Abstract
The limited vessel-forming capacity of infused endothelial progenitor cells (EPCs) into patients with cardiovascular dysfunction may be related to a misunderstanding of the biologic potential of the cells. EPCs are generally identified by cell surface antigen expression or counting in a commercially available kit that identifies “endothelial cell colony-forming units” (CFU-ECs). However, the origin, proliferative potential, and differentiation capacity of CFU-ECs is controversial. In contrast, other EPCs with blood vessel-forming ability, termed endothelial colony-forming cells (ECFCs), have been isolated from human peripheral blood. We compared the function of CFU-ECs and ECFCs and determined that CFU-ECs are derived from the hematopoietic system using progenitor assays, and analysis of donor cells from polycythemia vera patients harboring a Janus kinase 2 V617F mutation in hematopoietic stem cell clones. Further, CFU-ECs possess myeloid progenitor cell activity, differentiate into phagocytic macrophages, and fail to form perfused vessels in vivo. In contrast, ECFCs are clonally distinct from CFU-ECs, display robust proliferative potential, and form perfused vessels in vivo. Thus, these studies establish that CFU-ECs are not EPCs and the role of these cells in angiogenesis must be re-examined prior to further clinical trials, whereas ECFCs may serve as a potential therapy for vascular regeneration.
Introduction
New blood vessel formation occurs via angiogenesis, vasculogenesis, or arteriogenesis.1,2 Since 1997, postnatal vasculogenesis has been purported to be an important mechanism for angiogenesis via marrow-derived circulating endothelial progenitor cells (EPCs).3 Based on this paradigm, EPCs have been extensively studied as biomarkers of cardiovascular disease and as a cell-based therapy for repair of damaged blood vessels.4–6 However, administration of EPCs or bone marrow-derived cell populations enriched for EPCs into subjects with cardiovascular disease has had limited efficacy, with regard to new vessel formation. Many investigators speculate that the paracrine effects of cultured EPCs are responsible for the modest effects in patients because there is no evidence of long-term engraftment of EPCs into newly formed vessels.7–9 These clinical observations are surprising given animal studies where EPC administration partially rescued cardiovascular dysfunction following ischemic hind limb or myocardial injury with some evidence for EPC contribution to new vessel growth.5,9
In most studies, EPCs are identified and enumerated via flow cytometric identification of cells expressing CD34, CD133, or the VEGF receptor 2 (KDR).3,10,11 Because these molecules are also expressed on hematopoietic stem/progenitor populations,12–15 the presence of hematopoietic contamination of EPCs should be expected. EPCs are also quantitated by counting in a commercially available kit that identifies “endothelial cell colony-forming units” (CFU-ECs). Identification of CFU-ECs from peripheral blood by use of colony-forming assays has formed the basis for use of these cells as a predictive biomarker of vascular disease and as a cell source for angiogenic therapies.4 The variability in defining the cells that give rise to CFU-ECs has contributed to the controversy in understanding the role these cells may play in neoangiogenesis.
Although less studied, we and others have identified endothelial colony-forming cells (ECFCs),16 which are also referred to as blood outgrowth endothelial cells (BOECs),17 from human peripheral blood. ECFCs are organized in a hierarchy of progenitor stages that vary in proliferative potential and can be identified in clonal plating conditions.16 Few studies have directly compared CFU-EC and ECFC functions and none have examined whether the cells are clonally related.
We tested the hypothesis that CFU-ECs are clonally derived from the hematopoietic system using cellular immunophenotyping, progenitor cell replating assays, an in vivo transplant assay for vessel-forming ability, and plating of blood cells from human patients harboring a known genetic mutation in a hematopoietic stem cell (HSC) clone. We provide data to demonstrate that CFU-ECs are descendents of HSCs that retain some myeloid progenitor activity with no ability to form secondary EC colonies or perfused vessels in vivo. Furthermore, CFU-ECs differentiate into phagocytic macrophages and not ECs. In contrast, we show that ECFCs are rare circulating EPCs with robust proliferative potential and vessel-forming activity in vivo. Thus, these studies establish that CFU-ECs are not EPCs but are hematopoietic-derived progeny committed to the myeloid lineage, whereas ECFCs are vessel-forming EPCs.
Patients, materials, and methods
Patients and adult peripheral blood samples
Blood samples were collected in a citrate phosphate dextrose (CPD) solution from 30 healthy volunteers (15 men, aged 19-50 years). Human umbilical cord blood from 10 healthy term newborns (5 male, 5 female) was collected in CPD. The Institutional Review Board at the Indiana University School of Medicine approved all protocols, and informed consent was obtained from adult donors in accordance with the Declaration of Helsinki.
Peripheral blood was collected from 11 patients with polycythemia vera (PV) who harbored the somatic mutation JAK2 1849G>T. The Institutional Review Board at the Baylor College of Medicine approved all protocols, and informed consent was obtained in accordance with the Declaration of Helsinki.
Preparation of mononuclear cells
Blood was diluted 1:1 with HBSS (Invitrogen, Grand Island, NY) and overlaid onto Ficoll-Paque PLUS (Amersham, Piscataway, NJ). Cells were centrifuged at 740g for 30 minutes. Buffy coat mononuclear cells (MNCs) were collected and washed 3 times in PBS (Invitrogen) with 2% FBS (Hyclone, Logan, UT).
