Abstract
Several approaches to target insulin-like growth factor-1 (IGF-1) signaling have resulted in the inhibition of the growth of a broad range of tumor cells. Malignant T cells are insensitive to the antiproliferative effects of the interferon-γ (IFN-γ)/signal transducer and activator of transcription 1 (STAT1) pathway because of the IGF-1–dependent internalization of the IFN-γR2 signaling chain. Here we show that human malignant T cells are also resistant to the growth inhibitory effect of both the IGF-1 receptor–specific inhibitor picropodophyllin (PPP) and retrovirus-mediated gene transfer of a dominant negative IGF-1 receptor. However, blockade of IGF-1 receptor perturbs IFN-γR2 internalization and induces its cell surface accumulation in malignant T cells. This allows the reinstatement of the IFN-γ–induced STAT1 activation, a high expression of proapoptotic molecules, and the suppression of malignant T-cell growth both in vitro and in vivo in a severe combined immunodeficiency (SCID) mouse model. These data indicate that the inhibition of IGF-1 signaling combined with IFN-γ administration could be a promising approach to suppress the growth of neoplastic T cells resistant to each treatment on its own.
Introduction
Interferon-γ (IFN-γ), a cytokine produced by T and natural killer (NK) cells, plays an essential role in cell-mediated immunity, and it contributes to the control of the expansion of many normal and neoplastic cell types.1 It exerts its biological activities by interacting with its specific cell-surface receptor (IFN-γR), which consists of 2 IFN-γR1 binding chains and 2 IFN-γR2 signal-transducing chains.2 The binding of IFN-γ to its receptor complex activates the Janus kinase (JAK)/signal transducer and activator of transcription (STAT) signal transduction pathway3–5 and induces the transcription of numerous sets of IFN-γ–inducible proapoptotic genes.6–11
However, IFN-γ does not always cause apoptosis or block proliferation. T lymphocytes that undergo T helper 1 (Th1) polarization or malignant transformation become resistant to the antiproliferative/apoptotic effects of the IFN-γ/STAT1 pathway.12–14 This refractoriness is mainly due to IFN-γR2 down-regulation,12–17 due to ligand-independent internalization within clathrin-coated pits.18–20
By inducing IFN-γR2 internalization, both insulin-like growth factor-1 (IGF-1) and iron are critical factors in limiting the IFN-γ/STAT1 pathway in T lymphocytes.20–22 Since IGF-1 induces cell-membrane accumulation of the transferrin receptor (TfR),23 which plays an obligatory role in the iron-induced IFN-γR2 internalization,22 blockade of IGF-1 signaling in T cells could perhaps hinder their intracellular IFN-γR2 trafficking and reinstate their sensitivity to IFN-γ/STAT1 apoptotic signaling.
IGF-1 is a growth factor mainly produced by the liver that plays an important role in the promotion of mitogenesis, transformation, and protection from apoptosis of many cell types.24–26 It acts by binding to IGF-1R, a type II tyrosine kinase receptor composed of 2 extracellular α subunits that bind ligand, and 2 trans-membrane catalytic β subunits.24,27,28 Upon ligand binding, the receptor is autophosphorylated on several tyrosine residues located in the intracellular domain of the β subunits, and this provides binding sites for Shc, insulin receptor substrate-1 (IRS-1) and IRS-,2 and other signaling molecules. IRS-1/IRS-2 and Shc are thus phosphorylated and activate the phosphoinositide 3 kinase (PI3K)/Akt and the mitogen-activated protein kinase (MAPK) pathways.29
Preclinical studies have demonstrated that genetic or pharmacological inhibition of IGF-1 signaling reverses the neoplastic phenotype of many tumor cells.30 The effects of specific blockade of IGF-1 signaling on malignant T cells, however, have not been investigated.
The effects of this blockade, alone or in combination with IFN-γ administration, on the growth and apoptosis of human malignant T cells have now been evaluated. A retrovirus-based approach was exploited to stably express a dominant-negative (DN) form of the IGF-1R in malignant ST4 T cells. Expression of IGF-1R DN did not affect their ability to proliferate in the presence of serum in vitro, nor in vivo in severe combined immunodeficiency (SCID) mice, whereas it perturbed IFN-γR2 internalization and induced its cell-surface accumulation. This reinstated ST4 cell sensitivity to the IFN-γ/STAT1 apoptotic pathway in vitro and in vivo. Moreover, we show that the exposure to a specific inhibitor of IGF-1R tyrosine kinase activity (picropodophyllin [PPP]) or to IFN-γ alone neither induced apoptosis nor suppressed the proliferation of 3 malignant T-cell lines (ST4, Jurkat, and PF382). By contrast, the combination of PPP and IFN-γ significantly induced STAT1-dependent apoptosis and completely suppressed the growth of all cell lines.
