Abstract
The antiapoptotic protein Mcl-1, a member of the Bcl-2 family, plays critical roles in promoting the survival of lymphocytes and hematopoietic stem cells. Although previous studies have implicated Mcl-1 in regulating the survival of neutrophils and macrophages, the in vivo function of Mcl-1 in these 2 cell lineages remained unclear. To address this, we have generated mice conditionally lacking Mcl-1 expression in neutrophils and macrophages. We show that Mcl-1 conditional knockout mice had a severe defect in neutrophil survival, whereas macrophage survival was normal. The granulocyte compartment in the blood, spleen, and bone marrow of Mcl-1 conditional knockout mice exhibited an approximately 2- to 3-fold higher apoptotic rate than control cells. In contrast, resting and activated macrophages from Mcl-1–deficient mice exhibited normal survival and contained up-regulated expression of Bcl-2 and Bcl-xL. These data suggest that Mcl-1 plays a nonredundant role in promoting the survival of neutrophils but not macrophages.
Introduction
The antiapoptotic Bcl-2 family plays critical roles in regulating apoptosis in immune cells.1,2 Mcl-1, initially isolated from a human myeloblastic leukemia cell line, is a member of the Bcl-2 family.3 Mcl-1 contains 3 Bcl-2 homology domains and inhibits apoptosis by interacting with the proapoptotic proteins Bim, Bak, and Bid.4-7 Overexpression of Mcl-1 in a myeloid progenitor cell line inhibited cell death induced by various apoptotic stimuli.8 Furthermore, transgenic expression of Mcl-1 in mice enhanced the survival of a wide range of hematopoietic cells including lymphocytes and myeloid cells at distinct developmental stages.9 Direct evidence demonstrating an essential role for Mcl-1 in promoting hematopoietic cell survival came from studies on conditional Mcl-1–deficient mice. Lymphocytes and hematopoietic stem cells lacking Mcl-1 expression undergo apoptosis and exhibit defective differentiation.10,11 These results suggest that Mcl-1 may play essential roles in the survival of a wide range of cells in vivo.
Neutrophils are polymorphonuclear (PMN) leukocytes with a short half-life in the circulation (6-18 hours) due to spontaneous apoptosis. Neutrophils express several proapoptotic members of the Bcl-2 family including Bax, Bad, Bak, Bid, and Bik as well as the antiapoptotic members Mcl-1 and A1.12,13 The antiapoptotic A1 gene plays a critical role in regulating the spontaneous apoptosis of neutrophils as A1-deficient neutrophils develop normally but exhibit an enhanced spontaneous apoptosis when cultured in vitro.14 Mcl-1 may also play an essential role in neutrophil survival. Numerous studies have demonstrated that various stimuli that promote or inhibit neutrophil apoptosis can modulate Mcl-1 expression in these cells.15-21 For example, GM-CSF promotes neutrophil survival and enhances Mcl-1 protein stablility.19 Antisense oligonucleotide-mediated reduction of Mcl-1 expression in human neutrophils induced apoptosis.22 In addition, Mcl-1 may also be essential for macrophage survival. Inhibition of Mcl-1 expression by antisense oligonucleotides in human monocyte-differentiated macrophages resulted in apoptosis.23 Furthermore, Stat3 was shown to promote macrophage survival by up-regulating Mcl-1 expression.24 Although this body of evidence implicated Mcl-1 in neutrophil and macrophage survival, it is not clear whether endogenous Mcl-1 expression is required in vivo.
To determine the role of Mcl-1 in neutrophils and macrophages, we have generated mice conditionally lacking Mcl-1 expression in these cells and examined their development. We show that the survival of neutrophils is severely impaired, whereas the survival of macrophages is apparently normal in the Mcl-1–deficient mice. The impaired neutrophil survival in Mcl-1–deficient mice is accompanied by an increased apoptosis rate in this population. In contrast, resting or activated Mcl-1–deficient macrophages exhibited normal survival and expressed elevated levels of the antiapoptotic proteins, Bcl-2 and Bcl-xL. These results demonstrate that Mcl-1 is essential for the survival of neutrophils but dispensable for macrophage survival in vivo.
