Abstract
We have recently demonstrated that IgDhi B cells can occupy an extravascular perisinusoidal niche in the bone marrow in addition to the well-established follicular niche in conventional secondary lymphoid organs. The spleen has long been considered to be the site at which newly formed B lymphocytes mature into IgDhi naive recirculating B cells, but the existence of mutant mice that have selectively lost mature B cells in the bone marrow prompted an examination of B-cell maturation at this latter site. Following a single pulse of BrdU in intact mice, sequential labeling of more mature B-cell populations in the bone marrow suggested ongoing maturation at this site. Further evidence for B-cell maturation in the bone marrow was obtained from analyses of transitional B cells in splenectomized lymphotoxin α-deficient mice that lack all secondary lymphoid organs. In these mice, antibody-secreting cells recognizing multivalent antigens were also observed in the bone marrow following an intravenous microbial challenge. These data suggest that newly formed B cells mature into IgDhi B cells simultaneously in the spleen and the bone marrow and establish in a stringent manner that humoral immune responses can be initiated in situ in the bone marrow.
Introduction
Newly formed B cells emigrate from the bone marrow and home initially to the spleen1 where they mature via transitional stages primarily into long-lived follicular B cells, and where they also differentiate into marginal zone (MZ) B cells.2–7 Naïfive follicular B cells express high levels of surface IgD and CD23 and have the ability to recirculate, entering and exiting follicular niches in secondary lymphoid organs in search of antigen.8,9 Although mature B cells have long been described in the bone marrow,10,11 our recent studies suggest that the bone marrow may provide an alternative perisinusoidal niche for follicular phenotype IgDhi B cells, wherein they have ready access to blood-borne pathogens and can thus contribute to T-independent IgM responses directed against such organisms.12 These studies describing bone marrow antibody-secreting cells generated in response to blood-borne pathogens were performed on splenectomized mice, but it remained formally possible that the IgM plasma cells in the bone marrow observed in these circumstances could have migrated to the bone marrow from some other secondary lymphoid organ
Immature B cells in the bone marrow acquire the ability to migrate via the blood specifically to the spleen, presumably because these cells receive inhibitory signals that attenuate their ability to respond to chemokines and to thus home to lymph nodes or inflammatory sites.13 The unique architectural organization of the spleen is important for MZ B-cell development and survival,5,14 and the evolutionary requirement for immature B cells to home to this organ may be linked to the need for such cells to differentiate into MZ B cells. However, the teleologic rationale for mature follicular B-cell maturation to apparently occur only in the spleen, as is generally assumed to be the case, is unclear. It is reasonable to entertain the possibility that B-cell maturation might also occur in the bone marrow, particularly because immature B cells are generated there, and because the perisinusoidal bone marrow niche does contain a large number of B cells with an IgDhi phenotype.12 Indeed, in splenectomized animals and humans, mature follicular phenotype B cells are abundant in lymph nodes and in the perisinusoidal bone marrow niche, and they presumably mature at some site distinct from the spleen, most likely within the bone marrow compartment itself.
Another reason to explore the maturation of IgDhi B cells in the bone marrow relates to the poorly understood issue of the loss of these cells in certain mutant mice. Mice deficient in Aiolos, CD22, and CD72, for instance, all lose mature B cells in the bone marrow but preserve their phenotypic counterparts in other conventional secondary lymphoid organs.15–19 These null mice lack distinct negative regulators of the BCR. Mice lacking VCAM-1 also lack B cells in the bone marrow,20,21 suggesting that VCAM-1 may contribute to entry into or retention within this compartment. Although it has been suggested that CD22 might facilitate homing to the bone marrow by recognizing α2,6-linked sialic acid containing ligands on the endothelium,22 this proposed mechanism seems unlikely to be important because B cells expressing mutant CD22 that cannot bind α2, 6-linked sialic acid have been shown to home to the bone marrow as efficiently as wild-type B cells.23 A potential common link bringing together some of these mutant mice is an enhancement in BCR signal strength, but why such an augmentation of antigen receptor signaling may contribute to the loss of IgDhi bone marrow B cells remains unclear. To begin to explore the genetic regulation of mature B-cell loss in the bone marrow, it is important to determine whether maturation of these cells might occur in this compartment itself, because this maturation process, distinct from a parallel process occurring in the spleen, could possibly be influenced by specific mutations. Genetic defects linked to perisinusoidal B-cell loss could therefore reflect defects in B-cell maturation specifically in the bone marrow, represent defective homing of these cells to the bone marrow, or reflect aberrant and exaggerated egress of mature B cells from this site. A critical exploration of whether the maturation of IgDhi B cells can normally occur in the bone marrow is clearly called for.