These data could be used in the elaboration of new therapeutic approaches based on IFN-γ administration to overcome the resistance of malignant cells to the inhibitory effect of IGF-1 signaling blockade.
Materials and methods
Media
The culture media were RPMI 1640 (BioWhittaker Inc., Walkersville, MD) containing gentamycin (Schering-Plough, Milan, Italy) with or without 10% fetal bovine serum (FBS; Invitrogen, Carlsbad, CA), designated complete medium, and serum-free medium, respectively. All in vitro cultures were maintained at 37°C in a 5% CO2 humidified atmosphere.
Malignant cells
Human ST4 T cells were derived from a childhood convoluted-type T-cell lymphoma, while PF382 cells were derived from a T-cell acute lymphoblastic leukemia. They were stabilized both in vitro and in nu/nu mice starting from bioptic material. Jurkat (CRL8161; ATCC, Manassas, VA) T cells were derived from an acute lymphoblastic leukemia. All of these malignant T-cell lines are incapable of producing IFN-γ.12
Construction of retroviral vectors, production of retroviral particles, and viral transduction
A cDNA fragment encoding the first 516 amino acids (486 after the removal of the signal peptide) of the human IGF1R called 486/STOP, created by introduction of a point mutation in codon 486 (not including the signal peptide) that results in a premature stop signal and consequently in the production of a truncated soluble receptor,31 and a cDNA fragment encoding the full-length IGF1R were excised from the plasmid pCvn (kindly donated by Dr R. Baserga, Thomas Jefferson University, Philadelphia, PA) by HindIII and EcoRV and cloned into the dicistronic green fluorescent protein (GFP)–expressing Pallino retroviral vector32 prepared by digestion with XhoI, filling by Klenow enzyme, and then digestion with HindIII.
Retrovirus production and cell infection
Retroviruses were obtained by cotransfection of Pallino containing IGF1R wild type (WT) or IGF1R DN or of empty Pallino with pMD2 VSV-G plasmid into 293GP packaging cells (Invitrogen). Supernatants (500 μL) containing retroviruses were collected 24 hours after transfection, filtered, supplemented with 8 μg/mL polybrene (Sigma-Aldrich, St Louis, MO), and added to 5 to 10 × 104 target ST4 cells. After 12 hours, 1 mL of complete medium was added, and the cells were cultured for another 2 days. The percentages of transduced cells were analyzed for GFP expression on a FACSCalibur flow cytometer (Becton Dickinson, Franklin Lakes, NJ). The CELLQuest software (Becton Dickinson) was used for the data acquisition and analysis. Highly fluorescent cells were then sorted for GFP expression by a fluorescence-activated MoFlo High-Performance Cell Sorter (DakoCytomation, Glostrup, Denmark). The cells thus obtained were called ST4-WT, ST4-DN, and ST4-E.
Cell proliferation assay
To evaluate the proliferative response to IGF-1, ST4-E, ST4-WT, and ST4-DN cells were cultured in serum-free medium with scalar doses of recombinant human IGF-1 (R&D Systems, Minneapolis, MN). At 24, 48, and 72 hours, cells were stained with trypan blue (Sigma-Aldrich) and counted in triplicate. To assess the effect of IFN-γ on cell growth, ST4-E, ST4-WT, and ST4-DN were cultured in complete medium with scalar doses of recombinant human IFN-γ (kindly provided by Dr M. Brunda, Hoffman-La Roche, Nutley, NJ) and counted. In all the other experiments performed, IGF-1 was used at 100 ng/mL and IFN-γ was used at 1000 U/mL. In parallel experiments, cells were cultured in complete medium with or without IFN-γ in the absence or presence of 1 μg/mL of a blocking anti-Fas monoclonal antibody (mAb; BD Biosciences Pharmingen, San Diego, CA) for 72 hours and counted. ST4, PF382, and Jurkat cells were cultured with scalar doses (0, 0.05, 0.5, and 5 μM) of PPP (Calbiochem, Merck KGaA, Darmstadt, Germany), in serum-free medium with or without IGF-1 or in complete medium with scalar doses of PPP with or without IFN-γ, and counted after 48 hours.