Culture of ECFCs
ECFCs were cultured as previously described.16 ECFC colonies appeared between 5 and 22 days of culture and identified as well-circumscribed monolayers of cobblestone-appearing cells. Colonies were enumerated by visual inspection using an inverted microscope (Olympus, Lake Success, NY) under × 40 magnification. Cells were passaged as previously described.16
Culture of CFU-ECs
CFU-ECs were cultured using the EndoCult Liquid Medium Kit (StemCell Technologies, Vancouver, BC, Canada) per the manufacturer's protocol. MNCs were resuspended in complete EndoCult medium and seeded at 5 × 106 cells/well on fibronectin-coated tissue culture plates (BD Biosciences, Mountain View, CA). After 48 hours, wells were washed with media and nonadherent cells were collected. Nonadherent cells were plated in their existing media at 106 cells/well in 24-well fibronectin-coated tissue culture plates for 3 days. Some colonies were seeded into granulocyte-macrophage colony-forming unit (CFU-GM) methylcellulose assays as previously described.18
Immunophenotyping and uptake of acetylated LDL
ECFCs or CFU-ECs were fixed in 4% formaldehyde (PolySciences, Warrington, PA) for 15 minutes, blocked with Image-iT FX Signal Enhancer (Molecular Probes, Eugene, OR), and incubated overnight with 1 to 10 μg/mL of the primary or isotype control antibody or lectins as outlined, in PBS at 4°C. Cells were incubated with secondary antibody for 2 hours at room temperature. Nuclei were counterstained with 100 ng/mL Hoechst 33342 (Molecular Probes). We used primary murine monoclonal antibodies against human CD14 (BD PharMingen, San Diego, CA), human CD31 (Abcam, Cambridge, MA), human CD45 (BD PharMingen), human CD105 (BD PharMingen), human CD144 (BD PharMingen or Abcam), human CD146 (BD PharMingen), and human KDR (Zymed, San Francisco, CA; Sigma, St Louis, MO; or Abcam), and primary rabbit monoclonal antibodies against human CD115 (Chemicon, Temecula, CA) and human von Willebrand factor (VWF; BD PharMingen or US Biologicals, Swampscott, MA). To detect VWF expression, cells were permeabilized with 0.2% Triton X-100 (Sigma). We used murine IgG1κ (BD Biosciences) or rabbit isotype antibody control (Zymed) as isotype controls. The following secondary antibodies (all from Molecular Probes) were used to detect primary antibody binding: goat anti–mouse IgG (H+L) directly conjugated to Alexa Fluor 488, Alexa Fluor 546, or Alexa Fluor 647 and goat anti–rabbit IgG directly conjugated to Alexa Fluor 488. To assess lectin binding, we used biotinylated Ulex europaeus Agglutinin-I (UEA-I; Vector Laboratories, Burlingame, CA) and streptavidin-Alexa Fluor 488 (Molecular Probes).
ECFCs or CFU-ECs were incubated with 10 μg/mL DiI-Ac-LDL (Molecular Probes) in serum-free EBM-2 (Cambrex, East Rutherford, NJ) or basal EndoCult, respectively, as previously described.16
RT-PCR
Total RNA was isolated from CFU-ECs and ECFCs by Purelink Micro-to-Midi Total RNA Purification System (Invitrogen). Then 100 ng RNA was either used in one-step reverse transcription–polymerase chain reaction (RT-PCR), or converted to cDNA through oligo(dT) primers using the SuperScript III First-Strand Synthesis System for RT-PCR (Invitrogen). For end-point expression analysis, PCR amplification was generated using the following gene-specific primers: CD14 forward 5′-CGGCGGTGTCAACCTAGAG-3′, reverse 3′-GCCTACCAGTAGCTGAGCAG-5′ (PrimerBank ID 4557417a2); CD115 forward 5′-TCCAAAACACGGGGACCTATC-3′, reverse 3′-TCCTCGAACACGACCACCT-5′ (Primerbank ID 27262659a2); CD45 forward 5′-AATGAGAATGTGGAATGTGG-3′, reverse 3′-TTGCGTTAGTAAACTTGTGG-5′. PCR conditions consisted of 1 cycle at 94°C for 2 minutes, 30 cycles at 94°C for 30 seconds, 30 seconds at 55°C, and 45 seconds at 72°C. PCR products were imaged under UV fluorescence of ethidium bromide in 2% agarose gels.
Phagocytosis of microbes
Phagocytosis capabilities of ECFCs and CFU-ECs were assayed using the Vibrant Phagocytosis Assay kit (Molecular Probes) per the manufacturer's protocol. J774 murine monocytes (American Type Culture Collection, Manassas, VA) were used as a control.
Confocal and phase-contrast imaging and quantitation
Fluorescence was examined using a Zeiss 510 Meta confocal microscope (Zeiss, Thornwood, NY) with 2-photon capabilities using 10×/0.30 NA, 40×/0.80 NA, or 64×/0.90 NA Zeiss C-Apochromat water dipping objectives in HBSS with Hoechst dye. Images were acquired with the manufacturer's software and 2-dimensional projections of z-stacks were compiled. Phase-contrast images were collected using a Zeiss Axiovert 2 inverted microscope with a × 5 CP-ACHROMAT/0.12 NA objective. Images were acquired using a SPOT RT color camera (Diagnostic Instruments, Sterling Heights, MI) with the manufacturer's software. Images were assembled in Adobe Photoshop CS version 8.0 (Adobe Systems, San Jose, CA).
Quantitation of cell surface antigen expression, acetylated-LDL (Ac-LDL) uptake, and phagocytosis were performed using Integrated Morphometry Analysis in Metamorph (Molecular Devices, Sunnyvale, CA) or by visual inspection.
Nonspecific esterase activity
Nonspecific esterase activity was determined using an α-naphthyl acetate esterase kit (Sigma) per the manufacturer's protocol.
Transplantation of ECFCs or CFU-ECs
Cellularized gel implants were cast as previously described with minor modifications.19 Cultured ECFCs or CFU-ECs (2 × 106 cells/mL) were suspended in a solution comprising 1.5 mg/mL rat tail collagen I (BD Biosciences), 100 ng/mL human fibronectin (Chemicon), 1.5 mg/mL sodium bicarbonate (Sigma), 25 mM HEPES (Cambrex), 10% FBS, 30% complete EGM-2, in EBM-2, pH 7.4. Then 1 mL of the cell suspension was pipetted into the tissue culture plate, allowed to polymerize at 37°C for 30 minutes, and covered with complete EGM-2 for overnight incubation at 37°C, 5% CO2. Gels were bisected and implanted into the flank of anesthetized 12-week NOD/SCID mice. At 14 to 30 days, mice were killed and the grafts were excised and analyzed by immunohistochemistry.
Immunohistochemistry
For anti–human CD31 staining formalin-fixed, paraffin-embedded tissue sections were deparaffinized and immersed in a retrieval solution (Dako, Carpenteria, CA) for 20 minutes at 95°C to 99°C. Slides were incubated at room temperature with anti–human CD31 (clone JC70A, Dako) for 30 minutes followed by 10-minute incubations with LSAB2 link-biotin and streptavidin-HRP (Dako), then developed with DAB solution (Dako) for 5 min.
For anti–mouse CD31 staining, gels with surrounding murine tissue were fixed in zine (BD Biosciences), dehydrated with ethanol, and embedded in paraffin. Sections (4 μm) were incubated for 1 hour with anti–mouse (clone mec13.3, BD Pharmingen), donkey anti–rat biotin (1:100) for 30 minutes, then LSAB2-streptavidin-HRP (Dako) for 30 minutes. Slides were developed with DAB and analyzed by visual inspection under × 100 magnification.
