |
| R1-14* | VH3-15 | 0/291 | 0 | 0/0 | 0/0 | 0/0 | 0/0 | 0/0 | 0:0 | D2-8 | JH6 | PR |
| CD27+ memory-derived CD138+ plasma cells | ||||||||||||
| R2-1 | VH5-51 | 10/285 | 3.5 | 3/1 | 1/0 | 0/0 | 0/0 | 4/1 | 8:2 | D1-7 | JH4 | PR |
| R2-2 | VH3-49 | 13/291 | 4.5 | 2/0 | 1/0 | 0/0 | 4/1 | 3/1 | 10:2 | D3-9 | JH3 | PR |
| R2-4 | VH1-2 | 24/285 | 8.4 | 4/0 | 2/0 | 0/1 | 4/0 | 7/4 | 17:5 | D5-5 | JH4 | PR |
| R2-5 | VH5-51 | 14/285 | 4.9 | 2/2 | 1/0 | 0/0 | 1/1 | 5/1 | 9:4 | D6-13 | JH4 | PR |
| R2-6 | VH3-23 | 16/285 | 5.6 | 2/1 | 2/0 | 0/0 | 5/0 | 1/2 | 10:3 | D2-21 | JH4 | PR |
| R2-7 | VH3-11 | 18/285 | 6.3 | 3/1 | 1/1 | 1/0 | 5/1 | 0/3 | 10:6 | D7-27 | JH6 | PR |
| R2-8 | VH5-51 | 19/285 | 6.7 | 3/1 | 2/0 | 0/1 | 3/1 | 6/0 | 14:3 | — | JH6 | PR |
| R2-9 | VH3-7 | 11/285 | 3.9 | 2/2 | 1/0 | 1/0 | 1/0 | 4/0 | 9:2 | D2-21 | JH4 | Non-PR |
| R2-10 | VH3-64 | 24/285 | 8.4 | 0/1 | 1/1 | 0/0 | 6/1 | 5/2 | 12:5 | — | JH6 | PR |
| R2-11 | VH3-13 | 14/282 | 5.0 | 0/2 | 1/0 | 1/1 | 0/1 | 3/1 | 5:5 | D5-5 | JH6 | PR |
| R2-13 | VH1-2 | 29/285 | 10.2 | 5/1 | 2/0 | 1/0 | 6/1 | 6/3 | 20:5 | D2-8 | JH4 | PR |
| R2-14 | VH3-21 | 14/285 | 4.9 | 0/0 | 2/1 | 0/1 | 3/3 | 0/1 | 5:6 | — | JH6 | PR |
| Transcript . | VH . | No. nt mutated/nt sequenced . | Mutation frequency, % . | No. of replacement/silent mutations . | Total R/S . | D . | JH . | Rearrangement . | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| FR1 . | CDR1 . | FR2 . | CDR2 . | FR3 . | ||||||||
| CD27− naive-derived CD138+ plasma cells | ||||||||||||
| R1-1* | VH3-73 | 0/291 | 0 | 0/0 | 0/0 | 0/0 | 0/0 | 0/0 | 0:0 | D6-13 | JH3 | PR |
| R1-2* | VH3-30.5 | 0/285 | 0 | 0/0 | 0/0 | 0/0 | 0/0 | 0/0 | 0:0 | D5-5 | JH4 | PR |
| R1-3 | VH3-30 | 34/285 | 11.9 | 1/1 | 3/0 | 0/0 | 9/2 | 12/1 | 25:4 | D6-25 | JH6 | Non-PR |
| R1-5 | VH3-33 | 8/285 | 2.8 | 2/0 | 0/0 | 1/0 | 2/1 | 1/0 | 6:1 | D6-13 | JH4 | PR |
| R1-6 | p1 | 37/285 | 13.0 | 3/2 | 2/0 | 3/0 | 5/3 | 9/2 | 22:7 | D5-24 | JH4 | PR |
| R1-7* | VH3-15 | 0/291 | 0 | 0/0 | 0/0 | 0/0 | 0/0 | 0/0 | 0:0 | D6-13 | JH6 | PR |
| R1-8 | VH3-53 | 28/282 | 9.9 | 2/1 | 1/0 | 2/0 | 7/1 | 8/2 | 20:4 | D3-3 | JH4 | Non-PR |