Flow cytometry
To measure surface expression of IFN-γR2 and IGF-1Rα, ST4-E, ST4-WT, and ST4-DN cells cultured in complete medium were collected, washed twice, and stained with unconjugated mouse IgG1 anti–human IFN-γR2 (PBL Biomedical Laboratories, New Brunswick, NJ) or anti–IGF-1R (R&D Systems) mAbs for 30 minutes, followed by phycoerythrin (PE)–conjugated rabbit anti–mouse Ig (DakoCytomation) as described.21 IGF-1–induced IFN-γR2 internalization was assessed by incubating cells deprived of serum for 24 hours with anti–IFN-γR2 or isotype-matched mouse IgG1 control mAbs (DakoCytomation) for 1 hour at 37°C or at 4°C with or without IGF-1. Cell-surface–associated mAb was removed by treating cells with acid pH (phosphate buffer saline [PBS] with 0.2% bovine serum albumin [BSA], 100 mM glycine, and 2 M urea [pH 2.5]) for 5 minutes at 4°C. Cells were then fixed and permeabilized as described18 and incubated for 30 minutes at 4°C with PE-conjugated rabbit anti–mouse Ig. IFN-γR2 endocytosis was measured as specific cell-associated mean fluorescence (ΔMFI) by flow cytometry. To evaluate IFN-γ–induced membrane expression of Fas and FasL, cells were cultured in complete medium with or without IFN-γ. After 24 hours, cells were recovered and incubated for 30 minutes with PE-conjugated anti-Fas or with biotin-conjugated anti-FasL mAbs (BD Biosciences Pharmingen), followed by PE-conjugated streptavidin (DakoCytomation). To evaluate IFN-γ–induced apoptosis, cells were cultured in complete medium with or without IFN-γ, and in some experiments with scalar doses of PPP, for 48 hours, and then stained with a PE-conjugated annexin-V kit (BD Biosciences Pharmingen) according to the manufacturer's instructions. All experiments were performed with a FACSCalibur flow cytometer (Becton Dickinson). Each plot represents the results from 10 000 events.
Western blot analysis
To evaluate protein amount of the WT and mutant forms of IGF-1Rα, 5 × 106 cells were lysed on ice with cold lysis buffer as previously described,21 and 50 μg of total proteins (as determined by the BCA Protein Assay reagent Kit; Pierce Biotechnology, Rockford, IL) were separated on a 3% to 8% gradient NuPAGE Novex Tris-Acetate Gel (Invitrogen) and transferred to nitrocellulose membranes (Bio-Rad, Hercules, CA). Blots were blocked with 5% nonfat dry milk in TTBS21 overnight and then incubated with a 1:1000 dilution of anti–IGF-1Rα (N-20) rabbit polyclonal Ab (Santa Cruz Biotechnology, Santa Cruz, CA) for 1 hour, followed, after 3 washes in TTBS, by a 1:2000 dilution of HRP-conjugated goat anti–rabbit Ig Ab (Santa Cruz Biotechnology). Ab binding was visualized by ECL Plus (GE Healthcare Europe GmbH, Milan, Italy) according to the manufacturer's instructions. Membranes were subsequently probed with an antiactin Ab (Sigma-Aldrich). Phosphorylation of STAT1, STAT3, Akt, and p44/42 MAPK, and the amount of IRF-1 protein induction in cells cultured in serum-free medium or with PPP (1 μM) for 4 to 6 hours and then treated with or without IFN-γ, IFN-α (2 ng/mL; Peprotech, Rocky Hill, NJ), or IGF-1 for different times were evaluated as previously described.21 Briefly, 10 μg of protein extracts were separated on a 10% NuPAGE Bis-Tris Gel (Invitrogen), electroblotted onto nitrocellulose membranes, and incubated overnight with a 1:1000 dilution of the corresponding rabbit polyclonal Abs anti–phospho-Tyr (701)–STAT1, anti-STAT1, anti–phospho-Tyr (705)–STAT3, anti-STAT3, anti–phospho-Ser (473)–Akt, anti-Akt, anti–phospho-Thr (202)/Tyr (204)–p44/42 MAPK, anti-p44/42 MAPK (Cell Signaling, Beverly, MA), and anti–IRF-1 (C20; Santa Cruz Biotechnology). Membranes were then incubated with 1:2000 HRP-conjugated goat anti–rabbit Ig, and Ab binding was visualized as described. Nonphosphorylated proteins and actin were used as control for equal protein loading. Images were acquired with an IMAGE Scanner (GE Healthcare Europe GmbH) and saved in TIFF format. Density scanning was performed with the IMAGE MASTER 2D, version 3.1 (GE Healthcare Europe GmbH) software for Windows.