Genotyping the JAK2 mutation
ECFC or CFU-EC colonies were isolated from the same donor and collected via cloning cylinders and washed twice with PBS. DNA was isolated as previously described.16
Assays for allele specific determination by a real-time PCR (PCR Applied Biosystems 7000 Sequence Detection System, Applied Biosystems, Foster City, CA) were designed to quantify the somatic mutation JAK2 1849G>T and the wild-type JAK2 allele. In allelic discrimination assays, PCR of the normal JAK2 1849G allele leads to the release of the VIC fluorescent label, whereas PCR of the mutated JAK2 1849T allele leads to the release of FAM fluorescent label. The assay mix contained the following probes and primers: (1) forward primer: 5′-AAGCTTTCTCACAAGCATTTGGTTT-3′; (2) reverse primer: 5′-AGAAAGGCATTAGAAAGCCTGTAGTT-3′; (3) VIC-labeled oligo: 5′-TCTCCACAGACACATAC-3′; (4) FAM-labeled oligo: 5′-TCCACAGAAACATAC-3′. The reaction was performed in 96-well optical PCR plates in a total volume of 25 μL following the manufacturer's protocol.
Results
Derivation of CFU-ECs and ECFCs
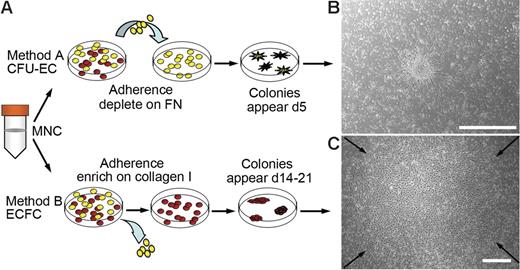
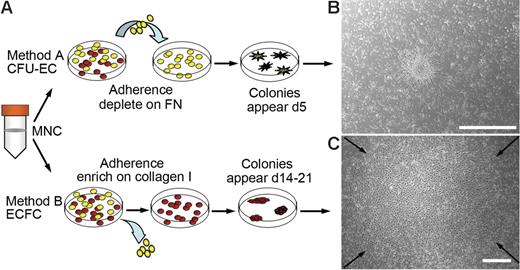
Diverse methods exist for isolating EPCs from peripheral blood. The 2 most widely used methods and their differences are outlined in Figure 1A. To compare EPC colonies generated by each method, MNCs were isolated from either adult peripheral or human umbilical cord blood.
Culture of EPCs from human peripheral blood. (A) Two methods for isolating and culturing EPCs from human peripheral blood. Yellow cells represent nonadherent cells and red cells represent adherent cells. FN indicates fibronectin. (B) Representative phase-contrast photomicrograph of a CFU-EC colony (day 5) cultured from adult peripheral blood MNCs by method A. Similar colonies were observed from 29 other adult peripheral and 10 cord blood donors. Scale bar represents 500 μm. (C) Representative phase-contrast photo-micrograph of an ECFC colony (day 19) cultured from adult peripheral blood MNCs by method B. Similar colonies were observed from 29 other adult peripheral and 10 cord blood donors. Arrows indicate colony boundary and scale bar represents 500 μm.
Culture of EPCs from human peripheral blood. (A) Two methods for isolating and culturing EPCs from human peripheral blood. Yellow cells represent nonadherent cells and red cells represent adherent cells. FN indicates fibronectin. (B) Representative phase-contrast photomicrograph of a CFU-EC colony (day 5) cultured from adult peripheral blood MNCs by method A. Similar colonies were observed from 29 other adult peripheral and 10 cord blood donors. Scale bar represents 500 μm. (C) Representative phase-contrast photo-micrograph of an ECFC colony (day 19) cultured from adult peripheral blood MNCs by method B. Similar colonies were observed from 29 other adult peripheral and 10 cord blood donors. Arrows indicate colony boundary and scale bar represents 500 μm.
For method A, MNCs were harvested and seeded onto fibronectin-coated tissue culture plates in media, which contained defined serum and growth factors to promote EPC outgrowth. After 48 hours, the nonadherent cells were collected and replated in the same culture media for an additional 3 days on fibronectin-coated tissue culture plates. At day 5 to 7, CFU-EC colonies were identified as elongated sprouting cells radiating from a central core of round cells. (Figure 1B). The number of cell colonies generated from 106 MNCs was 4.35 ± 2 (n = 13 adult donors).
For method B, MNCs were harvested and seeded onto collagen-coated tissue culture plates in endothelial growth media (EGM-2). In contrast to method A, nonadherent cells were discarded daily and fresh EGM-2 media was added to the cultures. As seen previously,16 on days 5 to 10 for cord blood or days 14 to 21 for adult peripheral blood samples, ECFC colonies were identified that originated from adherent cells (Figure 1C). The number of colonies generated from 106 MNCs was 0.017 ± 0.004 (n = 14 adult donors).
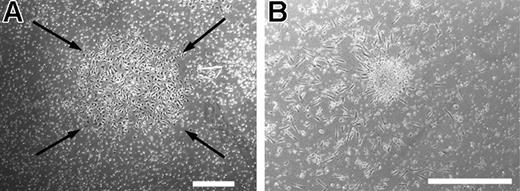
Use of method A or B produced 2 colony types though both colonies are considered to be EPC subtypes and potentially clonally related. ECFCs originate from an adherent cell and CFU-ECs from a nonadherent cell, suggesting clonally distinct cell populations. To test this hypothesis, we cultured the discarded adherent cells described in method A in EGM-2 media for 21 days. In 10 different experiments, we grew ECFC colonies (Figure 2A), which were identical to those produced using method B. Similarly, the discarded nonadherent cells described in method B gave rise to CFU-ECs if cultured in the growth media optimized for CFU-EC outgrowth as described in method A (Figure 2B). We never observed an ECFC colony emerging from a CFU-EC or vice versa (data not shown). Therefore, we hypothesized that CFU-ECs and ECFCs are distinct colony types that originate from different cell populations.
CFU-EC and ECFC colonies generated from discarded cells from methods A and B. (A) Representative photomicrograph phase-contrast of an ECFC colony, which arose when the adherent cells discarded from method A were cultured in EGM-2 medium (method B). Similar colonies were observed from 4 other adult peripheral and 5 cord blood donors. Arrows indicate colony boundary and scale bar represents 500 μm. (B) Representative phase-contrast photomicrograph of a CFU-EC colony, which arose when the nonadherent cells discarded from method B were cultured by method A. Similar colonies were observed from 4 other adult peripheral and 5 cord blood donors. Scale bar represents 500 μm.