Materials and methods
Mice
Eight- to 12-week old BALB/c, C57BL/6, and lymphotoxin α (LTα)–deficient mice were obtained from Jackson Laboratories (Bar Harbor, ME). Aly/aly mice24 were kindly made available by Dr Gilles Benichou at Massachusetts General Hospital (MGH; Boston, MA). Age- and sex-matched mice were used in all experiments. Animal procedures were cleared by the Subcommittee on Research Animal Care at MGH.
Continuous bromodeoxyuridine labeling
Continuous labeling with bromodeoxyuridine (BrdU; Sigma, St Louis, MO) was performed as described earlier.25 Briefly 0.25 mg/mL BrdU and 2 mg/mL glucose was administered in drinking water for 22 days, and the decay in labeling was followed for 4 weeks after cessation of BrdU administration. The BrdU decay data curves were generated by the least squares nonlinear regression analysis method using MacCurveFit 1.5 (Kevin Raner Software, Mount Waverly, VC, Australia).
Pulse BrdU labeling
Pulse labeling was achieved by administering a single intraperitoneal injection of BrdU (5 mg in 1 mL), followed by the analysis of bone marrow and peripheral B-cell populations.
Antibodies, staining, and flow cytometry
The following murine monoclonal antibody conjugates were used: allophycocyanin (APC) and Cy-chrome-RA3-6B2 (anti-CD45R/B220, rat IgG2a, κ), fluorescein isothiocyanate (FITC)–DS-1 and R-PE-DS-1 (anti-IgMa [Igh-6a], mouse IgG1b), R-PE/biotinylated (BI)–R6-60.2 (anti-IgM, rat IgG2a), FITC/BI-AMS 9.1 (anti-IgDa [Igh-5a], mouse IgG2bb), BI/FITC-11-26c.2a (anti-IgD, rat IgG2a, κ), FITC-B44 (anti-BrdU, mouse IgG1, κ), FITC-AA4.1 (early B-lineage antibody, rat IgG2bκ biotinylated (BI)–J2 (anti-CD95, hamster IgG), and unlabeled 2.4G2 (anti-CD16/CD32 [FcγIII/II receptor], rat IgG2b, κ, culture supernatant) all from BD PharMingen (San Diego, CA); APC-AA4.1 (eBiosciences, San Diego, CA); R-PE-1B4B1 (anti-IgM, rat IgG) and BI-11-26 (anti-IgD, rat IgG2a, all from Southern Biotechnology, Birmingham, AL; FITC-peanut agglutinin (PNA; Vector Laboratories, Burlingame, CA); biotinylated antibodies were revealed by streptavidin-fluorochrome conjugates (BD PharMingen).
Flow cytometry was performed using standard methodology as described elsewhere.25,26 Intracellular staining to detect BrdU incorporated into DNA was performed as described earlier.25 Absolute number calculations were made as described before.12
The cytometers used were: Epics Elite ESP and FC500 (both from Coulter, Hialeah, FL), MoFlo (DakoCytomation, Fort Collins, CO), and FACSAria and LSR II (both from Beckton Dickinson, San Jose, CA). Gates in the spleen were set according to Hardy et al8 and Cariappa et al,12 and the gates in the bone marrow were set according to Li et al,27 Hendriks et al,28 and Cariappa et al.12 Processed samples were analyzed using Epics Elite, and RXP (both from Beckman Coulter) and FloJo v8.1.1 (Treestar, San Carlos, CA) analysis software.
Bacterial challenge studies
ELISPOT assays
Enzyme-linked immunospot (ELISPOT) assays were performed as described earlier12 using Salmonella flagellin (2.5 μg/mL, a kind gift from Dr Bobby J. Cherayil at MGH), and lipopolysaccharide from S typhimurium (LPS, 50 μg/mL; List Biological Laboratories, Campbell, CA).
Statistical analyses
P values for differences between groups were determined by the Mann-Whitney U test using StatView version 5.0.1 (SAS, Cary, NC). For the BrdU decay data points, curves of best fit by the least squares method were generated using MacCurveFit 1.5 (Kevin Raner Software).
Results
Transitional B cells in the bone marrow
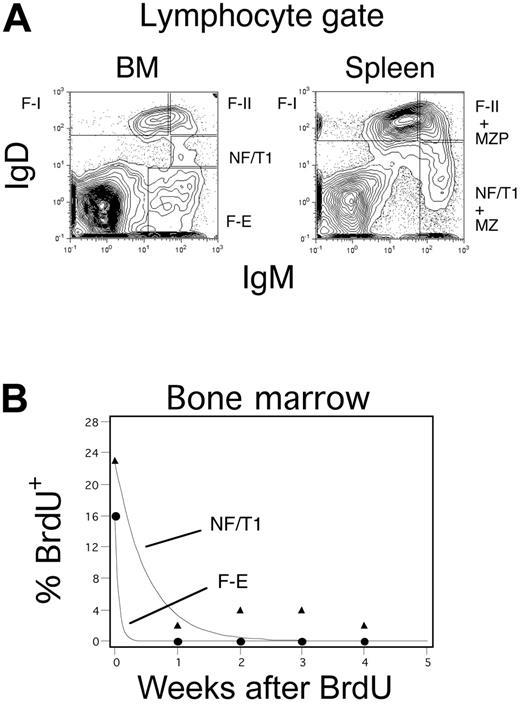
We have delineated B-cell populations using IgM, IgD, and CD21 or by using these markers in combination with the AA4.1 antibody. We initially confirmed that newly formed/transitional type 1 (NF/T1) IgMhi IgDlo/−CD21lo B cells as well as IgMhiIgDhiCD23hiCD21int (F-II) and IgDhiIgMlo (F-I) follicular phenotype B cells are found both in the bone marrow as well as in the spleen (Figure 1A).6,8,10,28,30 Although immature B cells in the bone marrow are occasionally referred to as B220loIgMlo B cells, we delineate fraction E/immature B cells in the bone marrow, using criteria used by Li et al27 and Hendriks et al,28 as IgM+IgD− cells to distinguish them from IgMhiIgDlo NF/T1 cells. As seen in Figure 1B, in long-term BrdU labeling and decay experiments, NF/T1 B cells have a slightly longer half-life than fraction E B cells, supporting the view that these may represent distinct stages in a continuum of B-cell development.
Categorization of B cells in the bone marrow and spleen. (A) B-cell populations in the bone marrow and spleen analyzed on the basis of surface IgM and IgD expression. Categorizations depend on analysis of CD21 expression as well (Figure 4A shows the CD21 levels in the bone marrow). F-I refers to IgDhiIgMloCD21int follicular B cells and F-II to IgDhiIgMhiCD21int follicular B cells. NF/T1 refers to IgMhiIgDloCD21lo B cells in the bone marrow and IgMhiIgDlo/− CD21lo B cells in the spleen. Fraction E refers to IgM+IgD− B cells in the bone marrow. MZP B cells are IgMhiIgDhiCD21hi presumed MZ precursors, and MZ B cells are IgMhiIgDlo/− CD21hi B cells both of which are present only in the spleen (for gating strategy, see Hardy et al,8 Cariappa et al,12 Li et al,27 and Hendriks et al28 ). (B) Regression analysis of BrdU decay data (from a continuous labeling experiment) reveals that NF/T1 B cells and fraction E B cells have distinct half-lives.
Categorization of B cells in the bone marrow and spleen. (A) B-cell populations in the bone marrow and spleen analyzed on the basis of surface IgM and IgD expression. Categorizations depend on analysis of CD21 expression as well (Figure 4A shows the CD21 levels in the bone marrow). F-I refers to IgDhiIgMloCD21int follicular B cells and F-II to IgDhiIgMhiCD21int follicular B cells. NF/T1 refers to IgMhiIgDloCD21lo B cells in the bone marrow and IgMhiIgDlo/− CD21lo B cells in the spleen. Fraction E refers to IgM+IgD− B cells in the bone marrow. MZP B cells are IgMhiIgDhiCD21hi presumed MZ precursors, and MZ B cells are IgMhiIgDlo/− CD21hi B cells both of which are present only in the spleen (for gating strategy, see Hardy et al,8 Cariappa et al,12 Li et al,27 and Hendriks et al28 ). (B) Regression analysis of BrdU decay data (from a continuous labeling experiment) reveals that NF/T1 B cells and fraction E B cells have distinct half-lives.
Some IgDhiCD23hiCD21int cells express relatively high levels of AA4.1 and are therefore also transitional B cells as defined using criteria established by Allman et al.31 Although IgMhiIgDhiCD23hiCD21hi B cells, seen exclusively in the spleen, were also once categorized by some groups as transitional B cells, they are now widely recognized as specialized precursors of MZ B cells (MZP B cells).26,32–34 The presence of similar spectral populations of B cells in the spleen and bone marrow (with the exception of MZP and MZ B cells) suggested that IgDhi B cells may well mature in the bone marrow as well as in the spleen. We have previously established that IgDhiIgMlo mature follicular B cells in the spleen and bone marrow are equally long-lived.12
Bone marrow and splenic B cells mature contemporaneously
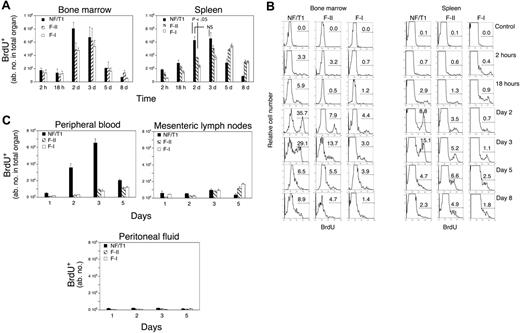
To ask whether mature bone marrow B cells are generated in situ contemporaneously with the maturation of B cells in the periphery, we injected mice with a single dose of BrdU, which primarily labels cycling pro-B and pre-B cells in a transient manner and dissipates extremely rapidly as these cells differentiate (data not shown). Further differentiation of these pulse-labeled cells was systematically followed by flow cytometric analysis of lymphocytes over an 8-day period in the bone marrow and the spleen (Figure 2A), as well as for 5 days in blood, lymph nodes, and peritoneum (Figure 2C). Our goal in these studies was to use a spike of BrdU incorporation in cycling cells as a marker to follow the short-term in vivo differentiation of a cohort of pulse-labeled B cells. This approach was not designed to examine half-lives. Robust labeling of pro-B cells was noted in the bone marrow, and up to 50% of all pro-B cells (Table 1) and distinct peaks of bone marrow and splenic mature B cells were sequentially labeled following the pulse. Table 1 shows that a single pulse of BrdU effectively labeled a large proportion of cells in the bone marrow and this labeling approach appears to be a valid method for following a cohort of cells.
B-cell differentiation in the bone marrow and spleen revealed by pulse labeling with BrdU of the pro-B/pre-B population. (A) A contemporaneous and wavelike progression of BrdU-labeled B cells was observed in the bone marrow and spleen. (B) Representative flow cytometric histograms following BrdU labeling in splenic and bone marrow B-cell fractions at various time points. For the 2-day time point, n = 7 mice. All other time points include results from 4 mice. Error bars represent ± SEM. (C) BrdU+ NF/T1 B cells are readily observed in the peripheral blood (left panel) but minimally in lymph nodes (center) or the peritoneal cavity (right).
B-cell differentiation in the bone marrow and spleen revealed by pulse labeling with BrdU of the pro-B/pre-B population. (A) A contemporaneous and wavelike progression of BrdU-labeled B cells was observed in the bone marrow and spleen. (B) Representative flow cytometric histograms following BrdU labeling in splenic and bone marrow B-cell fractions at various time points. For the 2-day time point, n = 7 mice. All other time points include results from 4 mice. Error bars represent ± SEM. (C) BrdU+ NF/T1 B cells are readily observed in the peripheral blood (left panel) but minimally in lymph nodes (center) or the peritoneal cavity (right).
We observed a major peak of BrdU-labeled immature IgM+IgDneg (fraction E) cells in the bone marrow at day 2 (Table 1). A smaller peak of IgMhiIgDlo (NF/T1) cells was also observed, which peaked around the same day in the spleen and bone marrow (Figure 2A). Peaks of IgMhiIgDhiCD21int (F-II) cells, and IgDhiIgMlo (F-I) cells, were observed later and contemporaneously in both the spleen and the bone marrow (Figure 2B). A decline in labeled cells is seen by day 5 in the bone marrow (Figure 2A-B), although more mature labeled cells persist in the spleen. These data suggest that newly generated B cells that have matured in the bone marrow may relatively rapidly egress this organ.
A major, albeit smaller, peak of BrdU-labeled NF cells in the peripheral blood was seen on day 3 (Figure 2C, left panel) confirming that the peripheral circulation is a major conduit for the transport of newly formed B cells between the bone marrow and spleen. In contrast, the mesenteric lymph nodes and peritoneal fluid showed very few BrdU+ NF/T1 cells (Figure 2C middle and right panels). Taken together, these results suggest that follicular B-cell maturation may occur contemporaneously in the spleen and bone marrow, although maturation exclusively in the spleen and rapid homing to the bone marrow (or the converse scenario) cannot be excluded based solely on these results.
Transitional B-cell populations are generated in the bone marrow in the absence of the spleen and lymph nodes
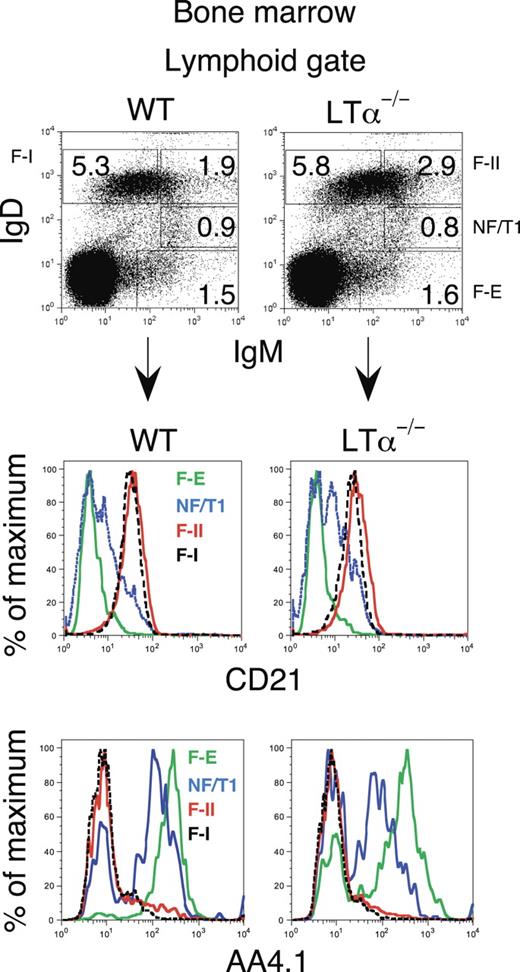
Although our BrdU studies confirmed that NF/T1 cells mainly travel from the bone marrow to the spleen, and not to lymph nodes (Figure 2), we recognized that it is formally possible that NF/T1 cells could perhaps mature in other peripheral lymphoid organs, and then return to the bone marrow. To more rigorously test this possibility, we studied 2 mutant mice that lack peripheral lymphoid tissue, LTα-deficient and aly/aly animals. LTα−/− mice lack lymph nodes and Peyer patches and are known to be permissive for B-cell maturation.36–38 We first established that bone marrow B-cell maturation was normal in the LTα−/− mice. As seen in Figure 3, the examination of B cells using AA4.1 and CD21 as indicators of maturation revealed that B cells in mice lacking LTα matured in a fashion similar to that observed in their wild-type counterparts. In both wild-type and LTα−/− animals the levels of AA4.1, a cell surface marker of immature and transitional B cells,31 were highest in fraction E followed by NF/T1 B cells and then IgDhiIgMhi (F-II) and IgDhiIgMlo (F-I) follicular phenotype B-cell populations (Figure 3, bottom row). These data supported the notion that the bone marrow contains transitional B cells and represents a distinct site of B-cell maturation.
Transitional B cells are observed in the bone marrow of wild-type and LTα−/− mice. B-cell maturation in the bone marrow proceeds normally in the LTα-deficient mouse as revealed using antibodies to IgM, IgD, and CD21. Comparison of the expression of AA4.1, a marker of recent B-cell generation, revealed similar numbers of transitional cells in the bone marrow in wild-type and LTα null mice. A representative experiment is shown; n = 3 mice in each group. WT indicates wild type.
Transitional B cells are observed in the bone marrow of wild-type and LTα−/− mice. B-cell maturation in the bone marrow proceeds normally in the LTα-deficient mouse as revealed using antibodies to IgM, IgD, and CD21. Comparison of the expression of AA4.1, a marker of recent B-cell generation, revealed similar numbers of transitional cells in the bone marrow in wild-type and LTα null mice. A representative experiment is shown; n = 3 mice in each group. WT indicates wild type.
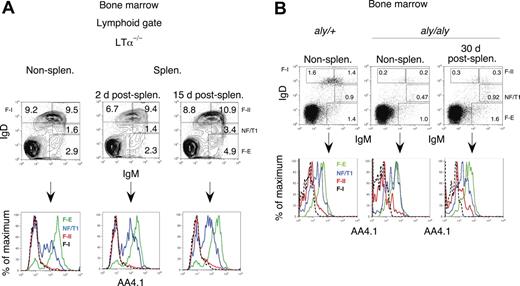
To establish that mature bone marrow B cells mature in situ and that the transitional B cells observed in the bone marrow do not represent cells that had migrated from the spleen, we splenectomized mutant mice and examined B-lineage cells in the bone marrow. We waited to perform analyses at least 2 weeks after splenectomy to ensure that transitional cells of recent splenic origin, which have a half-life of around 4 days, could be excluded as a source of any transitional B cells observed in the bone marrow.35 As can be seen in Figure 4A and Table 2, AA4.1hi cells were abundant in the bone marrow of splenectomized LTα−/− animals both 2 and 15 days after surgery. Similar results were obtained with splenectomized wild-type mice (data not shown). B cells mature in nonsplenectomized and splenectomized LTα−/− mice in a similar fashion. These data indicate that the bone marrow supports in situ maturation in the complete absence of peripheral lymphoid tissue.
The spleen and other peripheral organs are not required for the maturation of B cells in the bone marrow. (A) B-cell maturation proceeds normally in the bone marrow in splenectomized LTα-deficient mice (lacking all secondary lymphoid organs). Bone marrow B cells were analyzed for AA4.1 expression 2 and 15 days after splenectomy. Recently generated AA4.1hi B-cell populations were observed after splenectomy and were comparable to those in nonsplenectomized LTα+ mice, although overall B-cell production was enhanced after splenectomy. A representative experiment is shown; n = 3 mice in each group. (B) B-cell maturation proceeds normally in the bone marrow in splenectomized aly/aly mice. Bone marrow B cells of aly/aly mice were analyzed for AA4.1 expression 30 days after splenectomy. Recently generated AA 4.1hi B-cell populations were observed in these mice even after splenectomy and are comparable to those in aly/+ mice. A representative experiment is shown; n = 3 mice per group.
The spleen and other peripheral organs are not required for the maturation of B cells in the bone marrow. (A) B-cell maturation proceeds normally in the bone marrow in splenectomized LTα-deficient mice (lacking all secondary lymphoid organs). Bone marrow B cells were analyzed for AA4.1 expression 2 and 15 days after splenectomy. Recently generated AA4.1hi B-cell populations were observed after splenectomy and were comparable to those in nonsplenectomized LTα+ mice, although overall B-cell production was enhanced after splenectomy. A representative experiment is shown; n = 3 mice in each group. (B) B-cell maturation proceeds normally in the bone marrow in splenectomized aly/aly mice. Bone marrow B cells of aly/aly mice were analyzed for AA4.1 expression 30 days after splenectomy. Recently generated AA 4.1hi B-cell populations were observed in these mice even after splenectomy and are comparable to those in aly/+ mice. A representative experiment is shown; n = 3 mice per group.
Aly/aly mice carry an inherited homozygous mutation in the NFκB-inducing kinase (NIK) gene whose product is required for activating the alternate pathway of NFκB activation downstream of the lymphotoxin β receptor and the BAFF receptor.39 These mice lack lymph nodes and Peyer patches24 and are known to be permissive for B-cell maturation, albeit in a somewhat diminished fashion.40,41 We examined the bone marrow of aly/aly mice 30 days after removal of the spleen. As seen in Figure 4B, B cells mature in nonsplenectomized and splenectomized aly/aly mice in a fashion similar to that observed in their heterozygous counterparts. Even though aly/aly mice have diminished B-cell production, they nevertheless still generate transitional B cells in the bone marrow in the absence of the spleen. The presence of continued B-cell maturation in the absence of the spleen in both LTα−/− and aly/aly mice strongly supports the notion that the bone marrow represents a distinct site of B-cell maturation.
Intravenous challenge with S typhimurium induces activated bone marrow B cells in splenectomized LTα−/− mice
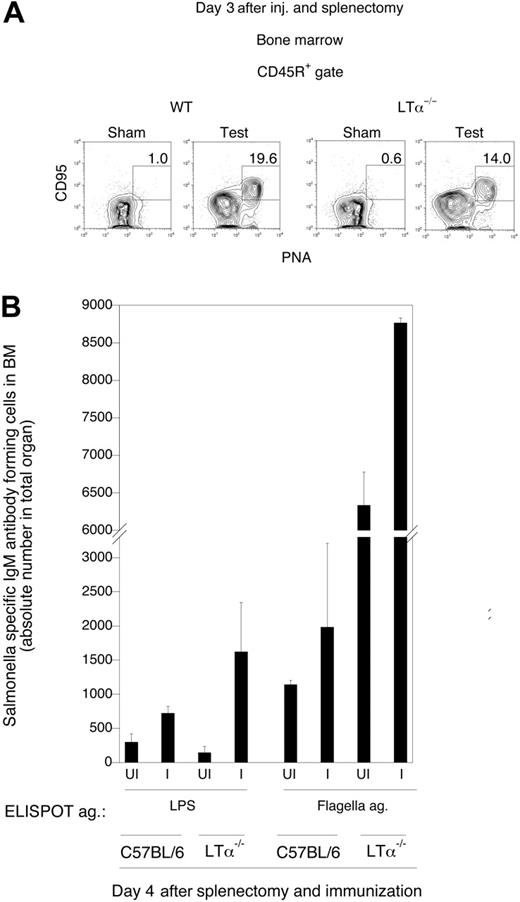
Collections of activated B220+ PNA+ Fashi B cells are seen in the bone marrow of wild-type mice within 2 days of an intravenous challenge with a blood-borne microbe.12 These activated B cells may represent precursors of the antibody-secreting cells seen in the bone marrow, or they might reflect abortive attempts to generate germinal centers in the bone marrow. As seen in Figure 5A, when splenectomized wild-type mice or splenectomized LTα−/− mice were challenged intravenously with a vaccine strain of S typhimurium (SL3261), B220+PNA+Fashi B cells were readily observed in the bone marrow. These data, obtained in the absence of all secondary lymphoid organs, indicate that activation of B cells in the bone marrow occurs in situ.
Bone marrow perisinusoidal B cells respond to multivalent antigens in an antigen-specific manner in the complete absence of lymphoid organs. (A) Analysis of CD95+PNA+CD45R+ B cells in the bone marrow of splenectomized wild-type and LTα−/− mice. Sham mice were injected with saline, whereas test mice were injected with SL3261 bacteria. The plots shown are representative of 3 mice in each group. (B) ELISPOT assay for IgM antibody-forming cells in the bone marrow against LPS and flagella antigen in splenectomized wild-type (C57BL/6) and LTα−/− mice previously challenged intravenously with SL3261 bacteria. UI indicates mice that were not challenged; I, mice that received an intravenous challenge. Error bars denote standard error of the mean.
Bone marrow perisinusoidal B cells respond to multivalent antigens in an antigen-specific manner in the complete absence of lymphoid organs. (A) Analysis of CD95+PNA+CD45R+ B cells in the bone marrow of splenectomized wild-type and LTα−/− mice. Sham mice were injected with saline, whereas test mice were injected with SL3261 bacteria. The plots shown are representative of 3 mice in each group. (B) ELISPOT assay for IgM antibody-forming cells in the bone marrow against LPS and flagella antigen in splenectomized wild-type (C57BL/6) and LTα−/− mice previously challenged intravenously with SL3261 bacteria. UI indicates mice that were not challenged; I, mice that received an intravenous challenge. Error bars denote standard error of the mean.
IgM antibody-forming cells against surface antigens of SL3261 in mice that lack all secondary lymphoid organs
Four days after intravenously challenging splenectomized wild-type and LTα−/− mice with SL3261 cells, IgM ELISPOT assays were performed with bone marrow cells using LPS and purified flagellin from S typhimurium as antigens. As seen in Figure 5B, robust generation of antibody-forming cells against Salmonella LPS and flagellin was observed in the bone marrows of mice lacking all secondary lymphoid organs. The reason for the heightened response in the LTα−/− mice, as compared with wild-type mice is not known at present, but presumably reflects the increase in bone marrow B-cell numbers and consequently increased numbers of antibody-forming cells in these mutant mice, which lack peripheral lymphoid tissue. These analyses complement our previous studies.12 They establish that antibody-forming B cells that appear in the bone marrow following intravenous microbial challenge do not reflect the migration of activated B cells from other lymphoid organs to the bone marrow, but indeed represent the in situ generation in this organ of IgM antibody-forming cells against multivalent microbial antigens.
