Abstract
Transfusion-related acute lung injury (TRALI) is the leading cause of transfusion-related mortality. Antibodies to HNA-3a are commonly implicated in TRALI. We hypothesized that HNA-3a antibodies prime neutrophils (PMNs) and cause PMN-mediated cytotoxicity through a two-event pathogenesis. Isolated HNA-3a+ or HNA-3a− PMNs were incubated with plasma containing HNA-3a antibodies implicated in TRALI, and their ability to prime the oxidase was measured. Human pulmonary microvascular endothelial cells (HMVECs) were activated with endotoxin or buffer, HNA-3a+ or HNA-3a− PMNs were added, and the coculture was incubated with plasma ± antibodies to HNA-3a. PMN-mediated damage was measured by counting viable HMVECs/mm2. Plasma containing HNA-3a antibodies primed the fMLP-activated respiratory burst of HNA-3a+, but not HNA-3a−, PMNs and elicited PMN-mediated damage of LPS-activated HMVECs when HNA-3a+, but not HNA-3a−, PMNs were used. Thus, antibodies to HNA-3a primed PMNs and caused PMN-mediated HMVEC cytotoxicity in a two-event model identical to biologic response modifiers implicated in TRALI.
Introduction
Transfusion-related acute lung injury (TRALI) is the most common cause of transfusion-related death in the United States.1 The pathogenesis of TRALI includes the infusion of specific antibodies from the donor directed against antigens (HLA class I or class II, or granulocyte specific) present on the recipient's leukocytes resulting in complement activation, neutrophil (PMN) sequestration, and activation in the lung, culminating in endothelial damage, capillary leak, and acute lung injury (ALI).2-5 An ex vivo lung model confirmed that antibodies against HNA-3a, together with HNA-3a+ PMNs, and plasma caused ALI.6,7 The HNA-3a locus is present in 95% of humans, and antibodies directed against this antigen are one of the most commonly implicated immunoglobulins in TRALI, including 3 reported fatalities and a “look-back” study of 1 donor with HNA-3a antibodies that demonstrated that a number of patients developed TRALI that were not reported; however, TRALI occurred in a minority of patients transfused.8,9
A two-event pathogenesis for TRALI has also been proposed such that the first event, related to the patient's underlying clinical condition, elicits activation of the pulmonary endothelium causing sequestration of PMNs in the microvasculature.10 The second event is the infusion of specific antibodies directed against antigens on the granulocyte or biologic response modifiers (BRMs), which activate the microbicidal arsenal of the sequestered PMNs resulting in endothelial damage, capillary leak, and ALI.10 This model has been confirmed in a rat lung model and in vitro using human pulmonary microvascular endothelial cells (HMVECs) as targets.11-13 We hypothesize that antibodies directed against HNA-3a prime PMNs and cause PMN-mediated cytotoxicity.
Patients, materials, and methods
All chemicals were purchased from Sigma Chemical (St Louis, MO). All solutions were made from sterile water or sterile 0.9% saline for intravenous administration (United States Pharmacopeia [USP], Baxter Healthcare, Deerfield, NY) as reported.10 Antibodies to CD18 or to intercellular adhesion molecule-1 (ICAM-1) were purchased from PharMingen (Torrey Pines, CA) and Ancell (Bayport, MN), respectively. Whole blood for plasma or PMN isolation was obtained from 4 disparate donors known to have antibodies to HNA-3a, as documented at BloodSource, The American Red Cross North Central Blood Services, and the Blood Center of Southeastern Wisconsin, or from healthy subjects after obtaining informed consent under protocols approved by the internal review boards at the pertinent medical institution.