CFU-EC and ECFC colonies generated from discarded cells from methods A and B. (A) Representative photomicrograph phase-contrast of an ECFC colony, which arose when the adherent cells discarded from method A were cultured in EGM-2 medium (method B). Similar colonies were observed from 4 other adult peripheral and 5 cord blood donors. Arrows indicate colony boundary and scale bar represents 500 μm. (B) Representative phase-contrast photomicrograph of a CFU-EC colony, which arose when the nonadherent cells discarded from method B were cultured by method A. Similar colonies were observed from 4 other adult peripheral and 5 cord blood donors. Scale bar represents 500 μm.
CFU-ECs are hematopoietic-derived monocyte/macrophage cell colonies
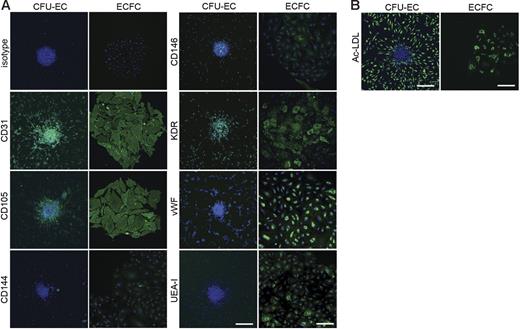
ECFC-derived cells expressed numerous cell surface antigens observed on ECs including CD31, CD105, CD144, CD146, VWF, KDR, and UEA-1 (Figure 3A). Some CFU-EC–derived cells also expressed CD31, CD105, CD144, CD146, VWF, KDR, and UEA-1 (Figure 3A). Table 1 indicates the percentage of both ECFC- and CFU-EC–derived cells, which express EC antigens. Antigen staining was corroborated by fluorescence-activated cell sorting (FACS) analysis (data not shown). Both ECFCs and CFU-ECs incorporated Ac-LDL, which is a functional phenotype of both ECs and macrophages (Figure 3B; Table 1).
Expression of endothelial cell antigens by CFU-ECs and ECFCs. (A) Immunophenotyping of CFU-EC and ECFC colonies by confocal microscopy. CFU-ECs and ECFCs express CD31, CD105, CD144, CD146, KDR, VWF, and UEA-I. Shown are isotype and antigen staining (green) representing 5 independent experiments using cells from different donors. Nuclei are stained with Hoechst 33342 (blue) and scale bar represents 200 μm. (B) CFU-ECs and ECFCs incorporate Ac-LDL. Shown is a confocal photomicrograph of cells that have taken up Ac-LDL (green) following a 4-hour incubation, representing 5 independent experiments using cells from different donors. Nuclei are stained with Hoechst 33342 (blue) and scale bar represents 200 μm.
Expression of endothelial cell antigens by CFU-ECs and ECFCs. (A) Immunophenotyping of CFU-EC and ECFC colonies by confocal microscopy. CFU-ECs and ECFCs express CD31, CD105, CD144, CD146, KDR, VWF, and UEA-I. Shown are isotype and antigen staining (green) representing 5 independent experiments using cells from different donors. Nuclei are stained with Hoechst 33342 (blue) and scale bar represents 200 μm. (B) CFU-ECs and ECFCs incorporate Ac-LDL. Shown is a confocal photomicrograph of cells that have taken up Ac-LDL (green) following a 4-hour incubation, representing 5 independent experiments using cells from different donors. Nuclei are stained with Hoechst 33342 (blue) and scale bar represents 200 μm.
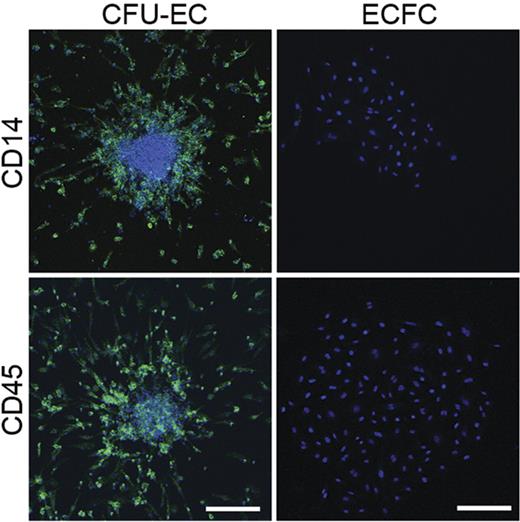
Given CFU-EC colony morphology and previous observations that monocytes and macrophages can express CD31, VWF, and UEA-1,20–22 we tested whether CFU-ECs expressed the hematopoietic-specific cell surface antigen, CD45, and the monocyte/macrophage cell surface antigen CD14. All CFU-EC colony-derived cells expressed CD45 and CD14 (Figure 4; Table 1). However, none of the ECFC colony-derived cells expressed CD45 or CD14 (Figure 4; Table 1). The fact that the CFU-ECs display cell surface antigens known to be restricted to the hematopoietic lineage implicates hematopoietic precursors in forming CFU-ECs.
Expression of hematopoietic-specific cell surface antigens by CFU-ECs and ECFCs. Immunophenotyping of CFU-EC and ECFC colonies by confocal microscopy. CFU-ECs but not ECFCs express CD14 and CD45 (green). Photomicrographs are representative of 5 independent experiments using cells from different donors. Nuclei are stained with Hoechst 33342 (blue) and scale bar represents 200 μm.
Expression of hematopoietic-specific cell surface antigens by CFU-ECs and ECFCs. Immunophenotyping of CFU-EC and ECFC colonies by confocal microscopy. CFU-ECs but not ECFCs express CD14 and CD45 (green). Photomicrographs are representative of 5 independent experiments using cells from different donors. Nuclei are stained with Hoechst 33342 (blue) and scale bar represents 200 μm.
We next tested whether the CFU-ECs expressed the macrophage-specific antigen, CD115 (c-fms) and contained sodium fluoride-inhibitable nonspecific esterase activity, which is a unique characteristic of monocytes and macrophages.23 CFU-EC colony-derived cells expressed CD115 and demonstrated nonspecific esterase activity that could be inhibited with sodium fluoride (Figure 5A-B; Table 1). ECFC-derived cells did not express CD115 (Figure 5A; Table 1) nor demonstrate nonspecific esterase activity (data not shown). Consistent with cell surface antigen expression, mRNA for CD14, CD45, and CD115 was detected in CFU-ECs but not ECFCs by RT-PCR (Figure 5C). Thus, CFU-EC progeny display macrophage features.