Discussion
These studies indicate that IgDhi B cells normally mature contemporaneously in the bone marrow and the spleen. They also formally establish that B cells in the bone marrow can be activated by blood-borne microbes in the absence of all secondary lymphoid organs.
One approach that we used to examine B-cell maturation in the bone marrow was the pulse labeling of cycling cells in this organ followed by the tracking of these labeled cells as they differentiate in vivo. The second approach was to use markers that are known to identify recently generated B cells and to examine differentiation in the bone marrow in wild-type mice, in mice that lack most secondary lymphoid organs (but still retain the spleen), and in mice that lack all secondary lymphoid organs. BrdU pulse labeling was used as a cohort labeling tool to follow the postmitotic differentiation of recently labeled pro-B and pre-B cells. It represents a very different technique from continuous labeling with BrdU, used generally to measure half-lives of B-cell populations. Continuous labeling has clearly established that perisinusoidal bone marrow B cells are as long-lived as their counterparts in the spleen.12,42,43 Pulse labeling in a temporally circumscribed manner results in a fairly rapid dissipation of the BrdU marker, which has no bearing on half-lives of B-cell populations. The pulse labeling of a cycling cohort, followed by a “chase” revealed the presence of a large pool of newly formed B cells in the bone marrow in the blood, and, after a brief delay, in the spleen. At the early time points studied, emigration of newly formed B cells into the blood (presumably en route to the spleen) was detected. Labeled B cells in populations known to acquire recirculating ability (fraction I and fraction II follicular B cells) began to be observed in blood and the lymph nodes only at the end of the chase period. These results are consistent with the emigration of a large fraction of the cells in the newly formed B-cell pool from the bone marrow to the spleen, and the maturation of roughly equivalent numbers of B cells simultaneously in the spleen and bone marrow.
The original stathmokinetic studies on B-cell maturation established that immature B cells emigrate to and mature in the spleen.1 Those studies did not simultaneously examine markers such as IgM and IgD that would have permitted the examination of maturation stages in the bone marrow as well. However, the observation was made that recently labeled surface IgM B cells accumulate within the sinusoids of the bone marrow, and the speculative suggestion was made that B cells might mature in the bone marrow possibly in an intravascular location.1
Recognizing that cohort labeling of cells, used in isolation, has its limitations, we chose to also examine B-cell differentiation in the bone marrow in mice that retain the spleen, but which lack all other secondary lymphoid organs, as well as in mice that have been splenectomized and also lack all secondary lymphoid organs. These studies demonstrated the presence of transitional maturing B-cell populations in the bone marrow, lending strong support to the results obtained from cohort studies.
Our current studies fill a long-standing lacuna in this field and suggest that B cells mature in the bone marrow at the same time that they mature in the spleen. Given the functional relevance of perisinusoidal B cells in the bone marrow, these studies open the door to a more detailed analysis of the mechanisms that control the generation, retention, entry, and emigration of mature B cells in the bone marrow.
One caveat of previous studies suggesting that bone marrow B cells may be activated by blood-borne pathogens was the failure to formally exclude the possibility that some B-cell activation might occur in splenectomized wild-type mice in lymph nodes and that this could be followed by the migration of these activated cells to the bone marrow.12 By analyzing splenectomized LTα−/− mice that lack all secondary lymphoid organs we have stringently established that B cells in the perisinusoidal niche of the bone marrow can be activated in situ and that these cells differentiate quite readily into antibody secreting cells in vivo. These studies support the notion that the bone marrow is an important site for T-independent immune responses to blood-borne pathogens. Given that the humoral immune repertoire resides largely in IgD-expressing B cells, IgM antibody generation in the bone marrow perisinusoidal niche may permit the harnessing of most of the B-cell repertoire in responses to life-threatening blood-borne pathogens.
Authorship
Contribution: A.C. conceptualized, designed, and performed research, collected data, analyzed data, and cowrote the paper; C.C. performed research; H.L. performed research; P.R. designed research; and S.P. conceptualized and designed research and wrote the paper. All authors checked the final version of the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Shiv Pillai, Massachusetts General Hospital Cancer Center, Bldg 149, 13th St, Charlestown Navy Yard, Boston, MA 02129; e-mail: pillai@helix.mgh.harvard.edu.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This work was supported by grants AI064930, CA102793, HL43340, and HL071932 from the National Institutes of Health and a grant from the Roche Organ Transplantation Research Foundation.
Joanne Yetz-Aldape, Michelle Connole, John Daley, and Suzan Lazo-Kallanian are thanked for help with flow cytometry.