Materials and methods
Generation of Mcl-1 conditional knockout mice
We generated a targeting construct for our Mcl-1 conditional knockout by cloning genomic fragments from a Mcl-1 BAC clone (Roswell Park Cancer Institute, Buffalo, NY) into the pGKneoF2L2DTA targeting vector. Exon 1 of the Mcl-1 gene encoding amino acid 1-179 was flanked by 2 loxP sites and the neomycin-resistant gene cassette was flanked by 2 FRT sites. After being linearized by NotI, the targeting construct was electroporated into TC1 embryonic stem (ES) cells. We screened homologously recombined ES clones with polymerase chain reaction (PCR) and confirmed by Southern blot. Seven ES clones with the correct targeting events were injected into C57BL/6 blastocysts. We bred chimeric male founder mice (Mcl-1fl/+) with FLPeR female mice25 to delete the neomycin cassette. We then bred Mcl-1fl/+ mice with LysMcre knock-in mice26 (Jackson Laboratory, Bar Harbor, ME) to generate Mcl-1fl/flLysMcre, Mcl-1fl/+LysMcre, and Mcl-1fl/fl mice. The phenotypes of Mcl-1fl/+LysMcre and Mcl-1fl/fl mice are indistinguishable from those of wild-type (Mcl-1+/+) C57BL/6 × 129 mice and were used as controls throughout the experiments. Animal usage was conducted according to protocols approved by the Duke University Institutional Animal Care and Use Committee.
Flow cytometry
Peritoneal macrophages were elicited with 1 mL 3% thioglycollate (Difco, BD Biosciences, San Jose, CA) intraperitoneally and the cells were recovered by lavage with 5 mL medium after 1 or 4 days. Peripheral blood was collected in PBS containing 5 mM EDTA after incision of the tail vain. Single-cell suspensions of spleen, bone marrow (BM), peritoneal exudates, and blood were lysed of red blood cells (RBCs), incubated with an Fc receptor blocker (2.4G2 hybridoma supernatant) followed by anti–Mac-1–FITC and anti–Gr-1–APC (BioLegend, San Diego, CA) on ice for 20 minutes, and washed with PBS containing 2% FCS. Data were collected on a FACScan or FACStarPLUS flow cytometer (BD Biosciences, San Jose, CA) and analyzed using CellQuest software (BD Biosciences). Apoptotic cells were determined by annexin V and 7-aminoactinomycin D (7-AAD) staining using an annexin V-PE kit (BD PharMingen, San Diego, CA) according to the manufacturer's instructions.
Western blot
BM-derived macrophages were obtained as described.27 Briefly, BM cells were cultured in complete RPMI 1640 (Gibco, Invitrogen, Carlsbad, CA) medium supplemented with 30% L929 conditioned medium. After 1 day the nonadherent cells were transferred to new dishes and cultured for 6 more days. The cells were lysed with 1 × SDS sample buffer. An equal amount of protein was separated on 10% polyacrylamide gel and transferred on a PVDF membrane (Perkin Elmer, Wellesley, MA). The primary antibodies used were rabbit anti–Mcl-1 (Rockland Immunochemicals, Gilbertsville, PA), hamster anti–Bcl-2 (BD Pharmingen), mouse anti–Bcl-xL (BD Pharmingen), and rabbit anti–Erk-2 (Santa Cruz Biotechnology, Santa Cruz, CA). The secondary antibodies were conjugated to HRP (Jackson Immunoresearch, West Grove, PA) and detected with West Pico Chemiluminescence substrate (Pierce, Rockford, IL). For the expression of Mcl-1 in neutrophils, Mac-1+Gr-1+ cells were purified by magnetic selection with Gr-1–biotin antibody (eBioscience, San Diego, CA) followed by streptavidin MicroBeads (Miltenyi Biotec, Auburn, CA) to a purity of more than 92%. The secondary antibody was antirabbit Alexa Fluor 680 (Molecular Probes, Invitrogen, Carlsbad, CA). It was detected with Odyssey system (LI-COR, Lincoln, NE).
Cytology
Peripheral blood and peritoneal exudate cells were lysed of RBCs and 5 × 105 cells in 100 μL PBS were centrifuged on microscope slides with Shandon cytocentrifuge (Shandon, Thermo Electron, Waltham, MA). The cells were stained with a Hema 3 staining kit (Fisher Scientific, Hampton, NH). The images were acquired with an Axiovert 200 inverted microscope equipped with a 40×/0.5 NA objective and an AxioCam MRC, and were analyzed with AxioVision AC 4.5 software (all from Zeiss, Thornwood, NJ).