Monocyte/macrophage function in CFU-ECs. (A) Detection of cell surface expression of CD115 on CFU-EC and ECFC colonies by immunofluorescent staining. CFU-ECs express CD115 (green). Confocal photomicrographs are representative of 5 independent experiments using cells from different donors. Nuclei are stained with Hoechst 33342 (blue) and scale bar represents 200 μm. (B) Representative phase-contrast photomicrograph of CFU-EC colonies exposed to α-naphthyl acetate esterase with and without NaF inhibition. Similar nonspecific esterase activity was seen in CFU-EC colonies from 4 other donors. Scale bar represents 500 μm. (C) RT-PCR analysis of whole peripheral blood MNCs (M), CFU-ECs (C), and ECFCs (E) for gene expression of CD14, CD45, CD115, and β-actin. Left 3 lanes show reactions absent for reverse transcriptase. Results are representative of 5 independent experiments using cells from different donors. (D) Percentage of cells derived from CFU-EC or ECFC colonies that phagocytose E coli. Results represent the mean percentage of cells that phagocytose E coli ± SEM of 5 independent experiments. J774 murine monocytes were used as a positive control. *P < .001 by Student paired t test. (E) CFU-EC–derived cells demonstrate the ability to phagocytose E coli. Representative confocal photomicrographs of J774 murine monocytes, and CFU-EC– and ECFC-derived cells exposed to fluorescein-labeled E coli. Similar results were seen in 4 other experiments with cells derived from different donors. Nuclei are stained with Hoechst 33342 (blue) and scale bar represents 50 μm.
Monocyte/macrophage function in CFU-ECs. (A) Detection of cell surface expression of CD115 on CFU-EC and ECFC colonies by immunofluorescent staining. CFU-ECs express CD115 (green). Confocal photomicrographs are representative of 5 independent experiments using cells from different donors. Nuclei are stained with Hoechst 33342 (blue) and scale bar represents 200 μm. (B) Representative phase-contrast photomicrograph of CFU-EC colonies exposed to α-naphthyl acetate esterase with and without NaF inhibition. Similar nonspecific esterase activity was seen in CFU-EC colonies from 4 other donors. Scale bar represents 500 μm. (C) RT-PCR analysis of whole peripheral blood MNCs (M), CFU-ECs (C), and ECFCs (E) for gene expression of CD14, CD45, CD115, and β-actin. Left 3 lanes show reactions absent for reverse transcriptase. Results are representative of 5 independent experiments using cells from different donors. (D) Percentage of cells derived from CFU-EC or ECFC colonies that phagocytose E coli. Results represent the mean percentage of cells that phagocytose E coli ± SEM of 5 independent experiments. J774 murine monocytes were used as a positive control. *P < .001 by Student paired t test. (E) CFU-EC–derived cells demonstrate the ability to phagocytose E coli. Representative confocal photomicrographs of J774 murine monocytes, and CFU-EC– and ECFC-derived cells exposed to fluorescein-labeled E coli. Similar results were seen in 4 other experiments with cells derived from different donors. Nuclei are stained with Hoechst 33342 (blue) and scale bar represents 50 μm.
A characteristic of macrophages is their ability to ingest and kill microbes.23 To further test whether CFU-EC progeny are hematopoietic-derived monocyte/macrophages, we incubated the CFU-EC colonies with fluorescently labeled Escherichia coli. Approximately 50% of CFU-EC–derived cells phagocytosed E coli similar to J774 murine macrophages (Figure 5D-E). ECFCs failed to ingest E coli consistent with functionally normal ECs (Figure 5D-E). Collectively, these data demonstrate that CFU-EC progeny are hematopoietic-derived monocytes and macrophages and not ECs.
CFU-ECs do not replate and form secondary CFU-EC colonies
CFU-ECs and ECFCs are considered to be proliferative subpopulations of EPCs, which give rise to EC progeny either in vitro or in vivo. Although we have previously shown that ECFC-derived cells can be replated to form secondary ECFC colonies,16 it is unclear whether cells isolated from CFU-EC colonies have replating potential.
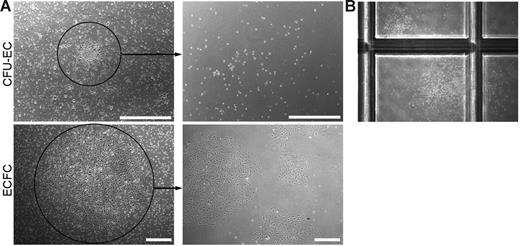
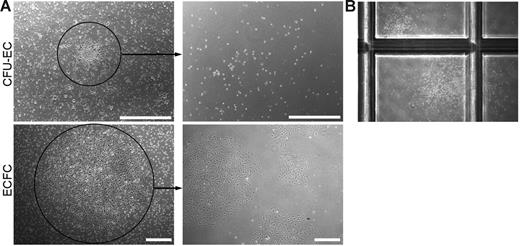
To address this question, we generated CFU-EC and ECFC colonies from the same adult donor (n = 7), and cloning cylinders were placed around individual colonies. Single cells contained within each colony were plated in limiting dilution assays to test for secondary colony formation. Consistent with the functional capacity of a progenitor cell, 31.90% ± 0.04 (n = 60) of the ECFC colonies replated into secondary colonies after 7 days in culture. However, none of the cells derived from individual CFU-EC colonies (n = 100) formed secondary EC colonies in the culture media used in either method A or method B (see “Derivation of CFU-ECs and ECFCs”). Representative photomicrographs of the primary ECFC or CFU-EC colony and the cell progeny derived from the colonies after secondary replating are shown in Figure 6A.
Secondary colony formation. (A) Representative phase-contrast photomicrographs of the cell progeny or secondary colonies (right) formed 7 days after primary CFU-EC and ECFC colonies (left) were plated at low cell density. Similar results were seen in 99 other CFU-EC colonies and 59 other ECFC colonies. Scale bar represents 500 μm. (B) Representative phase-contrast photomicrograph (original magnification, × 10) of secondary CFU-GM colonies formed 14 days after a primary CFU-EC colony was plated in a methylcellulose colony-forming assay. Primary ECFC colonies did not form CFU-GMs in the same assay (data not shown).
Secondary colony formation. (A) Representative phase-contrast photomicrographs of the cell progeny or secondary colonies (right) formed 7 days after primary CFU-EC and ECFC colonies (left) were plated at low cell density. Similar results were seen in 99 other CFU-EC colonies and 59 other ECFC colonies. Scale bar represents 500 μm. (B) Representative phase-contrast photomicrograph (original magnification, × 10) of secondary CFU-GM colonies formed 14 days after a primary CFU-EC colony was plated in a methylcellulose colony-forming assay. Primary ECFC colonies did not form CFU-GMs in the same assay (data not shown).