In vivo experiments
Five-week-old female immunodeficient SCID (CB17 scid/scid) mice (Charles River Laboratories, Calco, Italy) were fed and maintained under specific pathogen–free conditions in the animal facility of the Department of Clinical and Biological Sciences, University of Turin, and treated in accordance with European guidelines.
ST4-WT and ST4-DN cells (107 per mouse) were washed with PBS, resuspended in 0.4 mL of PBS containing 0.01% murine serum albumin (MSA; Sigma-Aldrich), and injected subcutaneously in the inguinal region. At 24 hours before tumor challenge, and then weekly, all mice received intraperitoneal injections with 0.2 mL of a 1:20 dilution of anti-asialo GM1 rabbit antiserum (Wako Chemicals GmbH, Dusseldorf, Germany) in PBS. Two hours after inoculation of cells, SCID mice received a subcutaneous injection of either 1000 U of IFN-γ in 0.4 mL PBS/MSA or PBS/MSA alone (vehicle) in the site of tumor challenge. This treatment was repeated daily for 5 days. In all the experiments, tumor incidence and growth were evaluated twice a week by a blinded observer. Neoplastic masses were measured with calipers along the 2 perpendicular diameters for 70 days. At the end of this period, tumor-free mice were classified as survivors. Latency time was considered as the period (in days) between challenge and the growth of neoplastic masses to a mean diameter of 3 mm. Mice were killed for humane reasons when the tumor exceeded 12 mm in mean diameter.
Statistical analysis
The Pearson t test (GraphPad Prism 3; GraphPad Software, San Diego, CA) was used to analyze the effect of IFN-γ on the in vivo growth of ST4 cells with a P value less than .05 as the significance cut-off.
Results
Expression of IGF-1R DN and characterization of IGF-1–dependent responses in human malignant T cells
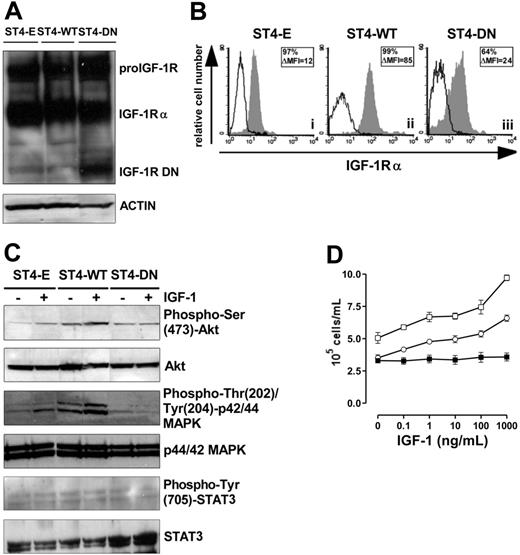
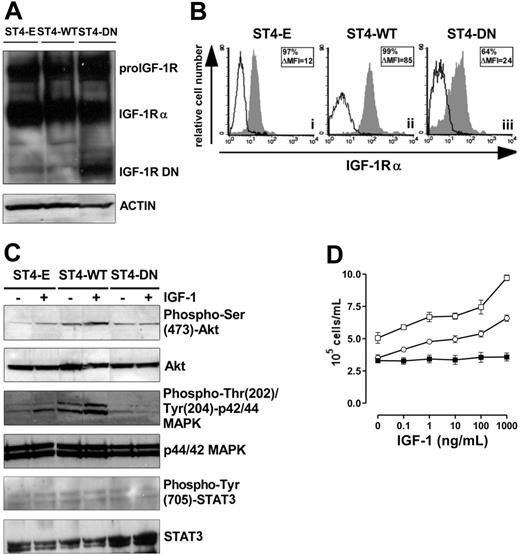
To achieve high transgene expression, ST4 cells were transduced with Pallino retroviruses32 encoding IGF1R WT or IGF1R DN31 or with empty Pallino as a control. The resulting cells (ST4-WT, ST4-DN, and ST4-E) were then fluorescence-activated cell-sorter (FACS)–sorted to select subpopulations expressing high GFP levels (> 98% of GFP-positive cells) and used for all the experiments described. IGF-1R DN is a truncated soluble receptor, a portion of which remains in the cytosol and associates with endogenous IGF-1R.31,33 Therefore, expression of IGF-1R DN was verified by Western blot analysis on total-cell lysates with an Ab to the α subunit of IGF-1R. Both the IGF-1 proreceptor and IGF-1Rα were detected in ST4-E, ST4-WT, and ST4-DN, whereas the IGF-1R truncated form (72 kDa) was detected only in ST4-DN cells (Figure 1A). ST4-WT, which express both endogenous and transduced IGF-1R, displayed higher surface IGF-1Rα compared with ST4-E and ST4-DN (Figure 1B).
Loss of IGF-1 responsiveness in IGF-1R DN–expressing cells. (A) Western blot analysis of WT and mutant IGF-1R expression in transduced ST4 cells. Total-cell lysates (50 μg) from ST4-E, ST4-WT, and ST4-DN were electrophoresed, electroblotted, and stained with an anti–IGF-1Rα Ab. Membranes were subsequently probed with an antiactin Ab to confirm equal protein loading in each lane of the gel. (B) Flow cytometric analysis of IGF-1Rα surface expression in ST4-E (i), ST4-WT (ii), and ST4-DN (iii) cultured in complete medium with expression of IGF-1Rα shown in the gray histograms and the background of mouse IgG1 negative control in the open histograms. Boxed results show percentage of positive cells and mean specific fluorescence, calculated by subtracting the positivity and the mean of fluorescence obtained with isotype-matched control Ig from that detected with the specific mAb. (C) Western blot evaluation of IGF-1–induced Akt, MAPK, and STAT3 activation on ST4-E, ST4-WT, and ST4-DN deprived of serum for 4 hours treated with IGF-1 (100 ng/mL) for 15 minutes. Phosphorylation was evaluated with anti–phospho-Ser (473)-Akt, anti–phospho-Thr (202)/Tyr (204)–p44/42 MAPK, or anti–phospho-Tyr (705)–STAT3 Abs. Membranes were subsequently probed with anti-Akt, anti-p44/42 MAPK, or anti-STAT3 Abs. All the experiments were performed independently at least 3 times. (D) ST4-E (○), ST4-WT (□), and ST4-DN (▪) were cultured in serum-free medium with scalar doses of IGF-1. After 72 hours, cells were harvested, stained with trypan blue, and counted. The graph illustrates the mean result of 4 independent experiments.
Loss of IGF-1 responsiveness in IGF-1R DN–expressing cells. (A) Western blot analysis of WT and mutant IGF-1R expression in transduced ST4 cells. Total-cell lysates (50 μg) from ST4-E, ST4-WT, and ST4-DN were electrophoresed, electroblotted, and stained with an anti–IGF-1Rα Ab. Membranes were subsequently probed with an antiactin Ab to confirm equal protein loading in each lane of the gel. (B) Flow cytometric analysis of IGF-1Rα surface expression in ST4-E (i), ST4-WT (ii), and ST4-DN (iii) cultured in complete medium with expression of IGF-1Rα shown in the gray histograms and the background of mouse IgG1 negative control in the open histograms. Boxed results show percentage of positive cells and mean specific fluorescence, calculated by subtracting the positivity and the mean of fluorescence obtained with isotype-matched control Ig from that detected with the specific mAb. (C) Western blot evaluation of IGF-1–induced Akt, MAPK, and STAT3 activation on ST4-E, ST4-WT, and ST4-DN deprived of serum for 4 hours treated with IGF-1 (100 ng/mL) for 15 minutes. Phosphorylation was evaluated with anti–phospho-Ser (473)-Akt, anti–phospho-Thr (202)/Tyr (204)–p44/42 MAPK, or anti–phospho-Tyr (705)–STAT3 Abs. Membranes were subsequently probed with anti-Akt, anti-p44/42 MAPK, or anti-STAT3 Abs. All the experiments were performed independently at least 3 times. (D) ST4-E (○), ST4-WT (□), and ST4-DN (▪) were cultured in serum-free medium with scalar doses of IGF-1. After 72 hours, cells were harvested, stained with trypan blue, and counted. The graph illustrates the mean result of 4 independent experiments.
The ability of ST4-DN cells to respond to IGF-1 was evaluated. IGF-1, following the interaction with its receptor, typically activates Akt and MAPK pathways, and in many cell types STAT3, by inducing their phosphorylation.29,34–37 Western blot analysis of Akt phosphorylation in cells treated with IGF-1 for 15 minutes showed that this pathway was activated in ST4-E (2-fold induction) and to a greater extent in ST4-WT cells (4-fold induction). By contrast, in ST4-DN cells IGF-1 did not induce Akt phosphorylation (Figure 1C). Similarly, IGF-1–induced phosphorylation of MAPK was detected in ST4-E (1.5-fold induction) and to a larger extent in ST4-WT (3-fold induction), but not in ST4-DN (Figure 1C). Instead, IGF-1–induced STAT3 phosphorylation was not observed in any cell types (Figure 1C).
The IGF-1–dependent proliferative response of ST4-E, ST4-WT, and ST4-DN cells was also analyzed. As serum deprivation is a simple and convenient way to deplete IGF-1 from cells in culture, cells were cultured in the absence of serum and in the presence of scalar doses of IGF-1 for 72 hours. While ST4-E and ST4-WT proliferated in a dose-dependent manner in response to IGF-1, this response was completely abolished in ST4-DN cells (Figure 1D).
These data indicate that the expression of IGF-1R DN in ST4 cells completely abrogates IGF-1–dependent Akt and MAPK activation and proliferation.
Effect of IGF-1R DN on IFN-γR2 internalization
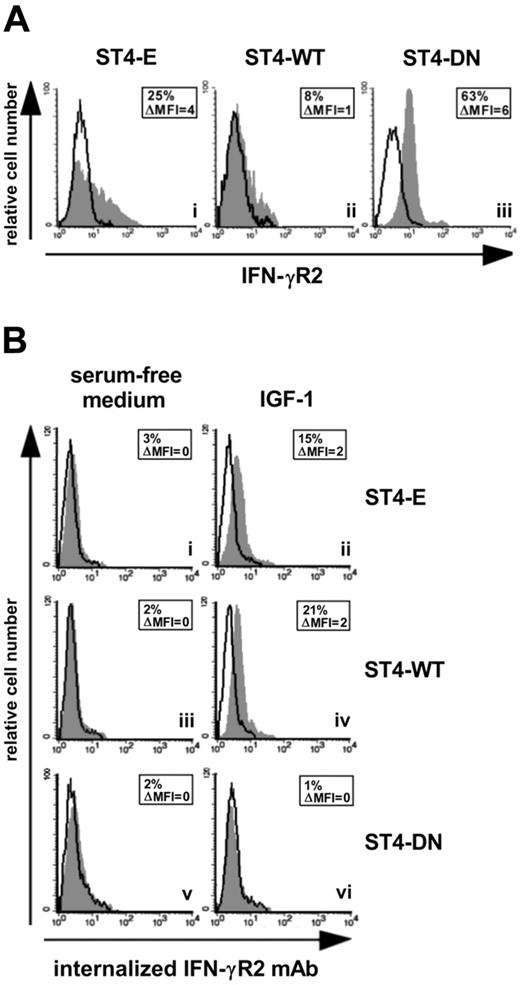
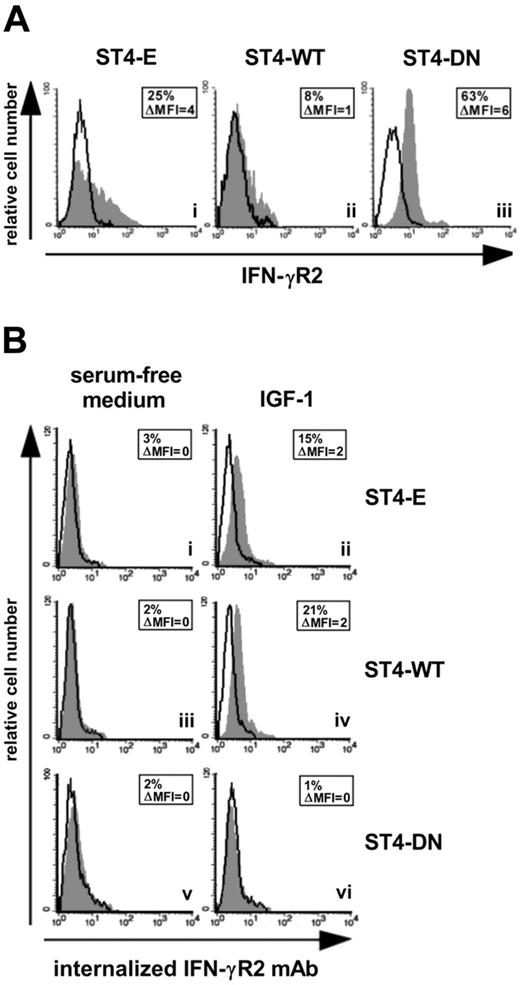
We had previously demonstrated that IGF-1 limits the IFN-γ/STAT1 pathway by delivering a signal for IFN-γR2 internalization.21 Since IFN-γR2 is mainly endocytosed in malignant T cells,8 we assessed whether the expression of IGF-1R DN caused the accumulation of IFN-γR2 at the cell surface in ST4 cells. ST4-E displayed low IFN-γR2 cell-surface accumulation (Figure 2Ai). This was further decreased in ST4-WT, but strongly enhanced in ST4-DN (Figure 2Aii-iii). These data indicate that IGF-1 present in serum induces IFN-γR2 internalization in ST4-E, which express endogenous IGF-1R only, and to a greater extent in ST4-WT, which express much higher amounts of IGF-1R (Figure 1B). By contrast, IGF-1 does not induce IFN-γR2 internalization in ST4-DN, since these cells express a nonfunctional IGF-1R (Figure 1C-D).