PMN priming assays
PMNs were isolated from whole blood drawn from healthy donors under a protocol approved by the Colorado Multiple Institutional Review Board at the University of Colorado School of Medicine.10 PMNs were incubated with buffer or 1% to 10% plasma from donors with antibodies to HNA-3a or fresh plasma from healthy individuals for 5 minutes at 37°C and activated with 1 μM fMLP, and the maximal rate of superoxide anion production was measured as previously described.10 Lipid extractions were completed as reported, and the measurement of soluble CD40 ligand (sCD40L) was completed via commercial enzyme-linked immunosorbent assay (ELISA; R&D Systems, Minneapolis, MN).14
A two-event in vitro model of PMN-mediated pulmonary endothelial damage
This two-event model of PMN-mediated cytotoxicity was performed as described.13 HMVECs were incubated with 2 μg/mL endotoxin (LPS) or buffer for 6 hours at 37°C in 5% CO2. PMNs (1 × 106) were added, allowed to settle (30 minutes), and then either PMN adherence was measured, as reported, or the coculture was incubated with anti–HNA-3a plasma or control plasma (fresh plasma [FP]) for 30 minutes, as described.13 PMN-mediated cytotoxicity was determined by 3 separate observers who counted viable, adherent HMVECs/mm2 to exclude bias.13
Statistics
All data are expressed as the mean ± the standard error of the mean, and statistical differences were determined by a paired analysis of variance (ANOVA) followed by a Bonferroni post hoc test for multiple comparisons.
Results and discussion
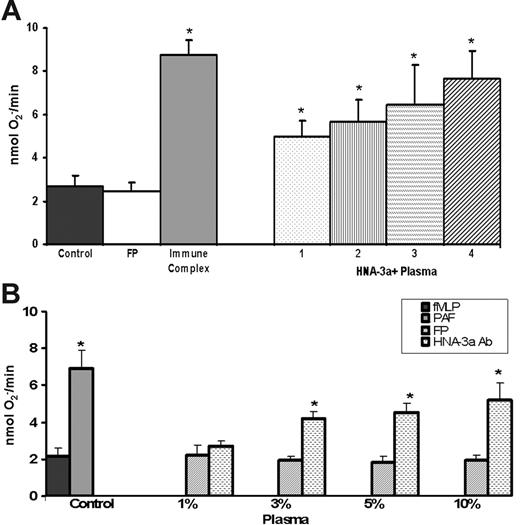
Incubation of HNA-3a+ PMNs with plasma [1%-10%]FINAL containing antibodies (brackets denote concentration) against HNA-3a (anti–HNA-3a plasma) demonstrated significant priming of the fMLP-activated oxidase at [3%-10%]FINAL compared with FP-treated controls (Figure 1). This priming activity was not significantly inhibited (10.4% ± 5.6%-19.2% ± 8.9%, n = 8, P = .32) by Fc blockade by PMN pretreatment (5 min at 37°C) with murine (Fab′)2 fragments against CD16, CD32, and CD64, respectively, whereas the priming activity of immune complexes was inhibited by 97.4% ± 8.3%. Anti–HNA-3a plasma did not cause activation of the PMN oxidase alone: FP, 0.2 ± 0.2; FP + HNA-3a antibodies (Abs), 0.1 ± 0.2 nmol O2−/min. Furthermore, anti–HNA-3a+ plasma did not prime the respiratory burst of HNA-3a− PMNs: FP/fMLP, 3.3 ± 0.4 nmol O2−/min; 10% anti–HNA-3a plasma/fMLP, 3.3 ± 0.4; platelet-activating factor (PAF) (PAF, positive control)/fMLP, 8.1 ± 0.7* (nmol O2−/min; *P < .05). To ensure that the activity was specific for HNA-3a antibodies and not due to biologic response modifiers (BRMs) implicated in TRALI, we (1) measured the amounts of sCD40L, and (2) extracted the lipophilic compounds and assayed priming activity.10,15 There were small amounts of sCD40L (< 10 ng/mL, the concentration required to prime PMNs) with more in the control plasma: FP, 7.2 ± 2.1 ng/mL versus anti–HNA-3a plasma 4.4 ± 1.1 ng/mL (n = 4).15 The lipid extracts also did not contain priming activity: FP, 1.9 ± 0.3 nmol O2−/min versus anti–HNA-3a plasma, 2.1 ± 0.4 nmol O2−/min (n = 4).