Given that CFU-ECs did not give rise to secondary EC colonies and are potentially hematopoietic-derived monocyte/macrophage colonies, we plated CFU-EC–derived cells in CFU-GM methylcellulose assays to test for secondary hematopoietic colony-forming cell activity. Strikingly, some of the cells derived from CFU-ECs formed CFU-GMs, though at low frequency (< 10%, n = 20; Figure 6B). Interestingly, replating of the round cells in the central core of the CFU-EC colony and not the spindle cells in the periphery resulted in CFU-GM colony formation, suggesting that the cells within the core are responsible for the hematopoietic colony formation (n = 4). Thus, some CFU-ECs display only myeloid-restricted hematopoietic colony-forming activity and none formed secondary CFU-ECs.
CFU-ECs and ECFCs are not clonally related
It remains unclear whether CFU-ECs and ECFCs are clonally related or derived from the same parent cell. To address this question, we generated both CFU-EC and ECFC colonies from 11 different patients with PV, a clonal HSC disorder characterized by hypersensitivity of hematopoietic progenitors to growth factors.24,25 The number of ECFC and CFU-EC colonies generated from 106 MNCs from donors with PV (0.016 ± 0.006 and 4.09 ± 1.8, respectively, n = 11) did not differ from the colony frequency of control donors.
Recently, a valine-to-phenylalanine substitution at position 617 (V617F) of the JAK2 gene has been identified in the majority of patients with PVs.26–28 The V617F JAK2 mutation can be detected by allele specific real-time PCR using DNA from the blood-derived cells. Therefore, if CFU-ECs and ECFCs are derived from the same parent cell, then DNA isolated from each individual colony should harbor no mutation or the same heterozygous or homozygous JAK2 mutation.
Consistent with our hypothesis that CFU-ECs are hematopoietic-derived cells, all of the genotyped CFU-EC colonies displayed either a homozygous or heterozygous JAK2 mutation (n = 541; Table 2)In contrast, only 3 of the 89 ECFC colonies tested displayed DNA evidence of a mutant heterozygous genotype (Table 2) with all other ECFC colonies being normal. All 3 mutant colonies (3% of the total ECFCs examined) were derived from the same patient, who presented with vascular thrombosis and only later developed classic hematologic signs of PV. Whether the genotypic abnormality in the ECFCs of this singular patient indicates a germline mutation, a novel vascular somatic mutation, contamination of ECFCs with hematopoietic cells, or a rare example of ECFC derivation from a hematopoietic clone will require additional vascular endothelial examination in this unique individual. Nonetheless, these experiments provide the first direct human genetic clonal data to demonstrate that CFU-ECs are derived from HSCs and that CFU-ECs and ECFCs are not clonally related.
ECFCs can be transplanted to form chimeric blood vessels in vivo
A characteristic of stem and progenitor cells is their ability to be adoptively transplanted into hosts in the absence or presence of host conditioning to repopulate the mature cells of the intended lineage with donor cells.15 Therefore, we tested whether CFU-ECs or ECFCs could form vascular structures de novo using a previously established methodology for subcutaneous transplantation of cellularized fibronectin/collagen gel implants into immunodeficient mice.19 Furthermore, we examined whether the human vessels would form anastomoses with murine vessels and participate in blood cell circulation.
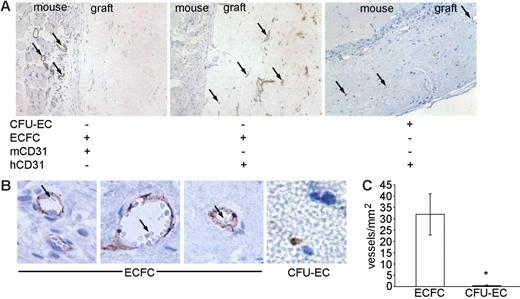
CFU-ECs and ECFCs were generated from the blood of each of 18 donors, suspended in collagen/fibronectin gels, and transplanted into immunodeficient mice. At 14 to 30 days, mice were killed and the grafts were analyzed for chimeric blood vessel formation. Grafts were stained with either a mouse or human anti-CD31 antibody to discriminate between murine and human blood vessels. Specifically, we examined the graft for the presence of human capillaries (expressing the human CD31 antigen and not the murine CD31 antigen), which were perfused with mouse red blood cells indicating anastomoses with the surrounding murine vasculature. In 18 independent experiments, CFU-ECs failed to produce chimeric blood vessels though individual CFU-EC–derived cells could be identified in the transplanted graft (Figure 7A-C). However, ECFCs formed chimeric vessels, which were perfused with mouse red blood cells (Figure 7A-C). Representative photomicrographs of cellularized gels and enumeration of vessels derived from human ECFCs are shown in Figure 7A-C. In addition, control anti-CD31 staining of murine vessels is illustrated in Figure 7A. Thus, in contrast to CFU-EC–derived cells, ECFC progeny can form functional human-murine chimeric vessels in a short-term xenograft model of blood vessel formation.
Transplantation of CFU-ECs and ECFCs into NOD/SCID mice. (A) Photomicrographs (original magnification, × 20) of cellularized grafts and surrounding murine tissue 28 days after implantation into NOD/SCID mice. Left and middle panels show consecutive sections of the same ECFC graft stained with anti–murine CD31 (mCD31) and anti–human CD31 (hCD31) to identify either murine or human blood vessels, respectively. mCD31 (left) does not cross-react with human endothelial cells within the graft and hCD31 (middle) does not cross-react with murine endothelial cells in the vessels outside the graft. Murine vessels were never identified in the cellularized graft (n = 18). Right panel shows a CFU-EC graft stained with anti–human CD31. Arrows indicate positive antigen staining. Results represent 9 other ECFC grafts and 2 other CFU-EC grafts. (B) Photomicrographs (original magnification, × 100) of ECFC and CFU-EC (far right) cellularized grafts stained with anti–human CD31 28 days after implantation. Vessels and capillaries in ECFC grafts are perfused with murine red blood cells (arrows) indicating anastomoses with murine blood vessels. CFU-EC grafts fail to form vessels or capillaries. Results represent 9 other ECFC grafts and 2 other CFU-EC grafts. (C) Quantitation of capillary density within ECFC (□) and CFU-EC (▪) cellularized grafts 28 days after implantation. Results represent the average number of capillaries containing murine red blood cells/mm2 of graft tissue ± SEM of 10 ECFC and 3 CFU-EC grafts. *P < .05 by Student unpaired t test.