Macrophage stimulation and apoptosis assays
Peritoneal macrophages were elicited by intraperitoneal injection of 1 mL 3% thioglycollate broth (Difco, BD Biosciences) 4 days before the experiment. The cells were recovered by peritoneal lavage with 5 mL 2% FBS in PBS, and 1.5 × 105 cells were seeded in 500 μL medium. The cells were left to adhere overnight and were stimulated with LPS (100 ng/mL), LTA (10 μg/mL), or polyI:C (100 μg/mL), all from Sigma-Aldrich (St Louis, MO) overnight. The supernatants were collected and assayed for proinflammatory cytokine production by enzyme-linked immunosorbent assay (ELISA). IL-6 and TNF-α production was assayed with ELISA kits (eBioscience). IL-12 production was determined following a standard ELISA protocol using the following pair of antibodies: 2 μg/mL anti–IL-12 capture antibody (BioLegend) with 1 μg/mL biotin anti–IL-12 antibody (BioLegend). The peritoneal macrophage viability was assessed with the LIVE/DEAD Viability/Cytotoxicity kit (Molecular Probes, Invitrogen) following the manufacturer's recommendations with minor modifications. Briefly, after the stimulation the cells were washed twice with prewarmed PBS (37°C), and then incubated with 1 μM Calcein-am and 4 μM Ehidium homodimer-1 in PBS for 30 minutes in a 37°C cell culture incubator. The cells were directly observed under a fluorescence microscope while still in staining solution. Images from the red and the green channel were obtained and overlaid with Adobe Photoshop (Adobe, San Jose, CA). For the in vitro neutrophil apoptosis assay, total BM cells were cultured in RPMI 1640 (Gibco, Invitrogen) complete medium alone or with G-CSF or GM-CSF (both from PeproTech, Rocky Hill, NJ) at 10 ng/mL for different periods of time and assayed for apoptosis and death as described (see “Flow cytometry”).
Results
Generation of Mcl-1 conditional KO mice
To generate mice specifically lacking Mcl-1 in macrophages and neutrophils, we constructed a targeting construct with exon 1 of Mcl-1 encoding amino acid 1-179 flanked by 2 loxP sites (Figure 1A). A neomycin-resistant gene cassette was flanked by 2 FRT sites (Figure 1A). We transfected the targeting construct into TC1 ES cells and selected homologously recombined clones by PCR. We further confirmed the correct targeting by Southern blot analysis (Figure 1B). Chimeric founder mice were generated by microinjecting targeted ES cell clones into C57BL/6 blastocysts. Male chimeric mice were bred with FLPeR female mice to delete the neomycin cassette in vivo (Figure 1C). The Mcl-1 floxed mice (Mcl-1f/f) were bred with LysMcre mice to induce Mcl-1 deletion in macrophages and neutrophils (herein referred to as Mcl-1−/− mice). LysMcre has been shown to induce efficient gene deletion in these cells.26 To examine the efficiency of Mcl-1 deletion in macrophages, we cultured BM-derived macrophages from Mcl-1−/− and control mice. BM-derived macrophages from Mcl-1−/− mice exhibited normal fluorescence-activated cell sorting (FACS) profiles of Mac-1 and F4/80 staining (data not shown). We analyzed Mcl-1 protein expression in these macrophages by Western blot analysis. Mcl-1 expression in macrophages from Mcl-1−/− mice was reduced by more than 95% when compared with that in control macrophages (Figure 1D), indicating that Mcl-1 was deleted with high efficiency.
Generation of mice conditionally lacking Mcl-1 expression in neutrophils and macrophages. (A) Schematic of targeting strategy for Mcl-1 allele. E1, E2, and E3 are exons 1-3 of Mcl-1. S indicates SpeI site. The size of alleles digested by SpeI and the probe are shown. (B) Southern blot analysis of gDNA from homologously recombined ES clones and parental ES cells (wild type). gDNA was digested with SpeI and hybridized with probes shown in panel A. (C) Southern blot analysis of gDNA from the tails of Mcl-1f/+ mice after crossing with FLPeR mice. gDNA from wild-type mice (+/+) served as a control for the wild-type allele. (D) Expression of Mcl-1 in BM-derived macrophages. Macrophages derived from 1-week culture of BM from Mcl-1−/− and control mice in the presence of L929-conditioned medium were lysed for Western blot analysis. Erk expression serves as a loading control.
Generation of mice conditionally lacking Mcl-1 expression in neutrophils and macrophages. (A) Schematic of targeting strategy for Mcl-1 allele. E1, E2, and E3 are exons 1-3 of Mcl-1. S indicates SpeI site. The size of alleles digested by SpeI and the probe are shown. (B) Southern blot analysis of gDNA from homologously recombined ES clones and parental ES cells (wild type). gDNA was digested with SpeI and hybridized with probes shown in panel A. (C) Southern blot analysis of gDNA from the tails of Mcl-1f/+ mice after crossing with FLPeR mice. gDNA from wild-type mice (+/+) served as a control for the wild-type allele. (D) Expression of Mcl-1 in BM-derived macrophages. Macrophages derived from 1-week culture of BM from Mcl-1−/− and control mice in the presence of L929-conditioned medium were lysed for Western blot analysis. Erk expression serves as a loading control.