Plasma containing HNA-3a antibodies prime the fMLP-activated respiratory burst of HNA-3a+ PMNs. (A) The maximal rate of superoxide anion production as a function of treatment group includes 10% plasma (final concentration = percentage, vol/vol) from 4 donors (numbered 1-4) with antibodies to HNA-3a, all of whom were implicated in TRALI reactions compared with 10% fresh plasma (FP; isolated from 4 healthy donors: 2 males and 2 multiparous females). Immune complexes generated by heat-treating human serum for 30 minutes at 56°C were used as a positive control and could be inhibited (by 97.4% ± 8.3%) by preincubation with a mixture of (Fab′)2 fragments from murine monoclonal antibodies against human CD16, CD32, and CD64. The data are expressed as the mean ± the standard error of the mean of 8 separate experiments using disparate donors. (*P < .05 compared with FP.) (B) The maximal rate of superoxide anion concentration as a function of concentrations (final percentage, vol/vol) of plasma with antibodies to HNA-3a. HNA-3a plasma (3%-10%) significantly primed the fMLP-activated respiratory burst compared with FP- and buffer-treated controls; however, 1% did not (*P < .05 compared with FP- or buffer-treated controls). PAF is used as a positive control and this figure is representative of the data from all 4 plasma samples that contained IgG antibodies to HNA-3a (Abs).
Plasma containing HNA-3a antibodies prime the fMLP-activated respiratory burst of HNA-3a+ PMNs. (A) The maximal rate of superoxide anion production as a function of treatment group includes 10% plasma (final concentration = percentage, vol/vol) from 4 donors (numbered 1-4) with antibodies to HNA-3a, all of whom were implicated in TRALI reactions compared with 10% fresh plasma (FP; isolated from 4 healthy donors: 2 males and 2 multiparous females). Immune complexes generated by heat-treating human serum for 30 minutes at 56°C were used as a positive control and could be inhibited (by 97.4% ± 8.3%) by preincubation with a mixture of (Fab′)2 fragments from murine monoclonal antibodies against human CD16, CD32, and CD64. The data are expressed as the mean ± the standard error of the mean of 8 separate experiments using disparate donors. (*P < .05 compared with FP.) (B) The maximal rate of superoxide anion concentration as a function of concentrations (final percentage, vol/vol) of plasma with antibodies to HNA-3a. HNA-3a plasma (3%-10%) significantly primed the fMLP-activated respiratory burst compared with FP- and buffer-treated controls; however, 1% did not (*P < .05 compared with FP- or buffer-treated controls). PAF is used as a positive control and this figure is representative of the data from all 4 plasma samples that contained IgG antibodies to HNA-3a (Abs).