Transplantation of CFU-ECs and ECFCs into NOD/SCID mice. (A) Photomicrographs (original magnification, × 20) of cellularized grafts and surrounding murine tissue 28 days after implantation into NOD/SCID mice. Left and middle panels show consecutive sections of the same ECFC graft stained with anti–murine CD31 (mCD31) and anti–human CD31 (hCD31) to identify either murine or human blood vessels, respectively. mCD31 (left) does not cross-react with human endothelial cells within the graft and hCD31 (middle) does not cross-react with murine endothelial cells in the vessels outside the graft. Murine vessels were never identified in the cellularized graft (n = 18). Right panel shows a CFU-EC graft stained with anti–human CD31. Arrows indicate positive antigen staining. Results represent 9 other ECFC grafts and 2 other CFU-EC grafts. (B) Photomicrographs (original magnification, × 100) of ECFC and CFU-EC (far right) cellularized grafts stained with anti–human CD31 28 days after implantation. Vessels and capillaries in ECFC grafts are perfused with murine red blood cells (arrows) indicating anastomoses with murine blood vessels. CFU-EC grafts fail to form vessels or capillaries. Results represent 9 other ECFC grafts and 2 other CFU-EC grafts. (C) Quantitation of capillary density within ECFC (□) and CFU-EC (▪) cellularized grafts 28 days after implantation. Results represent the average number of capillaries containing murine red blood cells/mm2 of graft tissue ± SEM of 10 ECFC and 3 CFU-EC grafts. *P < .05 by Student unpaired t test.
Discussion
We have used several approaches to conduct a comparative analysis of EPC as assayed by the CFU-EC and ECFC assays. The data indicate that CFU-ECs and ECFCs are different progenitor populations giving rise to functionally divergent progeny (Table 3). CFU-ECs and their progeny display hematopoietic-restricted and macrophage-specific cellular proteins, possess limited hematopoietic colony-forming activity, and function as macrophages to ingest bacteria. CFU-ECs are derived from HSCs as proven by a novel clonal analysis and do not possess the ability to form vascular structures in vivo. In contrast, ECFCs and their progeny express endothelial but not hematopoietic cell surface proteins, are clonally distinct from HSCs, display proliferative potential, and form functional human-murine chimeric vessels when implanted in vivo. These data led us to conclude that ECFCs function as EPCs and CFU-ECs do not.
The origins of the CFU-EC assay can be traced back to the original description of EPCs in 1997.3 Asahara et al3 reported that CD34- or KDR-expressing cells from human peripheral blood would form clusters of cells comprised of round cells centrally and sprouts of spindle-shaped cells at the periphery within 5 days, when the cells were plated on fibronectin-coated dishes. The purity of these starting populations was 15.7% and 27.6%, respectively (10.8% coexpressed these antigens). CD45 was expressed on 94.1% of the freshly plated cells and 27.2% of adherent cells continued to express this antigen after 7 days. Increasing expression of CD31, CD34, KDR, Tie-2, and E selectin was observed in the attached cells, along with mRNA expression of endothelial nitric oxide synthase (eNOS). Few cells (6.0%) expressed the macrophage marker CD68. Subsequently, Ito et al29 modified the isolation protocol in plating human peripheral blood nucleated cells on fibronectin-coated plates. After a 24-hour adherence depletion, the nonadherent cells were replated on fibronectin-coated plates and observed for the appearance of clusters of cells expressing CD31, Tie2, and KDR at 7 days. Hill et al4 further modified the culture conditions, to include a 48-hour preplating step on fibronectin-coated dishes, prior to culturing the nonadherent cells on fibronectin-coated culture plates. Confirmation that the EPC colony-forming cells gave rise to ECs was conducted via immunohistochemical staining with KDR, CD31, and BS-1 lectin and uptake of acetylated-LDL. Hill et al4 then presented evidence that the number of EPC CFUs was reduced in subjects with elevated serum cholesterol, hypertension, and diabetes. Further, an inverse correlation between circulating EPC CFUs and Framingham risk score was reported with those patients at highest risk having the fewest EPC CFUs. Thus, the EPC CFU assay (now called CFU-EC) appears to serve as a predictor for patient cardiovascular risk.
Although the CFU-EC assay may predict patient risk for developing cardiovascular complications, the composition of the cells giving rise to the CFU-ECs have remained largely unexplored. As noted, the original report describing the appearance of the CFU-ECs used a heterogenous population of cells to initiate the cultures that contained CD34- and KDR-expressing cells (known hematopoietic progenitor cell markers).3 Others have reported that CD133-expressing cells give rise to EPCs.10,11 We report that myeloid progenitor colony-forming activity can be recovered in some CFU-ECs and that CFU-ECs are clonally related to the HSC. The finding that CFU-ECs possess myeloid progenitor colony-forming activity is novel. Although CD34- and KDR-expressing cells are reported to give rise to CFU-ECs and both of these proteins are expressed on hematopoietic progenitors, we are not aware of any prior data where CFU-ECs were isolated and plated in hematopoietic assays. The presence of myeloid progenitor activity after 1 week of in vitro culture and the clonal marking data suggest that these progenitors may be derived from a more primitive hematopoietic progenitor cell that initiated the CFU-ECs. At present, it is unclear which specific stage of hematopoietic progenitor may give rise to the CFU-ECs. It is interesting that the presence of CD14-expressing monocytes is required for the formation of CFU-ECs, suggesting that monocyte-derived cytokines may play an important role.20 Colony-stimulating factor 1 (CSF-1) is known to promote monocyte/macrophage differentiation and is an important inducer of VEGF production, increased EPC mobilization, and enhanced angiogenesis in vivo.30–32 We report that most CFU-ECs express CD115, the receptor for CSF-1, suggesting that CFU-ECs may be CSF-1 responsive. Although we have not pursued a phenotypic approach to identifying which particular stage of hematopoietic progenitors gives rise to the CFU-ECs, these studies would be informative.
Following the fate of cells isolated from patients with PV to discern the clonal relationship of EPCs and the HSC is novel. PV is a myeloproliferative disorder that can develop as a somatic event in a single HSC clone.25,33 Although early studies used glucose-6-phosphate isoenzyme or other X-chromosome inactivation strategies to determine clonality, Kralovics et al33 reported that uniparental disomy of chromosome 9p was a frequent stem cell (myeloid and lymphoid lineages) defect in patients with PV. The identified mutant region of chromosome 9 contained several candidate genes that led to the identification the V617F JAK2 mutation.26–28 This gain-of-function mutation is frequent in PV patients but is also represented in other myelodysplastic syndromes.34 In the present studies, we isolated peripheral blood cells from PV patients carrying the V617F JAK2 mutation and plated cells in the CFU-EC and ECFC assays. All CFU-ECs displayed the mutation indicating their clonal derivation from the mutant HSC clone. Of note, the absolute number of CFU-ECs and ECFCs from PV patients did not differ from control donors.
Gunsilius et al35 previously used peripheral blood or bone marrow from patients with another clonal HSC disorder, chronic myelogenous leukemia (CML) to examine the origins of EPC. CML is characterized by a unique chromosomal translocation, t(9;22), that results in a BCR/ABL fusion gene product. This translocation is present in a multipotent HSC clone and all the derived progeny. This feature permitted Gunsilius et al35 to examine hematopoietic progenitor cells and cultured ECs for this genetic mutation. They reported that patient MNCs plated for 5 days on fibronectin-coated dishes formed clusters of cells with spindle-shaped progeny that expressed a panel of proteins including CD34, CD31, CD144, E-selectin, factor VIII, and VWF but not CD14, and the cells ingested acetylated LDL. In 5 of 6 patients, these cultured ECs displayed the translocation, suggesting to Gunsilius et al35 that cells of the endothelial lineage are part of the malignant clone in CML and may arise from a hemangioblastic precursor. The data presented herein would challenge these conclusions with respect to the definition of the in vitro cultured cells as belonging to the endothelial lineage. Further analysis of CML patients using the assays and conditions described in this paper may be informative as to whether a hemangioblastic precursor can be detected.
Although others have suggested that EPCs may be derived from monocytes and macrophages,20,21,36,37 the data presented herein for the CFU-ECs are definitive (clonal marking) and do not rely on a cell surface phenotype alone to identify lineage. We provide supporting data that the CFU-EC progeny function as macrophages by ingesting bacteria in addition to expressing molecules typical for macrophages. Recently, Zhang et al37 used an alternative method for EPC isolation where peripheral blood cells were plated on fibronectin-coated dishes and cells were cultured in endothelial growth factor-containing media. Under these conditions, no CFU-ECs formed in culture; however, adherent cells did express molecules and morphology consistent with EPC definitions. All the adherent cells grown in these cultures displayed features of monocytes/macrophages throughout the culture period despite up-regulating eNOS, KDR, vascular endothelial cadherin, and E-selectin expression. Similar to the results obtained herein, the adherent progeny ingested India ink consistent with macrophage phagocytic activity. Thus, whether using the CFU-EC assay or a more general adherence protocol, it is apparent that the use of cell surface antigens alone as a method to confirm that cells belong to the endothelial lineage may be inadequate because macrophages express many of the “endothelial-specific” proteins.
We determined that the most stringent method to assess whether the CFU-ECs or ECFCs displayed the ability to function as postnatal vasculogenic cells was to implant the cells and quantitate the formation of blood vessels in vivo. Many studies to date have infused human EPCs into immunocompromised mice in which acute vascular injuries have been induced to test for the ability of the EPCs to home to the injury site, improve perfusion, and incorporate into existing vascular structures or form new vessels.38 Although these approaches are useful, most studies fail to reveal significant new vessel formation by the infused cells. Schechner et al19 have described a technique of inoculating human ECs into type 1 collagen and fibronectin gels and implanting the gels subcutaneously into immunocompromised mice. As compared to Matrigel implantation, collagen and fibronectin gels fail to recruit substantial host murine vessel ingrowth. Thus, formation of human-murine chimeric vessels is a function of human vascular outgrowth to the host vessels surrounding the implanted gels. In the present studies, addition of CFU-ECs to the type 1 collagen and fibronectin gels resulted in cell aggregation and persistence of the cells on implantation (up to 30 days) but CFU-EC progeny failed to form microvessels within the gels. In contrast, ECFC formed capillary-like structures in vitro and microvessels on implantation that functionally connected with host murine vessels and participated in murine blood flow. These results demonstrate the ability of ECFCs to participate as postnatal vasculogenic cells, whereas CFU-ECs lack such activity.
Given the extensive literature on the positive effects of circulating EPCs on vascular function in animal and human subjects, how can we interpret the findings that CFU-ECs are hematopoietic and not ECs? A growing body of literature is emerging that hematopoietic myeloid progenitor cells and their progeny facilitate neoangiogenesis without directly participating in the process of postnatal vasculogenesis (incorporation into the endothelial intima).39–47 Furthermore, coinjection of early and late outgrowth colony-forming cells has been demonstrated to synergistically improve neurovascularization in mice following hind limb ischemic injury.48 Thus, we may have reached a time to clarify the terminology and restrict the term EPC to those cells that can function to form vessels in vivo (ECFC) and simply identify the proangiogenic hematopoietic progenitor cells (or their myeloid progeny) by a combination of their cell surface phenotype and in vitro hematopoietic colony-forming activity (which will define their stage of differentiation). The methods presented in this paper permit clarification of EPC terminology and may now facilitate interpretation of the roles of various circulating cell populations in neoangiogenesis and determination of postnatal vasculogenesis as a relevant biologic mechanism.
Authorship
Contribution: M.C.Y. codesigned experiments and wrote the manuscript; L.E.M. coperformed in vitro assays; D.P. coperformed in vivo assays; T.R.K. grew colonies from all donors; K.N.M. coperformed all macrophage assays; F.L. coperformed in vivo assays; R.D. coperformed all macrophage assays; C.J.T. performed immunohistochemical analysis; J.T.P. provided PV patients and cowrote the manuscript; and D.A.I. codesigned experiments and wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: David A. Ingram or Mervin C. Yoder, Indiana University School of Medicine, Herman B. Wells Center for Pediatric Research, 1044 W Walnut St R4/470, Indianapolis, IN 46202; e-mail: dingram@iupui.edu or myoder@iupui.edu.
An Inside Blood analysis of this article appears at the front of this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This work was supported by the National Institute of Neurological Disorders and Stroke grant P50 NS052606 (D.A.I.), grant NF043019 from the Department of Defense (D.A.I.), W81XWH-05-1-0161, Riley Children's Foundation (D.A.I. and M.C.Y.), P30 CA82709 (D.A.I. and F.C.Y.), National Institutes of Health grants 1P01 HL085036 (M.C.Y. and D.A.I.) and 1P01CA108671-O1A2 MPD Consortium, Project no. 1 (J.T.P.).
We thank Dr Pober and members of his laboratory for teaching us how to perform the in vivo transplant assays and Janice Walls for her expert administrative assistance in preparation of the manuscript.