IGF-1R blockade up-regulates IFN-γR2 surface expression. (A) Flow cytometric analysis of IFN-γR2 surface expression in ST4-E (i), ST4-WT (ii), and ST4-DN (iii) cells maintained in complete medium. Expression of IFN-γR2 (gray histograms) and the background of mouse IgG1 negative control (open histograms) are shown for 1 representative experiment of 3 independently performed. (B) IGF-1-induced IFN-γR2 internalization was evaluated by flow cytometry. ST4-E (i-ii), ST4-WT (iii-iv), and ST4-DN (v-vi) cells, cultured for 24 hours in serum-free medium to eliminate the IGF-1 present in serum, were incubated with or without IGF-1 (100 ng/mL) and with unconjugated anti–IFN-γR2 mAb or with an isotype-matched mAb at 37°C. After 1 hour, cell surface–associated mAb was removed, and cells were permeabilized and stained with PE-conjugated rabbit anti–mouse Ig. Mean specific internalized fluorescence was calculated by subtracting the mean internalized fluorescence obtained with isotype-matched control Ig from that detected with the anti–IFN-γR2 mAb.
IGF-1R blockade up-regulates IFN-γR2 surface expression. (A) Flow cytometric analysis of IFN-γR2 surface expression in ST4-E (i), ST4-WT (ii), and ST4-DN (iii) cells maintained in complete medium. Expression of IFN-γR2 (gray histograms) and the background of mouse IgG1 negative control (open histograms) are shown for 1 representative experiment of 3 independently performed. (B) IGF-1-induced IFN-γR2 internalization was evaluated by flow cytometry. ST4-E (i-ii), ST4-WT (iii-iv), and ST4-DN (v-vi) cells, cultured for 24 hours in serum-free medium to eliminate the IGF-1 present in serum, were incubated with or without IGF-1 (100 ng/mL) and with unconjugated anti–IFN-γR2 mAb or with an isotype-matched mAb at 37°C. After 1 hour, cell surface–associated mAb was removed, and cells were permeabilized and stained with PE-conjugated rabbit anti–mouse Ig. Mean specific internalized fluorescence was calculated by subtracting the mean internalized fluorescence obtained with isotype-matched control Ig from that detected with the anti–IFN-γR2 mAb.
IGF-1–dependent internalization of IFN-γR2 was further analyzed in anti–IFN-γR2 mAb uptake experiments. ST4-E, ST4-WT, and ST4-DN deprived of serum for 24 hours were incubated for 1 hour at 37°C with or without IGF-1 in the presence of anti–IFN-γR2 or isotype-matched control mAbs. If IFN-γR2 was internalized in response to IGF-1, anti–IFN-γR2 mAb would be taken up and result in an increase of specific cell–associated fluorescence.18 In the absence of IGF-1, ST4-E, ST4-WT, and ST4-DN showed no IFN-γR2 specific cell–associated fluorescence (Figure 2Bi, iii, v). IGF-1 induced an increase in such fluorescence in ST4-E and ST4-WT cells (Figure 2Bii, iv), indicating that anti–IFN-γR2 mAb had been internalized. In contrast, IGF-1 did not induce any anti–IFN-γR2 mAb uptake in ST4-DN cells (Figure 2Bvi). No cell-associated specific fluorescence was detected in any of the cells maintained for 1 hour at 4°C in the presence or absence of IGF-1 (data not shown). These data indicate that the expression of IGF-1R DN hampers internalization of IFN-γR2.