| R1-9* | VH3-23 | 1/285 | 0.4 | 0/0 | 0/0 | 0/0 | 0/0 | 1/0 | 1:0 | D6-25 | JH4 | PR |
| R1-11* | VH3-30.5 | 0/285 | 0 | 0/0 | 0/0 | 0/0 | 0/0 | 0/0 | 0:0 | D5-5 | JH4 | PR |
| R1-12* | VH3-21 | 0/285 | 0 | 0/0 | 0/0 | 0/0 | 0/0 | 0/0 | 0:0 | D5-5 | JH4 | PR |
| R1-13 | VH3-30.5 | 3/285 | 1.1 | 0/0 | 1/0 | 0/0 | 1/1 | 0/0 | 2:1 | D3-10 | JH4 | PR |
| R1-14* | VH3-15 | 0/291 | 0 | 0/0 | 0/0 | 0/0 | 0/0 | 0/0 | 0:0 | D2-8 | JH6 | PR |
| CD27+ memory-derived CD138+ plasma cells | ||||||||||||
| R2-1 | VH5-51 | 10/285 | 3.5 | 3/1 | 1/0 | 0/0 | 0/0 | 4/1 | 8:2 | D1-7 | JH4 | PR |
| R2-2 | VH3-49 | 13/291 | 4.5 | 2/0 | 1/0 | 0/0 | 4/1 | 3/1 | 10:2 | D3-9 | JH3 | PR |
| R2-4 | VH1-2 | 24/285 | 8.4 | 4/0 | 2/0 | 0/1 | 4/0 | 7/4 | 17:5 | D5-5 | JH4 | PR |
| R2-5 | VH5-51 | 14/285 | 4.9 | 2/2 | 1/0 | 0/0 | 1/1 | 5/1 | 9:4 | D6-13 | JH4 | PR |
| R2-6 | VH3-23 | 16/285 | 5.6 | 2/1 | 2/0 | 0/0 | 5/0 | 1/2 | 10:3 | D2-21 | JH4 | PR |
| R2-7 | VH3-11 | 18/285 | 6.3 | 3/1 | 1/1 | 1/0 | 5/1 | 0/3 | 10:6 | D7-27 | JH6 | PR |
| R2-8 | VH5-51 | 19/285 | 6.7 | 3/1 | 2/0 | 0/1 | 3/1 | 6/0 | 14:3 | — | JH6 | PR |
| R2-9 | VH3-7 | 11/285 | 3.9 | 2/2 | 1/0 | 1/0 | 1/0 | 4/0 | 9:2 | D2-21 | JH4 | Non-PR |
| R2-10 | VH3-64 | 24/285 | 8.4 | 0/1 | 1/1 | 0/0 | 6/1 | 5/2 | 12:5 | — | JH6 | PR |
| R2-11 | VH3-13 | 14/282 | 5.0 | 0/2 | 1/0 | 1/1 | 0/1 | 3/1 | 5:5 | D5-5 | JH6 | PR |
| R2-13 | VH1-2 | 29/285 | 10.2 | 5/1 | 2/0 | 1/0 | 6/1 | 6/3 | 20:5 | D2-8 | JH4 | PR |
| R2-14 | VH3-21 | 14/285 | 4.9 | 0/0 | 2/1 | 0/1 | 3/3 | 0/1 | 5:6 | — | JH6 | PR |
V gene sequence analysis was performed on the CD138+ cells derived from CD27− (naive) and control CD27+ (memory) B cells. Seventy percent of the CD138+ cells derived from CD27− B cells were germ-line configuration without somatic mutations. None of the control, CD138+ cells derived from the CD27+ B cells, were germ-line configuration. nt indicates nucleotide; FR, framework region; CDR, complementarity-determining region; R/S, replacement-to-silent mutation ratio; PR, productive gene rearrangement; and non-PR, nonproductive gene rearrangement.