Decreased neutrophil numbers in Mcl-1−/− mice
We examined the development of neutrophils and monocytes/macrophages in Mcl-1−/− mice. FACS analysis demonstrated that Mac-1+Gr-1+ granulocytes in the peripheral blood and spleen of Mcl-1−/− mice were reduced by 80% and 86%, respectively, when compared with control mice (Figure 2A). The reduction of Mac-1+Gr-1+ granulocytes was also observed in the BM of Mcl-1−/− mice (Figure 2A), indicating a general lack of granulocytes. In contrast, the percentages of Mac-1+Gr-1−/low population in the blood, spleen, and BM of Mcl-1−/− mice were relatively increased when compared with controls (Figure 2A). This Mac-1+Gr-1−/low population consisted mostly of eosinophils as determined in cell differential counts and FACS analysis (data not shown).
Impaired neutrophil development in Mcl-1–deficient mice. (A) FACS profiles of blood, spleen, and BM of Mcl-1−/− and control mice. Single-cell suspensions were stained with FITC–anti–Mac-1 and APC–anti–Gr-1 monoclonal antibodies. Shown are cells gated on granulocytes based on their forward and side scatter. Numbers indicate the percentage of cells in the gated regions. Data are representative of 3 independent experiments from a group of 9 mice. (B) Percents of lymphocytes, PMN cells, and monocytes in the blood of Mcl-1−/− and control mice. Blood smears were stained with Hema 3 and counted under light microscopy; n = 6 for each group. Data are mean + standard deviation. (C) Expression of Mcl-1 in purified BM neutrophils from Mcl-1−/− and control mice as determined by Western blot. Erk2 serves as a loading control. Data are representative of 2 experiments.
Impaired neutrophil development in Mcl-1–deficient mice. (A) FACS profiles of blood, spleen, and BM of Mcl-1−/− and control mice. Single-cell suspensions were stained with FITC–anti–Mac-1 and APC–anti–Gr-1 monoclonal antibodies. Shown are cells gated on granulocytes based on their forward and side scatter. Numbers indicate the percentage of cells in the gated regions. Data are representative of 3 independent experiments from a group of 9 mice. (B) Percents of lymphocytes, PMN cells, and monocytes in the blood of Mcl-1−/− and control mice. Blood smears were stained with Hema 3 and counted under light microscopy; n = 6 for each group. Data are mean + standard deviation. (C) Expression of Mcl-1 in purified BM neutrophils from Mcl-1−/− and control mice as determined by Western blot. Erk2 serves as a loading control. Data are representative of 2 experiments.
To further examine neutrophils and monocytes in the blood of Mcl-1−/− mice, we performed cell differential counts of peripheral blood smears. Corresponding to FACS analysis, results from cell differential counts demonstrated that the percent of PMN cells in Mcl-1−/− mice was reduced by 80%, whereas the percentages of lymphocytes and monocytes in these mice were comparable to those in control mice (Figure 2B). Taken together, these results demonstrate that the numbers of neutrophils but not monocytes were decreased in Mcl-1−/− mice.
Although the neutrophils in Mcl-1−/− mice were severely reduced in numbers, there were still some Mac-1+Gr-1+ PMN cells in every organ examined. These cells could either have escaped Cre-mediated deletion of Mcl-1 or developed in a Mcl-1–independent pathway. To resolve this issue, we purified Mac-1+Gr-1+ cells from the BM of Mcl-1−/− and control mice and examined the Mcl-1 protein in cell lysates. All the surviving neutrophils in Mcl-1−/− mice had an amount of Mcl-1 similar to that of controls (Figure 2C). These data suggest that Mcl-1 is essential for neutrophil survival. Neutrophils that delete Mcl-1 undergo apoptosis rapidly, but neutrophils that escape deletion survive.
Impaired neutrophil but not macrophage influx in peritonitis
Although monocyte development in Mcl-1−/− mice was apparently normal and macrophages can be derived from the BM of these mutant mice, it was not clear whether macrophage responding to inflammation requires Mcl-1. To address this, we examined leukocyte recruitment in thioglycollate-induced peritonitis in Mcl-1−/− and control mice. Peritoneal cells from control mice 1 day after thioglycollate injection contained a large fraction of Mac-1+Gr-1+ neutrophils (Figure 3A). Consistent with the decreased neutrophil numbers at steady state in Mcl-1−/− mice, peritoneal cells from Mcl-1−/− mice injected with thioglycollate had a 80% reduction in the number of Mac-1+Gr-1+ neutrophils compared with that of control mice (Figure 3A,C). Furthermore, in contrast to control peritoneal cells, most peritoneal cells from Mcl-1−/− mice did not exhibit PMN morphology (Figure 3A). The few surviving peritoneal neutrophils in Mcl-1−/− mice did not show any evidence of Mcl-1 deletion as demonstrated by Western blot of purified Mac-1+Gr-1+ peritoneal exudate cells (Figure 3D). These findings further confirm that mature neutrophils cannot survive without Mcl-1.