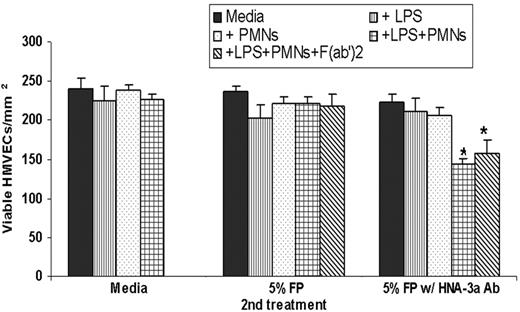
In the two-event model of PMN-mediated HMVEC cytotoxicity, HMVECs were stimulated with 2 μg/mL LPS, which resulted in widespread PMN adherence to HMVECs compared with media-treated controls (LPS, 24.2% ± 4.3% versus media control, 1.4% ± 0.2% of the total PMNs added). Moreover, antibody blockade of adhesion molecules including the PMN β2-integrin subunit CD18 or the HMVEC ICAM-1 significantly inhibited LPS-mediated PMN adhesion (CD18, 98% ± 3.2%; ICAM-1, 99% ± 2.2%; P < .05). Additionally, antibody neutralization of the chemokines released from LPS-activated HMVECs (IL-8, epithelial neutrophil activating protein-78 [ENA-78], and growth-related oncogene-α [GROα]), which are also required for PMN adhesion to LPS-treated HMVECs, significantly inhibited PMN adhesion (96% ± 3.1%, P < .05, n = 4) compared with LPS-treated HMVECs.13 The addition of 3% or 5% anti–HNA-3a plasma, but not FP, to LPS-activated HMVECs + HNA-3a+ PMNs caused cytotoxicity, which was unaffected by Fc blockade (Figure 2). Importantly, anti–HNA-3a plasma (1) did not evidence HMVEC damage to control or LPS-activated HMVECs and (2) did not elicit PMN-mediated HMVEC damage to control HMVECs (Figure 2). Anti–HNA-3a plasma also did not cause PMN-mediated damage when HNA-3a− PMNs were used: LPS/PMNs/FP, 256 ± 10 viable HMVECs/mm2; LPS/HNA-3a− PMNs/FP, 286 ± 5 viable HMVECs/mm2; LPS/PMNs/anti–HNA-3a plasma, 140 ± 15* viable HMVECs/mm2; LPS/HNA-3a− PMNs/anti–HNA-3a plasma, 280 ± 12* viable HMVECs/mm2 (*P = .005, n = 3).
Plasma containing HNA-3a antibodies can serve as the second event in a two-event, in vitro model of PMN-mediated pulmonary endothelial damage. The number of viable HMVECs/mm2 (mean ± SEM) as a function of the second event: media-treated controls (left), fresh plasma (FP)–treated HMVECs (middle), and plasma with HNA-3a antibodies (right). HMVECs incubated with media, LPS, PMNs, or LPS + PMNs as the first events were unaffected by media (control, left group) or FP (middle group). However, PMN-mediated damage occurred only with LPS activation, the addition of HNA-3a+ PMNs, and incubation with 5% plasma containing antibodies to HNA-3a. Importantly, Fc blockade, by preincubation with (Fab′)2 fragments from murine monoclonal antibodies against human CD16, CD32, and CD64 did not affect HNA-3a antibody-induced PMN-mediated damage of LPS-activated HMVECs nor did Fc blockade affect the number of viable HMVECs activated with LPS and incubated with PMNs + FP. *P < .05 compared with all other treatment groups.
Plasma containing HNA-3a antibodies can serve as the second event in a two-event, in vitro model of PMN-mediated pulmonary endothelial damage. The number of viable HMVECs/mm2 (mean ± SEM) as a function of the second event: media-treated controls (left), fresh plasma (FP)–treated HMVECs (middle), and plasma with HNA-3a antibodies (right). HMVECs incubated with media, LPS, PMNs, or LPS + PMNs as the first events were unaffected by media (control, left group) or FP (middle group). However, PMN-mediated damage occurred only with LPS activation, the addition of HNA-3a+ PMNs, and incubation with 5% plasma containing antibodies to HNA-3a. Importantly, Fc blockade, by preincubation with (Fab′)2 fragments from murine monoclonal antibodies against human CD16, CD32, and CD64 did not affect HNA-3a antibody-induced PMN-mediated damage of LPS-activated HMVECs nor did Fc blockade affect the number of viable HMVECs activated with LPS and incubated with PMNs + FP. *P < .05 compared with all other treatment groups.