0.99% germ-line identity of the rearranged VH gene.
Discussion
In this report, we demonstrate that CD27− naive human B cells will undergo CpG DNA–induced activation and proliferation under in vitro conditions promoting cell-cell contact, and can subsequently be driven to a bone marrow–resident, CD138+, plasma-cell phenotype. The majority of plasma cells derived from CD27− precursors had germ-line heavy chain variable region gene sequences, excluding the possibility of selective expansion by contaminating CD27+ precursors. These results are consistent with recent studies that demonstrated CpG-induced proliferation in CD27− cord blood B cells32,33 and others showing that CpG stimulation drives naive B cells into IgM-producing plasmablasts.6
Our results and those of others suggest that CD27− B cells have low-level constitutive expression of intracellular TLR-9, with a positive-feedback mechanism to up-regulate protein expression after CpG stimulation. Bernasconi et al reported that CD27− B cells have a low level of TLR-9 mRNA expression, but at the same time TLR-9 ligation by CpG DNA induced CD69 and CD86 up-regulation as soon as 4 hours after exposure, indicating activation of the naive CD27− B cells.24 Similarly, we found that intracellular TLR-9 in unstimulated CD27− B cells increased at 96 hours after activation coincident with cell proliferation. It remains unclear, however, if the increase in TLR-9 protein after activation is due to an increase in mRNA transcription or simply increased protein synthesis from existing transcripts. While some groups have shown an increase in TLR-9 mRNA in naive B cells after CpG DNA stimulation,34,35 others report decreased transcript copy numbers.6 Further molecular work will be necessary to define the regulatory pathway interactions responsible for TLR-9 regulation in CD27− B cells.
Our results also demonstrate that CpG DNA–induced proliferation of CD27− B cells can indeed occur when they are cultured at high density with close cell-cell contact. This finding complements other reports that CD27− human B cells do not proliferate under low cell density culture conditions after CpG DNA exposure unless BCR cross-linking was also present.1,25,26 Several recent reports by other groups suggest that CD40-CD154 costimulation between activated B cells may be a possible mechanism for the enhanced proliferative capacity of naive B cells with cell-cell contact.14,36 Engagement of CD40 on B cells by CD40 ligand (CD154) results in clonal expansion and differentiation into immunoglobulin-secreting cells.37 CD40 ligand is expressed by human B cells, and CD40-CD40L (CD154) interaction between B cells can costimulate B-cell responses.38 Moreover, recent data suggest that the level of exposure of B lymphocytes to CD154 affects proliferation, global expansion of CD19+ cells, and emergence of CD38++ CD138+ cells, as well as IgG and IgM secretion.36 Thus, we hypothesize that the close cell-cell contact that occurs in our culture system optimizes the ability of CD40-CD40 ligand interaction between B cells and thereby enhances their proliferation and differentiation after TLR-9 signaling.
In contrast to previous reports, we demonstrate that both naive and memory B cells can be driven to a CD138+ bone marrow–resident plasma-cell phenotype. While most studies use the CD38+ IgD− phenotype to define the plasma-cell state with in vitro differentiation systems, our data suggest that this phenotypic classification may be suboptimal. Plasma-cell differentiation, which we define by the CD20− CD38+ IgD− CD138+++ phenotype, appears to be driven primarily by the postactivation microenvironment rather than the initial TLR-9–mediated activation event. In our system, several growth factors known to up-regulate CD138 (hyaluronate, IFN-α, and human hepatocyte growth factor) are required for this step. HLA-DR, CD20, and CD19 down-regulation occurred only with CD138 expression. In addition, some groups have hypothesized that CD138 is responsible for localization of long-lived plasma cells to the bone marrow extracellular matrix.11 As B cells differentiate from plasmablasts (CD38+ IgD− phenotype) to terminally differentiated, nondividing plasma cells (CD138 phenotype), expression of genes involved in proliferation, survival, homing, and bone remodeling continues to change.39
We observed that even without initial T-cell–derived costimulation, we were able to drive naive B cells into IgM-producing plasma cells and memory B cells into plasma cells that produce primarily IgG. It is believed that after antigen encounter naive marginal zone B cells and follicular B cells may develop into IgM-producing short-lived plasma cells, whereas germinal center B cells and memory B cells will develop into either long-lived or short-lived plasma cells.40 We hypothesize that the IgM-producing plasma cells derived from naive CD27− B cells after CpG DNA stimulation are short-lived plasma cells and that the IgG-producing plasma cells derived from CD27+ B cells may be long-lived plasma cells. This view is supported by our findings that in phase III the percentage of living cells does not decline precipitously and that bcl-2 protein levels decrease only modestly. The bcl-2 family of proteins is known to be important for plasma-cell survival, and induction of some of these proteins, such as Mcl-1 by human hepatocyte growth factor and IL-6, may contribute to plasma-cell survival independent of bcl-2 itself.41-43 However, further studies are necessary to confirm this hypothesis, including viability studies of CD138+ cells over weeks to months, profiling of plasma-cell survival factors other than bcl-2, and a more extensive analysis of plasma-cell phenotypes (eg, CD9, CD31).