Impaired neutrophil but not macrophage influx in peritonitis. (A) FACS analysis of day 1 peritoneal cells. Peritoneal cells were stained with FITC–anti–Mac-1 and APC–anti–Gr-1. Numbers indicate the percentages of cells in the gated regions. Also shown are cytospin images of peritoneal cells from Mcl-1−/− and control mice. (B) FACS analysis and cytospin images of day 4 peritoneal cells. (C) Numbers of total peritoneal cells, neutrophils, and macrophages at day 1 and day 4 after thioglycollate injection. The numbers of Mac-1+Gr-1+ and Mac-1+Gr-1− cells were calculated by multiplying the percents of cells with the total numbers (n = 6). Data shown are mean + standard deviation. (D) Expression of Mcl-1 in purified peritoneal neutrophils from Mcl-1−/− and control mice as determined by Western blot. Erk2 serves as a loading control. Data are representative of 2 experiments.
Impaired neutrophil but not macrophage influx in peritonitis. (A) FACS analysis of day 1 peritoneal cells. Peritoneal cells were stained with FITC–anti–Mac-1 and APC–anti–Gr-1. Numbers indicate the percentages of cells in the gated regions. Also shown are cytospin images of peritoneal cells from Mcl-1−/− and control mice. (B) FACS analysis and cytospin images of day 4 peritoneal cells. (C) Numbers of total peritoneal cells, neutrophils, and macrophages at day 1 and day 4 after thioglycollate injection. The numbers of Mac-1+Gr-1+ and Mac-1+Gr-1− cells were calculated by multiplying the percents of cells with the total numbers (n = 6). Data shown are mean + standard deviation. (D) Expression of Mcl-1 in purified peritoneal neutrophils from Mcl-1−/− and control mice as determined by Western blot. Erk2 serves as a loading control. Data are representative of 2 experiments.
We then examined the peritoneal cells from Mcl-1−/− and control mice 4 days after thioglycollate injection. At this time point, a majority of the induced peritoneal cells were Mac-1+Gr-1− macrophages, whereas a small fraction of these cells were Mac-1+Gr-1+ neutrophils in control mice (Figure 3B-C). Interestingly, the percent of peritoneal macrophages in Mcl-1−/− mice was comparable to that in control mice (Figure 3B). In addition, the percent of neutrophils in Mcl-1−/− mice was not significantly different from that in control mice (Figure 3B). These results demonstrate that macrophage influx to inflammation does not depend on Mcl-1.
Enhanced apoptosis in Mcl-1−/− neutrophils
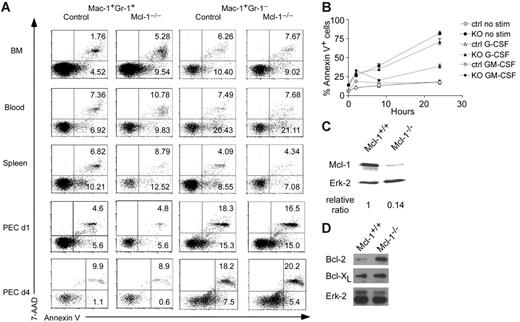
Given that Mcl-1 may have an essential role in neutrophil survival, we examined the apoptosis of Mac-1+Gr-1+ granulocytes in Mcl-1−/− mice by staining with annexin V and 7-AAD. As shown in Figure 4A, Mac-1+Gr-1+ granulocytes in the BM of Mcl-1−/− mice exhibited 2- to 3-fold higher apoptotic rates than those in control mice as judged by annexin V+ and annexin V+7-AAD+ staining. The apoptosis of Mac-1+Gr-1+ granulocytes in day 1 and day 4 peritoneal cells was similar to that of control cells, in agreement with our data showing the lack of Mcl-1 deletion in these cells (Figure 4A). As expected, Mac-1+Gr-1− myeloid cells in BM, blood, spleen, and day 1 and day 4 peritoneal cells had a similar apoptotic rate to that of control cells (Figure 4A). These results demonstrate that Mcl-1 is critical for the survival of neutrophils but not macrophages.