As presented, 4 different anti–HNA-3a plasma samples from donors implicated in TRALI primed the fMLP-activated respiratory burst at concentrations of 3% to 10%. This priming activity could not be inhibited by Fc receptor blockade, indicating that this activity was not due to nonspecific Fc activation. Moreover, the anti–HNA-3a plasma, but not FP, caused PMN-mediated cytotoxicity of activated pulmonary HMVECs, and the anti–HNA-3a plasma alone had no affect on HMVEC viability. The plasma concentrations (3%-5%) employed appear physiologically relevant because a 70-kg human has a plasma volume of approximately 3 L, and if one infuses 100 mL of FFP containing HNA-3a antibodies, then the resultant concentration of the infused plasma would be 3% to 5%. These data support a two-event pathogenesis for TRALI because a first event, LPS-mediated HMVEC activation that resulted in PMN sequestration to the HMVEC surface, was required for injury with anti–HNA-3a plasma. These adherent, primed PMNs are functionally hyper-reactive because agents that normally do not elicit oxidase activation (HNA-3a antibodies, lipids, or sCD40L) may activate these adherent PMNs, resulting in HMVEC damage.13,15
Recently, infusion of monoclonal antibodies to HNA-2a with HNA-2a+ PMNs caused mild ALI in an ex vivo rat lung model, and high concentrations (4.5 mg/kg) of monoclonal antibodies directed against major histocompatibility complex (MHC) class I antigens in mice caused ALI with a 50% mortality.16,17 In the first report, ALI was caused by antibodies alone, dependent upon the PMN expression of HNA-2a, and these antibodies caused oxidative changes in PMNs.17 However, this ex vivo lung model required the perfusion of PMNs through tubing, which causes nonspecific PMN priming when used in cardiopulmonary bypass or dialysis that could lead to mechanical sequestration of PMNs, and together with the murine HNA-2a antibody may result in TRALI.18-23 The second report used high concentrations of antibodies because ALI was reproducible only at 4.5 mg/kg, which may have little physiologic relevance because of the unlikelihood of attaining such concentrations clinically.16 In addition, antigranulocyte antibodies did not cause ALI in this murine model, the mortality was inconsistent with human TRALI (50% vs 5%-10%), and ALI was not caused by the binding of antibody to PMNs but rather the binding of antibody to antigens on pulmonary endothelium that activate the PMN Fc receptors.17 To prove that antibodies did not affect PMNs, a flow-based assay of O2− production was used that would not be affected by agents that prime but do not activate the oxidase, including lipids, sCD40L, and HNA-3A antibodies.16
The presented data reinforce the hypothesis that patient factors are vital for the genesis of TRALI and explain why the majority of patients who received anti–HNA-3a antibodies did not develop TRALI.8,10,24 These data are disparate from the work of Sachs and Looney, for we employed human antibodies (polyclonal not monoclonal) from donors implicated in TRALI, demonstrated that two events were required for PMN-induced endothelial injury, and used physiologically relevant plasma concentrations.10,14,16,17 Further testing of clinically relevant animal models is required to determine the relevance of antibodies in TRALI.
Authorship
Contribution: C.C.S. contributed to the experimental design, conducted data analyses, and wrote the manuscript; B.R.C. contributed vital reagents, contributed to the experimental design, and assisted in writing/reviewing the manuscript; P.M.K. contributed vital reagents, contributed to the experimental design, conducted data analyses, and assisted in writing/reviewing the manuscript; S.Y.K. performed vital assays and assisted in writing/reviewing the manuscript; M.R.K. performed vital assays and assisted in writing/reviewing the manuscript; R.M.S. contributed vital reagents and assisted in writing/reviewing the manuscript; B.S. was instrumental in the isolation and testing of HNA-3a− PMNs both in vitro and in the HMVEC damage assay and performed all lipid extractions and assays to assess lipid activity; and D.R.A. contributed to the experimental design and assisted in writing/reviewing the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Christopher C. Silliman, Associate Medical Director, Bonfils Blood Center, 717 Yosemite St, Denver, CO 80230; e-mail: christopher.silliman@uchsc.edu.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This work was supported by Bonfils Blood Center grants; HL59355-07 from National Heart, Lung, and Blood Institute, National Institutes of Health (NHLBI, NIH); and P50 GM49222-12 from the National Institute of General Medical Sciences (NIGMS), NIH.