We were surprised to find that antibody production rates (pg antibody/live cell per 24 hours) declined when blasts differentiated into CD138+ plasma cells, after initially rising at the end of activation (phase I) and expansion (phase II). Unfortunately, we are not aware of any current literature describing differences between antibody secretion rates of normal human B-cell blasts and CD138+ plasma cells for comparison with our results. We speculate, however, that the elevated antibody secretion rate of activated blasts may aid in the rapid increase in antibody titers during an acute immune response, while lower Ig secretion rates by long-lived plasma cells might be adequate to maintain protective antibody titers, perhaps mediated by increased sensitivity to down-regulation by the inhibitory FcγRIIB.44 Further experiments comparing Ig secretion rates at various stages of differentiation and quantitative modeling of antibody kinetics are currently under way in our laboratory.
In summary, we have demonstrated that human CD27− (naive) peripheral blood B cells can be activated via TLR-9 signaling and, under conditions of cell-cell contact with the appropriate cytokine and trophic factor support, differentiate into CD138+ plasma cells with germ-line heavy chain gene sequences. This in vitro culture system provides a tool for further understanding of plasma-cell differentiation. For example, it could be used to study class switching recombination control, as there are almost no published data in human B cells examining the time course of activation-induced cytidine deaminase (AID) protein levels in resting and activated naive and memory B cells. In addition, it may aid in the analysis of antigenic reactivity of naive B cells in healthy subjects and those with autoimmune B-cell dysfunction. Such knowledge may provide insights into identifying therapeutic targets for the treatment of differentiated B-cell disorders such as SLE and multiple myeloma.
Authorship
J.H. designed and performed experiments, collected and analyzed data, and wrote the paper; T.P. performed experiments and collected data; R.E.F. assisted in flow cytometry B-cell phenotyping experiments; M.B. performed experiments and collected data; B.Z. performed experiments; C.W. and E.C.B.M. designed and performed the sequencing experiments and assisted in experimental design and data analysis; S.H.B. performed Western blot experiments; I.S. assisted in experimental design and data analysis; M.S.Z. designed experiments, analyzed data, and wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Martin S. Zand, Associate Professor of Medicine, Microbiology and Immunology, Medical Director, Kidney and Pancreas Transplant Programs, University of Rochester Medical Center, 601 Elmwood Ave, Box 675, Rochester, NY 14642; e-mail: martin_zand@urmc.rochester.edu.
An Inside Blood analysis of this article appears at the front of this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors would like to thank Rosemary Ziemba-Ball for her assistance with the intracellular Ig staining and Kimberly Morse for her assistance with the Western blotting experiments.
This work was supported in part by grants from the Renal Research Institute (M.S.Z.); The Alternative Research and Development Foundation (M.S.Z.); NIH grants N01-AI-50020 (M.S.Z., I.S., E.C.B.M.), R01 AI049660 (I.S.), and U19 AI56390 (I.S.); and a training grant from the Allergy, Immunology and Rheumatology Unit (J.H.).

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal