Abstract
Tumor necrosis factor-α (TNF-α) binds to 2 distinct cell-surface receptors: TNF-α receptor-I (TNFR-I: p55) and TNF-α receptor-II (TNFR-II: p75). TNF-α induces leukocyte adhesion molecules on endothelial cells (ECs), which mediate 3 defined steps of the inflammatory response; namely, leukocyte rolling, firm adhesion, and transmigration. In this study, we have investigated the role of p75 in TNF-α–induced leukocyte adhesion molecules using cultured ECs derived from wild-type (WT), p75-null (p75−/−), or p55-null (p55−/−) mice. We observed that p75 was essential for TNF-α–induced E-selectin, vascular cell adhesion molecule 1 (VCAM-1), and intercellular adhesion molecule 1 (ICAM-1) expression. We also investigated the putative role of p75 in inflammation in vivo using an intravital microscopic approach with a mouse cremaster muscle model. TNF-α–stimulated leukocyte rolling, firm adhesion to ECs, and transmigration were dramatically reduced in p75−/− mice. Transplanted WT cremaster in p75−/− mice showed a robust leukocyte rolling and firm adhesion upon TNF-α activation, suggesting that the impairment in EC-leukocyte interaction in p75−/− mice is due to EC dysfunction. These results demonstrate, for the first time, that endothelial p75 is essential for TNF-α–induced leukocyte–endothelial-cell interaction. Our findings may contribute to the identification of novel p75-targeted therapeutic approaches for inflammatory diseases.
Introduction
Tumor necrosis factor α (TNF-α) and its 2 receptors, p55 and p75, are expressed in a variety of cell types. p55 is noninducible, whereas p75 is inducible at the transcriptional level by various external stimuli.1 The receptors contain a conserved N-terminal extracellular TNF-α binding domain, a small transmembrane region, and a nonconserved cytoplasmic domain.1 Due to the lack of homology in the cytoplasmic region, it has been postulated that these receptors may trigger distinct signaling cascades upon TNF-α ligation. Experiments in cultured cells have demonstrated that the 2 TNF-α receptors are able to induce both common and distinct signaling pathways. For example, although p55 showed a stronger response, both receptors, upon TNF-α activation, were shown to be capable of translocating nuclear factor (NF) κB from the cytosol to the nucleus and to induce the transcription of NFκB-responsive genes.2,3 Also, both receptors use TRAF-2 (TNFR-associated protein), a common signaling intermediate that along with other adaptor proteins form signaling complexes and activate several signaling cascades.4 On the other hand, caspase activation and subsequent induction of apoptosis by TNF-α is an exclusive feature of p55 activation by virtue of a well-defined death domain, which is absent in p75. Recently, Pan et al5 determined that Etk/Bmx (endothelial/epithelial tyrosine kinase) binds constitutively to p75, and implicated it in TNF-α–induced endothelial-cell (EC) migration and angiogenesis.
Activation of ECs by TNF-α induces the expression of multiple leukocyte adhesion molecules, such as E-selectin, P-selectin, intercellular adhesion molecule 1 (ICAM-1), and vascular cell adhesion molecule 1 (VCAM-1). Regulated expression of these adhesion molecules and their interactions with specific leukocyte receptors results in rolling, firm adhesion, and transmigration of leukocytes from the blood to the interstitial tissue, a critical cellular response to pro-inflammatory agents.6 Leukocyte rolling is mediated by a reversible and transient interaction between constitutively functional leukocyte adhesion proteins (eg, L-selectin and P-selectin glycoprotein ligand-1) and their respective EC counter receptors. This initial rolling event, with the assistance of specific chemoattractants displayed at the EC surface, helps to induce or activate secondary adhesion receptors such as β2 and β1 integrins on leukocytes. Leukocyte adhesion receptors, in turn, interact with constitutive or induced counter receptors expressed on ECs; namely, ICAM-1, ICAM-2, and VCAM-1,7 inducing firm and sustained attachment of leukocytes. Recently, endothelial E-selectin has also been implicated in agonist-induced firm adhesion of leukocytes to ECs.8,9 Adherent leukocytes subsequently transmigrate to the interstitial space through EC tight junctions, a complex process that is mediated by multiple adhesion molecules on both leukocytes and ECs.10,11 Using receptor-specific agonistic antibodies or TNF-α mutants, previous studies in human ECs have demonstrated that p55, not p75, is critical for TNF-α–mediated up-regulation of leukocyte adhesion molecules.12 In an in vivo TNFR-null mouse model, Lucas et al13 showed that p75 is critical for ICAM-1 up-regulation and cerebral malarial susceptibility. More recently, Vielhauer et al14 demonstrated a role of endothelial p75 in the progression of immune-mediated glomerulonephritis.
In the present study, we have investigated the role of p75 in TNF-α–induced E-selectin, VCAM-1, and ICAM-1 induction in cultured ECs. We have identified a critical role for p75 in TNF-α–induced E-selectin, VCAM-1, and ICAM-1 expression in ECs. We further extended this study to understand the role of p75 in TNF-α–induced inflammation in vivo by measuring leukocyte rolling, firm adhesion, and transmigration in TNF-α–treated postcapillary venules of live mice. We employed an in vivo cremaster muscle-flap microcirculatory model, using intravital microscopy, to analyze the wild-type (WT) situation, as well as that in which either one (p75−/− or p55−/−) or both (D−/−) of the TNF-α receptor genes was absent. We identified for the first time that p75 is critical for TNF-α–induced leukocyte rolling, firm adhesion, and transmigration to the endothelium.
Materials and methods
Animals
Mice (C57BL/6 background) with null mutations either in TNF-α receptor-I (p55−/−), TNF-α receptor II (p75−/−), or both p55 and p75 (D−/−) were generated by Peschon et al.15 The p55−/− mice were generated using a C57/BL/6 ES cell line. The p75−/− mice and D−/− mice in a random C57BL/6 × 129 hybrid background were backcrossed to C57BL/6 for 5 (B6N5) and 4 (B6N4) generations, respectively. The mice were bred and maintained at Taconic (Germantown, NY). Taconic provided us with the WT (C57BL/6, control) and the TNF-α receptor–null mice at ages 8 to 10 wks. Twelve-week-old mice were used for the experiments. Genotypes of these mice were confirmed by polymerase chain reaction (PCR). The Animal Care and Use Committee of Cleveland Clinic Foundation Lerner Research Institute, Cleveland, OH, approved the procedures in this study.
Mouse aortic EC (MAEC) isolation
ECs from the thoracic aorta were isolated by an explant technique as described previously.16 EC authenticity was verified by staining for von Willebrand factor and endothelial-specific gene-expression profiling was verified by real-time PCR.
RNA isolation and real-time PCR assay
First-strand cDNA was synthesized from total RNA (1 μg) using Taqman Reverse Transcription Reagents (Applied Biosystems, Branchburg, NJ) according to the manufacturer's instructions. Reaction mixture (2 μL) was used to perform PCR reactions with specific primer pairs corresponding to particular genes of interest. RPL-32 served as an internal control to ensure the quality and efficiency of the PCR. All the real-time PCR data reported was verified to be derived from a single amplicon, and authenticity of each amplicon was verified by DNA sequencing. Real-time PCR was performed using SYBR Green PCR Core Reagents (PE Applied Biosystems, Foster City, CA) and a Perkin Elmer (Wellesley, MA) ABI PRISM 7700 Sequence Detector, according to the manufacturer's instructions. p55-specific siRNA (cat no. 51320) was obtained from Ambion (Austin, TX).
Quantitation of E-selectin, VCAM-1, and ICAM-1 on EC surface
Surface expression of E-selectin, VCAM-1, and ICAM-1 were measured using a biotin-labeled antibody/[125I]-streptavidin technique.17 Mouse aortic ECs were plated in 24-well plates and grown to confluence. Biotin-conjugated rat anti–mouse E-selectin (1:50 dilution), rat anti–mouse VCAM-1 (1:50), or hamster anti–mouse ICAM-1 (1:200 dilution) primary antibodies (BD Pharmingen International, San Diego, CA) were used. Biotin-labeled nonspecific immunoglobulin G (IgG) was used as a negative control to ascertain the specificity of the experimental antibodies.
In vivo microcirculatory observation
Mouse cremaster muscle preparation was performed as previously described.18 The exposed tissue was superfused with warm (37°C) Ringer solution. The microcirculatory measurements were taken and recorded using an intravital microscope (Optiphot-2; Nikon, Tokyo, Japan) with a ×40 objective lens (Nikon Optiphot-2) and a ×10 eyepiece was used to examine the cremaster microcirculation. The microscope was equipped with a color camera, a 19-inch monitor, and a videotape recorder. The magnification of the monitor was ×1800.
Data collection and analysis
Following the cremaster muscle flap preparation, the flap was kept for equilibration in warm Ringer solution for 1 hour. Unbranched venules (25 μm to 40 μm in diameter) with stable flow were selected for observation. Control readings of rolling and adhering leukocytes were taken at the end of the equilibrium period for a time window of 2 minutes and a viewing distance of 100 μm. Leukocytes that remained stationary for at least 30 seconds in the viewing segment (100 μm) of the venule were considered firm adherent leukocytes. Mouse TNF-α (2 ng/mL, 25 μL) was then applied directly to the exposed muscle flap of a 25-mm2 area and readings were taken every 15 minutes for a window of 2 minutes, for up to 180 minutes. The TNF-α response was expressed as the fold change in the number of rolling and adherent leukocytes compared with the control readings in the respective mice. Rolling leukocyte flux was determined by counting the number of rolling leukocytes per 0.5 minutes, passing a reference point in the microvessel and expressed as cells per minute. We analyzed transmigrated leukocytes in the cremaster tissue 3 hours after TNF-α application, using hematoxylin-and-eosin staining.
Cremaster transplantation
Mouse cremaster transplantation was performed as previously described.18 Briefly, the cremaster muscle was exposed with an anterior incision, and the testicular contents were removed. The muscle was isolated on the iliac artery and vein, with external diameters of 250 μm and 350 μm, respectively. Anastomoses to the recipient vessels were performed at this level. The muscle flap was not detached until preparation of the recipient site was complete, to ensure a shorter ischemia time. As the recipient site, we used the carotid artery and the external jugular vein. A skin incision was made at the anterolateral neck region. The sternocleidomastoid muscle was detached from the clavicle to expose the carotid artery. Anastomoses between the carotid and iliac artery as well as the external jugular and iliac veins were performed by using an 11.0 ethilon and surgical microscope. Anastamoses were performed by using a standard end-to-end technique with interrupted sutures. After clamp release, the tube flap was opened along its frontal wall by using thermal cautery. The animal was secured in a Plexiglas tissue bath (Altaglas International, Philadelphia, PA), and the muscle was spread out and fixed with silk sutures over a glass in the bottom of the bath. The muscle was sealed with oxygen-impermeable plastic film (Saran Wrap; S. C. Johnson, Racine, WI).
Immunohistochemistry and hematoxylin-eosin staining
Mouse cremaster muscles were superfused with mouse TNF-α or vehicle (phosphate-buffered saline [PBS]) for 3 hours. The cremaster was dissected and frozen using optimal cutting temperature compound (OCT; TissueTek, Elkhart, IN). Cryostat sections (8 μm) were immunostained with Ventana ES (Ventana Medical Systems, Tucson, AZ) using purified hamster anti–mouse CD54 (ICAM-1) antibody (BD Pharmingen), biotin-labeled secondary antibody, and streptavidin-linked horseradish peroxidase (HRP). Sections were counterstained with hematoxylin. Cremaster samples were fixed in 10% buffered formalin, embedded in paraffin, and examined after hematoxylin-and-eosin staining. Images were visualized using a Leica DMR microscope (Leica, Heidelberg, Germany) equipped with a 10×/0.3 numerical aperture (NA) dry objective (Figures 2D, 5) or a 40×/0.7 NA dry objective (Figure 5 insets). Images were acquired using a QImaging Retiga EX camera (QImaging, Burnaby, BC, Canada) and Image-Pro software version 5.0 (Media Cybernetics, Silver Spring, MD).
Statistical analysis
The Wilcoxon rank sum test was used to compare p55−/− and p75−/− and WT on median baseline rolling and adhering cell counts, and on median peak 1 (early)/baseline and peak 2 (late)/baseline ratios. The significance level for each test was set at P values less than .05.
Results
p75 is critical for TNF-α–induced E-selectin, VCAM-1, and ICAM-1 expression in cultured mouse ECs
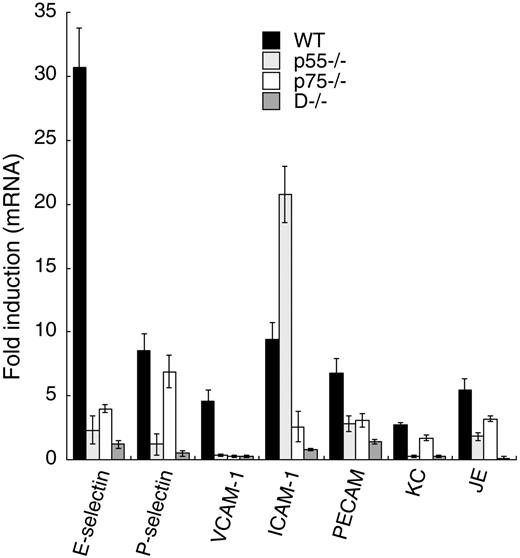
To study the role of individual TNF-α receptors in TNF-α–mediated leukocyte adhesion molecule expression in ECs, we measured mRNA levels of a variety of adhesion molecules in control and TNF-α–treated cultured mouse aortic ECs isolated from WT mice or mice null for p55, p75, or both. We performed real-time PCR analysis to quantify the target mRNAs from total RNA using gene-specific primers. Our results, depicted in Figure 1, suggest that the 2 TNF-α receptors may initiate both common and distinct signaling pathways to elicit the expression of leukocyte adhesion molecules. For example, TNF-α–induced P-selectin expression (8.5-fold in WT) was mediated by p55 receptor alone, whereas expression of platelet-endothelial cell adhesion molecule-1 (PECAM) and the chemokines KC and JE required both p55 and p75. E-selectin and VCAM-1 mRNA induction in TNF-α–treated WT ECs (∼30- and 5-fold, respectively) was substantially blocked in p55−/− or p75−/− ECs, suggesting that both receptors are critical for their expression. In WT ECs, TNF-α induced ICAM-1 expression approximately 9-fold. This induction increased to approximately 20-fold in TNF-p55–null ECs, and in TNF-p75–null ECs, ICAM-1 expression was marginally induced, suggesting that p75, not p55, is required for ICAM-1 induction. In double-null ECs (D−/−), TNF-α did not induce any of the genes studied. Further, we determined p75-specific expression of adhesion molecules in p55-depleted cultured human umbilical vein ECs. We used p55-specific siRNA to deplete (> 90%) p55 expression in the ECs. Quantitative PCR analysis showed that TNF-α induction of E-selectin and VCAM-1 did not occur in the p55 siRNA-treated human ECs, whereas ICAM-1 induction was comparable to control ECs (not shown). Thus, human and mouse ECs appear to show a similar functional dependency on p75. Taken together, these results suggest that the 2 TNF-α receptors have both distinct and overlapping roles in the induction of leukocyte adhesion molecules in ECs.
Relative importance of p55 and p75 in the induction of leukocyte-EC adhesion molecules. (A) Quantitative real-time PCR was performed using total RNA (1 μg) isolated from TNF-α–treated or untreated cultured mouse aortic ECs isolated from WT, p55−/−, p75−/−, or D−/− mice. PCR reactions were performed with specific primer pairs corresponding to the specific genes of interest. RPL-32 (a TNF-α nonresponsive gene) served as an internal control to ensure the quality and efficiency of the PCR. Each value represents the average of 4 independent experiments. Error bars represent SD.
Relative importance of p55 and p75 in the induction of leukocyte-EC adhesion molecules. (A) Quantitative real-time PCR was performed using total RNA (1 μg) isolated from TNF-α–treated or untreated cultured mouse aortic ECs isolated from WT, p55−/−, p75−/−, or D−/− mice. PCR reactions were performed with specific primer pairs corresponding to the specific genes of interest. RPL-32 (a TNF-α nonresponsive gene) served as an internal control to ensure the quality and efficiency of the PCR. Each value represents the average of 4 independent experiments. Error bars represent SD.
We proceeded to measure TNF-α–induced E-selectin, VCAM-1, and E-selectin protein levels in WT, p75−/−, or p55−/− ECs isolated from mouse aorta. We quantified TNF-α–induced E-selectin, VCAM-1, and ICAM-1 surface expression in cultured aortic ECs isolated from WT, p75−/−, and p55−/− mice using biotin-labeled primary antibody and [125I]-labeled streptavidin.17 As shown in Figure 2A, TNF-α–induced E-selectin surface expression (approximately 3-fold) in WT cells was abolished in either p75−/− or p55−/− cells. Similarly, TNF-α–induced VCAM-1 cell-surface expression in WT cells (approximately 2-fold) was severely compromised when either of the TNF-α receptors was absent (Figure 2B). Figure 2C shows the ICAM-1 surface expression profile upon TNF-α treatment in WT, p75−/−, or p55−/− ECs. TNF-α induced ICAM-1 cell-surface expression approximately 2-fold in WT ECs, whereas in p75−/− ECs, TNF-α failed to induce ICAM-1 induction. We consistently observed a more than 3-fold induction of ICAM-1 cell-surface expression in TNF-α–treated p55−/− ECs.
The p75 TNF-α receptor is critical for TNF-α–induced E-selectin, VCAM-1, and ICAM-1 expression on mouse ECs. Quantification of E-selectin (A), VCAM-1 (B), and ICAM-1 (C) on the mouse endothelial cell surface. Biotin-conjugated primary antibodies and the [125I]-streptavidin system, as described in “Materials and methods,” were used to determine surface expression of E-selectin, VCAM-1, and ICAM-1 on untreated or TNF-α–treated (mouse TNF-α, 3 hours) cultured mouse aortic ECs isolated from WT, p75−/−, or p55−/− mice. (D) Mouse cremaster muscles treated with mouse TNF-α (3 hours) or vehicle (PBS) were dissected and frozen using OCT. Cryostat sections were immunostained for ICAM-1 as described in “Materials and methods.” Arrowheads show ICAM-1–stained ECs. (E) Cultured WT mouse aortic ECs were treated with mouse TNF-α (2 ng/mL, ▪) or human TNF-α (2 ng/mL, □) and after 2 hours total RNA was isolated and real-time PCR was performed as described in “Materials and methods.” Values are expressed as fold induction with respect to controls. Error bars indicate SD.
The p75 TNF-α receptor is critical for TNF-α–induced E-selectin, VCAM-1, and ICAM-1 expression on mouse ECs. Quantification of E-selectin (A), VCAM-1 (B), and ICAM-1 (C) on the mouse endothelial cell surface. Biotin-conjugated primary antibodies and the [125I]-streptavidin system, as described in “Materials and methods,” were used to determine surface expression of E-selectin, VCAM-1, and ICAM-1 on untreated or TNF-α–treated (mouse TNF-α, 3 hours) cultured mouse aortic ECs isolated from WT, p75−/−, or p55−/− mice. (D) Mouse cremaster muscles treated with mouse TNF-α (3 hours) or vehicle (PBS) were dissected and frozen using OCT. Cryostat sections were immunostained for ICAM-1 as described in “Materials and methods.” Arrowheads show ICAM-1–stained ECs. (E) Cultured WT mouse aortic ECs were treated with mouse TNF-α (2 ng/mL, ▪) or human TNF-α (2 ng/mL, □) and after 2 hours total RNA was isolated and real-time PCR was performed as described in “Materials and methods.” Values are expressed as fold induction with respect to controls. Error bars indicate SD.
We performed immunohistochemical analysis of the mouse cremaster microvessels in the presence or absence of TNF-α, and observed that the adhesion molecule expression pattern was similar to that in cultured mouse aortic ECs. ICAM-1 expression in the WT, p55−/−, and p75−/− cremaster muscle microvessels using immunohistochemistry is shown in Figure 2D. WT muscle treated with TNF-α showed a substantial increase in ICAM-1 expression (Figure 2Di vs Figure 2Dii), whereas p75−/− cremaster muscle treated with TNF-α (Figure 2Div) did not show ICAM-1 expression. Further, compared with WT, TNF-α–induced ICAM-1 expression was robust in the p55−/− cremaster muscle postcapillary venule ECs. TNF-α–treated WT tissue showed VCAM-1 and E-selectin expression, whereas no expression was observed in TNF-α–treated p55−/− or p75−/− tissue (data not shown). Further, we took advantage of the fact that human TNF-α is not as efficient as mouse TNF-α in activating the mouse p75 receptor,19 to substantiate the critical role of this receptor in E-selectin, VCAM-1, and ICAM-1 induction. As shown in Figure 2E, in WT mouse ECs, human TNF-α was significantly less efficient in its induction of E-selectin, VCAM-1, and ICAM-1 mRNA compared with mouse TNF-α. In contrast, induction of E-selectin, VCAM-1, and ICAM-1 mRNA expression in human umbilical vein ECs by mouse and human TNF-α was similar (data not shown). These cell-surface expression studies on cultured ECs further support the critical role of the p75 receptor in TNF-α–mediated E-selectin, VCAM-1, and ICAM-1 expression.
p75 is essential for TNF-α–induced leukocyte rolling and firm adhesion
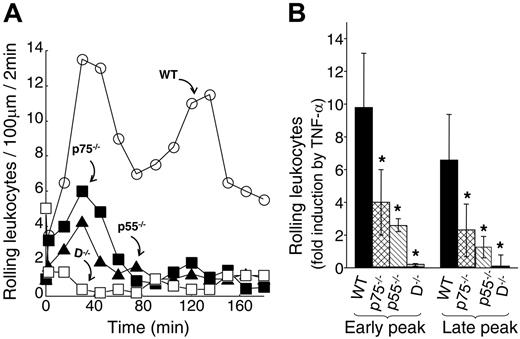
Using cultured mouse ECs, we have identified a unique leukocyte adhesion molecule expression pattern when either p55 or p75 TNF-α receptors are deleted. We have identified that the p75 TNF-α receptor is critical for TNF-α–induced expression of E-selectin, VCAM-1, and ICAM-1. To directly test the role of p75 on TNF-α–induced leukocyte-EC interaction, we performed an intravital microscopy-based inflammation model on the cremaster muscle-flap of WT, p75−/−, and p55−/− mice. In WT mice, TNF-α (2 ng/mL) induced 2 temporal peaks of leukocyte rolling (one at 30 to 60 minutes and the other at 90 to 135 minutes; Figure 3A). We observed a substantial reduction in both peaks of leukocyte rolling when either one of the receptors was absent. The early peak of leukocyte rolling in a viewing segment of 100 μm was reduced from 9.8-fold ± 2.5-fold induction in WT to 2.6-fold ± 0.4-fold (P = .02) and 4.1-fold ± 2-fold (P < .05) in p55−/− and p75−/− mice, respectively (Figure 3B). The late peak of leukocyte rolling was significantly reduced both in p55−/− and in p75−/− mice: 6.6-fold ± 2.8-fold in WT to 1.8-fold ± 0.9-fold (P < .05) in p55−/− and 2.3-fold 1.8-fold in p75−/− mice. Compared with the WT mice, the rolling flux, calculated as the number of rolling leukocytes per minute passing a reference point in the microvessel, was also substantially reduced in both p55−/− and p75−/− mice. During the early peak, the rolling flux in the WT mice was 6.1 min−1 ± 1.9 min−1 versus 3.3 min−1 ± 0.6 min−1 and 3.9 min−1 ± 1.5 min−1 in the p55−/− and p75−/− mice, respectively. In the late peak, the rolling flux in the WT mice was 5.3 min−1 ± 0.8 min−1 versus 2.2 min−1 ± 0.4 min−1 and 3.1 min−1 ± 1.1 min−1 in the p55−/− and p75−/− mice, respectively. Double-null mice (D−/−) did not respond to TNF-α. These results suggest that both TNF-α receptors contribute to the complete induction of leukocyte rolling in TNF-α–activated ECs.
Both TNF-α receptors are critical for TNF-α–induced leukocyte rolling. (A) Representative leukocyte rolling profile following mouse TNF-α treatment of WT (○), p75−/− (▪), p55−/− (▴), and D−/− (□) mice. One hour after surgery, TNF-α (2 ng/mL in 25 μL) was applied directly to a 25-mm2 area of the cremaster muscle flap. Number of rolling leukocytes in a 100-μm viewing segment was counted in a time window of 2 minutes. Control counts were obtained prior to TNF-α application. (B) Average fold change in early-phase and late-phase rolling leukocytes upon TNF-α treatment of WT (▪), p75−/− (⊡), p55−/− (▧), and D−/− (⊡) mice. Group size equals 4, error bars represent standard deviations (SD), the asterisk indicates statistical significance (P < .05). Leukocyte flux was calculated as described in “Materials and methods” and as follows: (i) Early peak: WT = 6.1 min−1 ± 1.9 min−1, p55−/−= 3.3 min−1 ± 0.6 min−1, p75−/−= 3.9 min−1 ± 1.5 min−1; (ii) Late peak: WT = 5.3 min−1 ± 0.8 min−1, p55−/−= 2.2 min−1 ± 0.4 min−1, p75−/−= 3.1 min−1 ± 1.1 min−1. Error bars indicate SD.
Both TNF-α receptors are critical for TNF-α–induced leukocyte rolling. (A) Representative leukocyte rolling profile following mouse TNF-α treatment of WT (○), p75−/− (▪), p55−/− (▴), and D−/− (□) mice. One hour after surgery, TNF-α (2 ng/mL in 25 μL) was applied directly to a 25-mm2 area of the cremaster muscle flap. Number of rolling leukocytes in a 100-μm viewing segment was counted in a time window of 2 minutes. Control counts were obtained prior to TNF-α application. (B) Average fold change in early-phase and late-phase rolling leukocytes upon TNF-α treatment of WT (▪), p75−/− (⊡), p55−/− (▧), and D−/− (⊡) mice. Group size equals 4, error bars represent standard deviations (SD), the asterisk indicates statistical significance (P < .05). Leukocyte flux was calculated as described in “Materials and methods” and as follows: (i) Early peak: WT = 6.1 min−1 ± 1.9 min−1, p55−/−= 3.3 min−1 ± 0.6 min−1, p75−/−= 3.9 min−1 ± 1.5 min−1; (ii) Late peak: WT = 5.3 min−1 ± 0.8 min−1, p55−/−= 2.2 min−1 ± 0.4 min−1, p75−/−= 3.1 min−1 ± 1.1 min−1. Error bars indicate SD.
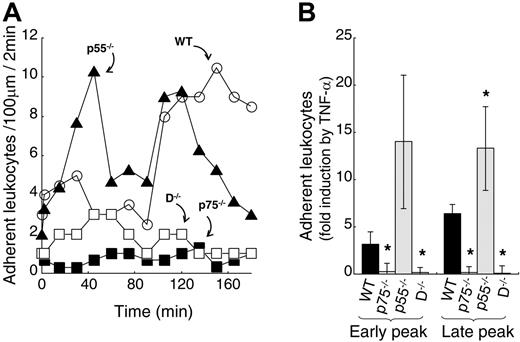
Leukocyte recruitment to sites of inflammation is a multistep process beginning with leukocyte capture and rolling, and leading to firm adhesion and transmigration. To investigate the role of TNF-α receptors in TNF-α–induced firm adhesion, we measured leukocytes that remained stationary for at least 30 seconds in the viewing segment of the venule (100 μm) for a time window of 2 minutes. As shown in Figure 4A, in WT mice, TNF-α induced 2 temporal peaks of leukocyte firm adhesion to ECs of postcapillary venules. A relatively small but consistent early induction of leukocyte adhesion was observed between 30 and 60 minutes following TNF-α treatment, and a defined second late peak occurred between 90 and 135 minutes. The majority of adherent leukocytes (> 95%) were neutrophils, as determined by cellular morphology. In p75−/− mice, TNF-α treatment did not increase leukocyte adhesion. In contrast, in p55−/− mice showed a robust leukocyte adhesion. The results of Figure 4B demonstrate the average number of leukocytes that have undergone firm adhesion, upon TNF-α treatment, from 4 independent experiments. In WT mice, TNF-α–induced leukocyte adhesion was 6.4-fold ± 0.9-fold during the late adhesion phase. In p75−/− mice, we did not observe TNF-α–induced leukocyte firm adhesion during either the early or late adhesion phases. In contrast, TNF-α treatment resulted in a 4.3- and 2-fold up-regulation of leukocyte firm adhesion in p55−/− mice compared with WT mice at early and late phases, respectively. Recently, Vielhauer et al14 reported a similar negative effect of p55 on the infiltration of a subset of leukocytes in immune serum-induced glomerulonephritis. Our results suggest that p75 alone is responsible for TNF-α–induced leukocyte firm adhesion, and simultaneous p55 activation induces a negative effect.
The p75 TNF-α receptor is essential for TNF-α–induced firm adhesion. (A) Representative leukocyte firm adhesion profile following mouse TNF-α treatment of WT (○), p75−/− (▪), p55−/− (▴), and D−/− (□) mice. One hour after surgery, TNF-α (2 ng/mL in 25 μL) was applied directly to a 25-mm2 area of the cremaster muscle flap. Number of adhering leukocytes was counted in a time window of 2 minutes in a 100-μm viewing segment. Control counts were obtained prior to TNF-α application. (B) Average fold change with respect to control in early- and late-phase firm adhesion of leukocytes upon TNF-α treatment of WT (filled bar), p75−/− (spotted bar), p55−/− (hatched bar), and D−/− (shaded bar) cremasters. Group size equals 4, error bars represent SD, the asterisk indicates statistical significance (P < .05).
The p75 TNF-α receptor is essential for TNF-α–induced firm adhesion. (A) Representative leukocyte firm adhesion profile following mouse TNF-α treatment of WT (○), p75−/− (▪), p55−/− (▴), and D−/− (□) mice. One hour after surgery, TNF-α (2 ng/mL in 25 μL) was applied directly to a 25-mm2 area of the cremaster muscle flap. Number of adhering leukocytes was counted in a time window of 2 minutes in a 100-μm viewing segment. Control counts were obtained prior to TNF-α application. (B) Average fold change with respect to control in early- and late-phase firm adhesion of leukocytes upon TNF-α treatment of WT (filled bar), p75−/− (spotted bar), p55−/− (hatched bar), and D−/− (shaded bar) cremasters. Group size equals 4, error bars represent SD, the asterisk indicates statistical significance (P < .05).
Both the p55 and p75 receptors play a role in TNF-α–induced transmigration
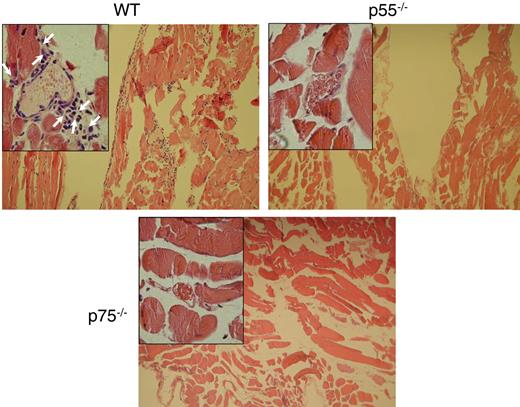
Leukocyte firm adhesion is considered to be a prerequisite for leukocyte transmigration through the EC layer of the vessel wall to the interstitium.20 Therefore, we also determined TNF-α–induced transmigration using the mouse cremaster muscle model. We analyzed transmigrated leukocytes in the cremaster tissue 3 hours after TNF-α application. Hematoxylin-and-eosin staining of the cremaster sections showed dramatic leukocyte accumulation in the interstitial space of the WT mice following TNF-α treatment (Figure 5). This infiltration was significantly reduced in either p75−/− or p55−/− mice, emphasizing the importance of both p75 and p55 TNF-α receptors in TNF-α–induced transmigration of leukocytes.
Both TNF-α receptors are critical for TNF-α–induced leukocyte transmigration. Cumulative effect of TNF-α on leukocyte infiltration to the interstitial space of the cremaster muscle. Following 3 hours of TNF-α treatment, acetone-fixed cremaster specimens were subjected to hematoxylin-and-eosin staining to observe infiltrated leukocytes. Arrows show the infiltrated leukocytes.
Both TNF-α receptors are critical for TNF-α–induced leukocyte transmigration. Cumulative effect of TNF-α on leukocyte infiltration to the interstitial space of the cremaster muscle. Following 3 hours of TNF-α treatment, acetone-fixed cremaster specimens were subjected to hematoxylin-and-eosin staining to observe infiltrated leukocytes. Arrows show the infiltrated leukocytes.
EC defect appears to be responsible for the aberrant leukocyte-EC interaction in TNF-α receptor-null mice
Topical application of TNF-α to the cremaster muscle tissue was employed to localize TNF-α and prevent or minimize its systemic effects. However, microinfusion of the topically applied TNF-α into the blood stream and activation of leukocytes, or alternatively, a developmental defect in leukocyte maturation in TNF-α receptor-null mice, could not be completely ruled out. Analysis of the differential blood cells (WBC counts) revealed that there were no significant differences among the mice of various genotypes employed (Table 1), indicating that there were no gross developmental defects in leukocytes in the null mice.
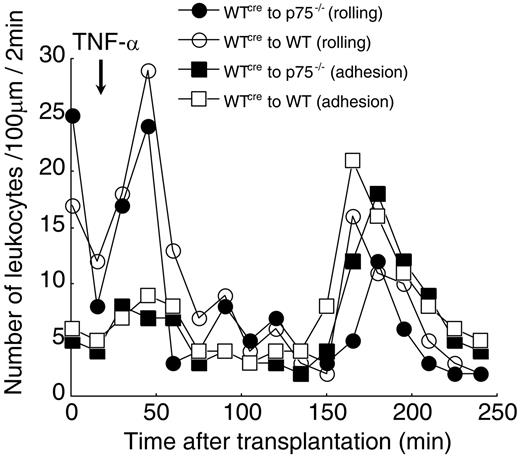
Further, we performed a cremaster transplantation experiment in which we transplanted WT cremaster muscle to p75−/− mice and determined the TNF-α–induced leukocyte rolling and adhesion profile. TNF-α treatment of WT cremaster muscle that had been transplanted to p75−/− mice or WT cremaster muscle transplanted to WT mice (control) gave identical leukocyte rolling and adhesion profiles (Figure 6). Further, the TNF-α–induced leukocyte rolling and adhesion, in the transplants, were comparable in profile and quantity to that in the TNF-α–treated WT nontransplanted mouse cremaster preparation. Since in the transplantation of WT cremaster muscle to p75−/− mice, the leukocytes originated from receptor-null mice and responded similarly to WT, we conclude that the impairment in EC-leukocyte interaction in p75−/− is most likely due to EC dysfunction.
TNF-α activation of ECs, not leukocytes, appears to be responsible for the induction of leukocyte-EC interaction upon TNF-α treatment. WT cremaster muscle was transplanted to either p75−/− or WT mice. Following cremaster transplantation, the animal was kept for an equilibration period of 15 minutes. TNF-α (2 ng/mL, 25 μL) was applied directly to a 25-μm2 area of the cremaster muscle flap. Number of rolling and adherent leukocytes was counted in a time window of 2 minutes, in a 100-μm viewing segment. Control counts were obtained prior to TNF-α application. Values shown are the average number of rolling leukocytes or adhering leukocytes from 3 independent experiments. (○) WT cremaster to WT mice (rolling), (•) WT cremaster to p75−/− mice (rolling), (□) WT cremaster to WT mice (adhesion), (▪) WT cremaster to p75−/− mice (adhesion).
TNF-α activation of ECs, not leukocytes, appears to be responsible for the induction of leukocyte-EC interaction upon TNF-α treatment. WT cremaster muscle was transplanted to either p75−/− or WT mice. Following cremaster transplantation, the animal was kept for an equilibration period of 15 minutes. TNF-α (2 ng/mL, 25 μL) was applied directly to a 25-μm2 area of the cremaster muscle flap. Number of rolling and adherent leukocytes was counted in a time window of 2 minutes, in a 100-μm viewing segment. Control counts were obtained prior to TNF-α application. Values shown are the average number of rolling leukocytes or adhering leukocytes from 3 independent experiments. (○) WT cremaster to WT mice (rolling), (•) WT cremaster to p75−/− mice (rolling), (□) WT cremaster to WT mice (adhesion), (▪) WT cremaster to p75−/− mice (adhesion).
Discussion
Earlier studies have, in general, reported that most of the cellular effects induced by TNF-α, including the inflammatory response, were mediated by the TNF-α p55 receptor.3,21 It has also been reported that p55, not p75, is important for expression of leukocyte adhesion molecules induced by TNF-α in ECs, a critical event in the initiation of inflammatory responses.1,12,22 Most of these studies have used receptor-specific antibodies or TNF-α muteins (mutated versions of TNF-α proteins that activate either the p55 or p75 receptor specifically) to differentially activate either the p55 or the p75 receptor in cultured ECs.23–25 Although the binding affinity of these reagents significantly favored a specific receptor, cross-reactivity cannot be completely ruled out. Recently, in cells lacking p55, TNF-α treatment was shown to result in activation of transcription factors and intracellular kinases known to induce leukocyte adhesion molecules,26,27 implicating the role of alternate TNF-α receptors in the expression of leukocyte adhesion molecules. In this study, we have used mouse ECs lacking either one or both TNF-α receptors to test the individual roles of p75 and p55 in TNF-α–induced expression of leukocyte adhesion molecules. We have determined that TNF-α–induced P-selectin expression was mediated by the p55 receptor alone, whereas expression of PECAM, KC, and JE required both p55 and p75, though p55 was predominant. Using mRNA measurements, cell-surface expression, and immunohistochemical studies, we have demonstrated for the first time that both receptors were critical for TNF-α–induced E-selectin and VCAM-1 expression, whereas p75-mediated signals are sufficient for ICAM-1 expression in ECs.
Multiple in vivo inflammatory models have recently been published employing genetically modified mice with one or both of the TNF-α receptors either deleted or overexpressed.28–30 Results from these studies are inconclusive in that essential roles for p75 or p55 and/or both in inducing the inflammatory response were reported. One report suggests that p75 receptor activity is antagonistic to p55 receptor activity in mediating inflammation.15 For the most part, these studies measured infiltrating leukocytes as a biologic read-out of the inflammatory response, which is a relatively late event in this multistep process. In this study, we took an alternative approach in which we analyzed the role of the TNF-α receptors in 3 distinct steps of the inflammatory response; namely, leukocyte rolling, firm adhesion, and transmigration in vessels in real-time in live animals. This 3-step experimental model helped us delineate the roles played by the individual receptors in each of these cellular processes. This approach led us to discover the critical role of p75 in TNF-α–induced leukocyte rolling, firm adhesion, and transmigration. Furthermore, we identified a negative effect on leukocyte adhesion when the p55 receptor was activated simultaneously. Vielhauer et al14 reported a negative effect of p55 on the infiltration of αβ T cells, γδ T cells, and macrophages in immune serum-induced glomerulonephritis. They observed an increase in the renal infiltration of T cells and macrophages in p55−/− mice (compared with WT mice) at 21 and 42 days following experimental renal injury and suggested an aberrant apoptosis in p55−/− mice as one of the possible mechanisms. In our study, we observed an increase in leukocyte adhesion within 2 hours of TNF-α treatment and no significant change in differential WBC counts in p55−/− mice (Table 1). Thus, we do not consider aberrant apoptosis of normal leukocytes as a possible mechanism for increased leukocyte firm adhesion in p55−/− mice. We are currently investigating alternate mechanisms for this increased leukocyte activity.
We performed cremaster muscle transplantation experiments to distinguish whether defects in ECs versus leukocytes were the cause of the aberrant leukocyte firm adhesion in the TNF-α receptor-null mice. When we transplanted WT cremaster to p75−/− mice, we observed a complete restoration of TNF-α–induced leukocyte firm adhesion. Since WT cremaster contained ECs with intact p75, we concluded that the defect in EC-leukocyte interactions in p75−/− mice was most likely due to EC dysfunction rather than leukocyte abnormalities, though we cannot rule out a role of other resident cells, such as smooth muscle cells. We demonstrate herein that in cultured ECs, the p75 TNF-α receptor plays an important role in the TNF-α induction of leukocyte adhesion molecules such as E-selectin, VCAM-1, ICAM-1, and, to a lesser extent, KC and JE. Using gene knock-out mice and specific blocking antibodies, others have shown that EC expression of such adhesion molecules and chemokines is critical for leukocyte rolling and firm adhesion.20,31
The molecular mechanisms underlying the early and late phases of leukocyte-EC interactions are well accepted to be quite distinct.32 P-selectin has been shown to be critically involved in leukocyte rolling.33 Upon stimulation of ECs by various agonists, P-selectin stored in Weibel-Palade bodies moves rapidly to the cell surface, accounting in part for the early phase, whereas P-selectin is stably induced at the transcriptional level during the late phase.20 Although we have determined that P-selectin mRNA was essentially unchanged in cultured p75−/− ECs at 2 hours after TNF-α treatment, the possibility of early leukocyte rolling via the activation of the stored P-selectin by p75 exists. In the late phase, we identified that TNF-α–induced P-selectin expression was essentially unchanged in the p75−/− mouse, therefore, a P-selectin–independent leukocyte rolling mechanism or mechanisms must have been impaired in these mice. Multiple in vivo studies have shown that E-selectin is required for TNF-α–induced leukocyte rolling.34,35 ICAM-1 has also been shown to be critical for selectin-mediated rolling of leukocytes during TNF-α–induced inflammation in mouse cremaster microvessels.36 We observed that TNF-α–induced E-selectin and ICAM-1 expression were severely impaired in p75−/− mice, which likely contributes to the observed reduction in leukocyte rolling in the late phase. Similarly, TNF-α–induced endothelial expression of E-selectin, ICAM-1, VCAM-1, and E-selectin are critical for late-phase leukocyte firm adhesion,32,37 and loss of expression of these molecules in the vessel wall of p75−/− mice is very likely responsible for the reduction in leukocyte-endothelial interaction in p75−/− mice. Taken together, we propose that p75-mediated EC expression of leukocyte adhesion molecules is critical for the regulation of TNF-α–induced leukocyte-EC interaction.
In conclusion, using TNF-α receptor-null ECs, we have identified that p75 is essential for TNF-α–induced leukocyte adhesion molecules in endothelium. Furthermore, we have demonstrated for the first time that p75, in the endothelium, is critically involved in the TNF-α–induced leukocyte-endothelial interaction. As p55 has been implicated as the primary TNF-α receptor in mediating caspase activation, cell death, and subsequent defense against microbial infection, p75 presents a promising therapeutic alternative with the potential of avoiding many of the common side effects associated with drugs targeting TNF-α or p55.
Authorship
Contribution: U.M.C. designed the research, performed research, analyzed the data, and wrote the paper; M.S. made a vital contribution to the methods used and analyzed the data; M.U., L.Y., J.B., K.O., and Z.Z. performed the research; E.P. contributed vital reagents; P.H.H. analyzed the data and gave insights to the data; M.P. contributed vital new technology; P.E.D. designed research, analyzed the data, and contributed in writing the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Paul E. DiCorleto, Department of Cell Biology, Lerner Research Institute and Cleveland Clinic Lerner College of Medicine of Case Western Reserve University, Cleveland Clinic Foundation, 9500 Euclid Ave, Cleveland, OH 44195; e-mail::dicorlp@ccf.org.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We thank J. J. Peschon (Amgen, Seattle, WA) for providing the TNF-α receptor-null mice, and L. Mavrakis, B. Davis, and P. Daher for mouse EC isolation and cell-culture assistance. Human umbilical vein endothelial cells were harvested through the Birthing Services Department at the Cleveland Clinic Foundation and the Perinatal Clinical Research Center at the Cleveland Metrohealth Hospital.
This work was supported by National Institutes of Health (NIH) grant HL29582 (P.E.D.), and NIH Research Center Award RR-00080.

![Figure 2. The p75 TNF-α receptor is critical for TNF-α–induced E-selectin, VCAM-1, and ICAM-1 expression on mouse ECs. Quantification of E-selectin (A), VCAM-1 (B), and ICAM-1 (C) on the mouse endothelial cell surface. Biotin-conjugated primary antibodies and the [125I]-streptavidin system, as described in “Materials and methods,” were used to determine surface expression of E-selectin, VCAM-1, and ICAM-1 on untreated or TNF-α–treated (mouse TNF-α, 3 hours) cultured mouse aortic ECs isolated from WT, p75−/−, or p55−/− mice. (D) Mouse cremaster muscles treated with mouse TNF-α (3 hours) or vehicle (PBS) were dissected and frozen using OCT. Cryostat sections were immunostained for ICAM-1 as described in “Materials and methods.” Arrowheads show ICAM-1–stained ECs. (E) Cultured WT mouse aortic ECs were treated with mouse TNF-α (2 ng/mL, ▪) or human TNF-α (2 ng/mL, □) and after 2 hours total RNA was isolated and real-time PCR was performed as described in “Materials and methods.” Values are expressed as fold induction with respect to controls. Error bars indicate SD.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/109/5/10.1182_blood-2006-05-020875/4/m_zh80050709080002.jpeg?Expires=1767724796&Signature=3t04lzHeeSJj3XhTFh1bN8qfWMP5LPBa0RGu8-FDNNp5UwbmeE06TxyZ~UuIcW11sXoh4LXe6CONvMrbP1cBhhYooZFFiHv8ChoJiYf3CKsRBUNnjA5CIe-~lXd4YMPTUVMiJD8PpbeuU~8lNxVEnwlp656yu6n1lCo1F8QxDIQcAvp5XQsdj4ud5YFrbzzG~pBn4wf2hd4OAGwMeNeTJiUvqnf5kp7S-w1fhOVJQlSwlseB4weFqrXgQMluVpI8z-ZFAGDIzwMC9PoW7besL4k~CKGrgn~ZZThvRF~bPDtV9wkodrQZsWLdPVXBAvYWGDn5QmK7jXhG8S1KQ0tUiw__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)