Enhanced apoptosis in Mcl-1−/− neutrophils. (A) Detection of apoptosis in granulocytes and macrophages of Mcl-1−/− mice. Apoptosis was detected by double staining of 7-AAD and annexin V. Mac-1+Gr-1+ and Mac-1+Gr-1− cells from BM, blood, spleen, and peritoneal cavity day 1 and day 4 after thioglycollate injection (PEC d1, d4) were gated for 7-AAD and annexin V staining. Numbers indicate percents in each region. The results are representative of 6 independent experiments from 12 mice. (B) Apoptosis rates of Mcl-1–deficient BM neutrophils with or without growth factor stimulation. BM cells from Mcl-1–deficient (KO) and control mice were stimulated with G-CSF or GM-CSF for different periods of time in triplicates. Mac-1+Gr-1+ cells were examined for apoptosis by flow cytometry. The graph shows the mean and standard deviation of the percentage of apoptotic and dead cells as defined by annexin V+. The results are representative of 3 independent experiments. (C) Mcl-1 expression in purified BM neutrophils treated with 10 ng/mL GM-CSF for 24 hours. Relative protein expression was normalized to Erk-2 expression levels. (D) Western blot analysis of Bcl-2 and Bcl-xL expression in Mcl-1−/− macrophages. BM macrophages were lysed and blotted with anti–Bcl-2 and Bcl-xL antibodies as shown in Figure 1D.
Enhanced apoptosis in Mcl-1−/− neutrophils. (A) Detection of apoptosis in granulocytes and macrophages of Mcl-1−/− mice. Apoptosis was detected by double staining of 7-AAD and annexin V. Mac-1+Gr-1+ and Mac-1+Gr-1− cells from BM, blood, spleen, and peritoneal cavity day 1 and day 4 after thioglycollate injection (PEC d1, d4) were gated for 7-AAD and annexin V staining. Numbers indicate percents in each region. The results are representative of 6 independent experiments from 12 mice. (B) Apoptosis rates of Mcl-1–deficient BM neutrophils with or without growth factor stimulation. BM cells from Mcl-1–deficient (KO) and control mice were stimulated with G-CSF or GM-CSF for different periods of time in triplicates. Mac-1+Gr-1+ cells were examined for apoptosis by flow cytometry. The graph shows the mean and standard deviation of the percentage of apoptotic and dead cells as defined by annexin V+. The results are representative of 3 independent experiments. (C) Mcl-1 expression in purified BM neutrophils treated with 10 ng/mL GM-CSF for 24 hours. Relative protein expression was normalized to Erk-2 expression levels. (D) Western blot analysis of Bcl-2 and Bcl-xL expression in Mcl-1−/− macrophages. BM macrophages were lysed and blotted with anti–Bcl-2 and Bcl-xL antibodies as shown in Figure 1D.
Next, we assessed the kinetics of neutrophil death in Mcl-1−/− mice in vitro. We incubated neutrophils for different periods of time and followed their spontaneous rate of apoptosis. We also incubated them with the growth factors G-CSF or GM-CSF because they are known to enhance neutrophil survival. Not surprisingly, the proportions of apoptotic and dead cells as defined by annexin V+ staining were increased in Mcl-1−/− cells at all time points (Figure 4B). The addition of G-CSF to the culture slightly decreased the apoptotic rate of control cells at 24 hours, but overall did not rescue the death of Mcl-1−/− neutrophils. In sharp contrast, GM-CSF treatment almost completely rescued the effects of Mcl-1 deletion and reduced the percentage of dead and apoptotic cells in Mcl-1−/− neutrophils close to these of the controls (Figure 4B). These results suggest that GM-CSF treatment prevents neutrophil apoptosis by up-regulation of other prosurvival molecules. Alternatively, GM-CSF may enhance the stability of Mcl-1 protein as shown previously.19
To test whether a non-Mcl-1–mediated survival pathway exists after GM-CSF treatment, we cultured BM cells from Mcl-1−/− mice and controls with GM-CSF for 24 hours and purified Gr-1+Mac-1+ cells to measure the protein levels of Mcl-1. The amount of Mcl-1 in granulocytes of Mcl-1–deficient mice was dramatically reduced compared with controls, suggesting that Mcl-1 was efficiently deleted, but the cells were still surviving well following GM-CSF treatment (Figure 4C). These findings strongly support the hypothesis that other GM-CSF–dependent prosurvival molecules in neutrophils can maintain their viability in the absence of Mcl-1.
Although previous data have implicated Mcl-1 in macrophage survival,23,24 the apparently normal survival of macrophages in Mcl-1−/− mice suggests that other Bcl-2 family members may compensate for the loss of Mcl-1 expression in these cells. To test this, we examined Bcl-2 and Bcl-xL expression in macrophages from Mcl-1−/− mice. Notably, the expression of Bcl-2 was dramatically up-regulated, whereas the expression of Bcl-xL was slightly enhanced in Mcl-1−/− macrophages (Figure 4D). These results suggest that the enhanced expression of these antiapoptotic proteins may compensate for Mcl-1 deficiency.
Survival and cytokine production of Mcl-1–deficient macrophages
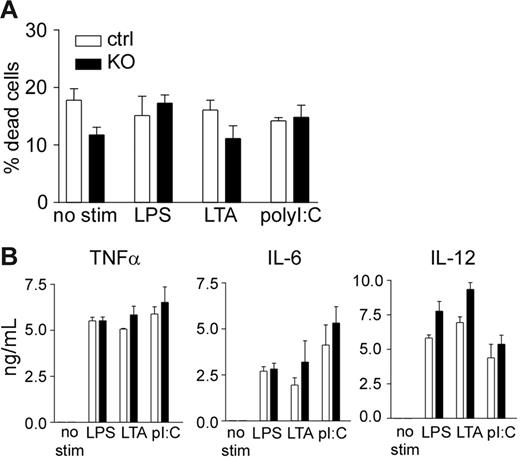
A recent study suggests that Mcl-1 may be a key regulator of macrophage survival on activation by bacterial pathogens.28 To test whether Mcl-1–deficient macrophages have a survival defect on activation, we stimulated peritoneal macrophages with different pathogen-associated molecular patterns (PAMPs). Stimulation of Mcl-1–deficient macrophages did not result in enhanced apoptosis when compared with control macrophages (Figure 5A). Furthermore, activation of Mcl-1–deficient and control macrophages resulted in similar levels of inflammatory cytokine production (Figure 5B). These results demonstrate that Mcl-1–deficient macrophages did not exhibit survival defect after toll-like receptor (TLR) stimulation.
Normal macrophage survival and cytokine production on TLR stimulation. (A) Apoptosis rate of Mcl-1–deficient macrophages with or without PAMP stimulation. Peritoneal macrophages from Mcl-1–deficient (KO) and control mice (ctrl) were stimulated with the indicated PAMPs for 16 hours and examined for apoptosis using the LIVE/DEAD Viability/Cytotoxicity kit. (B) Inflammatory cytokine production by Mcl-1–deficient macrophages after PAMP stimulation. Peritoneal macrophages from Mcl-1–deficient (▪)and control (□)mice were stimulated with the indicated PAMPs overnight and measured for cytokine productions by ELISA. Shown are mean and SD of triplicate determinations. Data are representative of 3 experiments.
Normal macrophage survival and cytokine production on TLR stimulation. (A) Apoptosis rate of Mcl-1–deficient macrophages with or without PAMP stimulation. Peritoneal macrophages from Mcl-1–deficient (KO) and control mice (ctrl) were stimulated with the indicated PAMPs for 16 hours and examined for apoptosis using the LIVE/DEAD Viability/Cytotoxicity kit. (B) Inflammatory cytokine production by Mcl-1–deficient macrophages after PAMP stimulation. Peritoneal macrophages from Mcl-1–deficient (▪)and control (□)mice were stimulated with the indicated PAMPs overnight and measured for cytokine productions by ELISA. Shown are mean and SD of triplicate determinations. Data are representative of 3 experiments.
Discussion
Although numerous studies have implicated Mcl-1 as a key antiapoptotic regulator in the survival of neutrophils and macrophages,15-24 it remained undetermined whether this is indeed the case in vivo. Furthermore, it is not clear whether Mcl-1 may regulate the spontaneous apoptosis or the development of mature neutrophils. Given that conventional Mcl-1 knockout mice die at early embryonic stages,29 we have generated mice with floxed Mcl-1 alleles and induced Mcl-1 gene deletion in neutrophils and macrophages by crossing them to LysMcre mice. LysM-driven Cre expression has been shown to induce efficient gene deletion in both neutrophils and macrophages with a higher deletion efficiency in neutrophils.26 As expected, we have observed efficient deletion of Mcl-1 in macrophages. In addition, the severely impaired neutrophil survival in Mcl-1 conditional knockout mice suggests that Mcl-1 is also efficiently deleted in this cell lineage.
Our results suggest that Mcl-1 is essential for the survival of mature neutrophils. A prominent feature of the neutrophil is its very short half-life in the circulation (6-18 hours). Correlated with this short half-life, proapoptotic proteins of the Bcl-2 family such as Bax, Bad, Bak, Bid, and Bik are constitutively expressed in neutrophils.12,13 However, only 2 members of the antiapoptotic Bcl-2 family, Mcl-1 and A1(Bfl-1), have been detected in neutrophils so far.13 Consistent with this expression pattern, mice lacking Bim had a 2-fold increase in granulocytes.30 Absence of Bim protected neutrophils from spontaneous apoptosis.31 In contrast, neutrophils from mice lacking the A1 gene had enhanced spontaneous apoptosis in vitro, although the development of these cells appeared to be normal.14 Our results demonstrated that mature neutrophils were reduced by 80% to 90% in the blood, spleen, and peritoneal exudates of Mcl-1–deficient mice. The lack of mature neutrophils in the periphery was most likely due to the decreased survival of these cells after the excision of Mcl-1. An alternative explanation is that there is a block in the development of mature neutrophils. These 2 possibilities are difficult to distinguish because cells that delete Mcl-1 die rapidly after that as suggested by the enhanced apoptosis among these cells. Interestingly, although the number of Gr-1+Mac-1+ neutrophils in day 1 peritoneal exudates was much reduced, these cells did not exhibit an obviously increased apoptosis. Furthermore, the number of Gr-1+Mac-1+ neutrophils in day 4 peritoneal exudates was similar between Mcl-1–deficient and control mice. We demonstrated that these residual neutrophils are derived from cells that escaped deletion of Mcl-1 gene. Interestingly, GM-CSF was able to rescue the dramatically increased death of Mcl-1–deficient neutrophils. Although the mechanism is unclear at present, the most likely explanation is that GM-CSF is up-regulating another antiapoptotic molecule such as A1 that is able to replace Mcl-1 in promoting cell viability. This hypothesis is supported by the fact that GM-CSF treatment renders Mcl-1 dispensable/redundant and Mcl-1 is efficiently deleted without loss of cell viability. Our results are consistent with the notion that Mcl-1 confers a critical prosurvival protection in the neutrophils and the function is nonredundant with A1 or other possible antiapoptotic genes. Whether Mcl-1 also plays a role in the development of immature neutrophils remains to be determined. Our data also point to a very interesting difference between G-CSF– and GM-CSF–mediated cell signaling and survival. In contrast with GM-CSF treatment, which almost completely rescued the cell death in Mcl-1−/− granulocytes, G-CSF was unable to do so. These data suggest that the only prosurvival target of G-CSF signaling in neutrophils is Mcl-1, whereas this is not the case for GM-CSF signaling.
Our results indicate that Mcl-1 is dispensable for the development and survival of macrophages. Previous work demonstrated that inhibition of the PI3K/Akt pathway in human monocyte-differentiated macrophages resulted in decreased expression of Mcl-1 and cell apoptosis.23 Inhibition of Mcl-1 expression by antisense oligonucleotides also resulted in macrophage apoptosis.23 Furthermore, STAT3 activation was shown to be essential for maintaining cell survival and the expression of Mcl-1 in human macrophages.24 However, Mcl-1–deficient mice had normal numbers of Mac-1+Gr-1− monocytes/macrophages in the blood, spleen, BM, and peritoneal exudates, indicating that Mcl-1 is not required for their development. Furthermore, we found that activated macrophages from Mcl-1–deficient mice exhibited similar levels of survival to those of control cells. The differential requirement for Mcl-1 in human and mouse macrophage survival may reflect a species difference. Alternatively, antisense oligonucleotide treatment of human macrophages may suppress the expression of other prosurvival genes. Nevertheless, the normal macrophage development and survival in Mcl-1–deficient mice is likely due to a compensatory mechanism provided by other antiapoptotic molecules Bcl-2 and Bcl-x through up-regulation of their expression. Taken together, our results have demonstrated that Mcl-1 plays a critical role in neutrophil survival but is dispensable for macrophage development and survival.
Authorship
Contribution: I.D. performed most of the experiments described inthe figures and analyzed the data; A.S.J. performed experimentsdescribed in Figure 4; and Y.-W.H. designed the experiments, analyzed the data, and wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: You-Wen He, Box 3010, Department of Immunology, Duke University Medical Center, Durham, NC 27710; e-mail: he000004@mc.duke.edu.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked advertisement in accordance with 18 USC section 1734.
Acknowledgments
This work was supported by grant RSG0125201 (Y.-W.H.) from the American Cancer Society and grant CA92123 (Y.-W.H.) from the National Institutes of Health. We thank Heather Hartig for critical review of this manuscript and Chia-Lin Hsu for help with multicolor flow cytometry.




This feature is available to Subscribers Only
Sign In or Create an Account Close Modal