Effect of IGF-1R DN on activation of the IFN-γ/STAT1 pathway
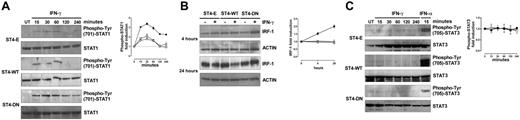
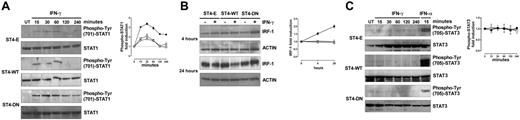
The extent of IFN-γ–induced STAT1 activation depends on the amount of IFN-γR2 surface expression.8 Since the expression of IGF-1R DN induces IFN-γR2 cell surface accumulation, the kinetics of IFN-γ–mediated STAT1 phosphorylation in ST4-E, ST4-WT, and ST4-DN was evaluated. Cells were treated with or without IFN-γ. At 15, 30, 60, 120, and 240 minutes, cells were harvested and protein cell extracts were analyzed by Western blot with a specific anti–phospho-Tyr (701)–STAT1 Ab. No STAT1 phosphorylation was observed in untreated cells. IFN-γ induced a weak STAT1 phosphorylation in ST4-E and ST4-WT cells. It was evident after 15 minutes and disappeared at 120 minutes. In contrast, a stronger and sustained IFN-γ–dependent STAT1 activation was detected in ST4-DN cells. It was evident after 15 minutes and lasted until 240 minutes (Figure 3A).
IGF-1R DN increases the activation of IFN-γ/STAT1 pathway in T cells. (A) ST4-E (○), ST4-WT (□), and ST4-DN (▪) cells were cultured in complete medium without (UN) or with IFN-γ (1000 U/mL) for the indicated time intervals. STAT1 activation was evaluated by Western blot analysis of protein cell extracts with an anti–phospho-Tyr (701)–STAT1 Ab. Membranes were subsequently probed with an anti-STAT1 Ab, and the fold induction of IFN-γ–dependent STAT1 phosphorylation was calculated as the ratio between the band intensities of IFN-γ–treated and untreated cells, quantitated after normalization with total STAT1. The fold induction values are shown as means ± SEM from 3 independent experiments at each time point. (B) Expression levels of IRF-1 were evaluated by Western blot on total lysates from ST4-E (○), ST4-WT (□), and ST4-DN (▪) cells treated with or without IFN-γ for 4 and 24 hours. IRF-1 induction, shown as means ± SEM from 3 independent experiments, was quantitated after normalization to actin expression. (C) Western blot evaluation of STAT3 phosphorylation in ST4-E (○), ST4-WT (□), and ST4-DN (▪) cells cultured in complete medium without (UN) or with IFN-γ for the indicated time intervals or with IFN-α for 15 minutes. Membranes were probed with anti–phospho-Tyr (705)–STAT3 and subsequently with anti-STAT3 Abs. Phospho-STAT3 induction, shown as means of fold induction ± SEM from 3 independent experiments, was quantitated as described after normalization to total STAT3 expression.
IGF-1R DN increases the activation of IFN-γ/STAT1 pathway in T cells. (A) ST4-E (○), ST4-WT (□), and ST4-DN (▪) cells were cultured in complete medium without (UN) or with IFN-γ (1000 U/mL) for the indicated time intervals. STAT1 activation was evaluated by Western blot analysis of protein cell extracts with an anti–phospho-Tyr (701)–STAT1 Ab. Membranes were subsequently probed with an anti-STAT1 Ab, and the fold induction of IFN-γ–dependent STAT1 phosphorylation was calculated as the ratio between the band intensities of IFN-γ–treated and untreated cells, quantitated after normalization with total STAT1. The fold induction values are shown as means ± SEM from 3 independent experiments at each time point. (B) Expression levels of IRF-1 were evaluated by Western blot on total lysates from ST4-E (○), ST4-WT (□), and ST4-DN (▪) cells treated with or without IFN-γ for 4 and 24 hours. IRF-1 induction, shown as means ± SEM from 3 independent experiments, was quantitated after normalization to actin expression. (C) Western blot evaluation of STAT3 phosphorylation in ST4-E (○), ST4-WT (□), and ST4-DN (▪) cells cultured in complete medium without (UN) or with IFN-γ for the indicated time intervals or with IFN-α for 15 minutes. Membranes were probed with anti–phospho-Tyr (705)–STAT3 and subsequently with anti-STAT3 Abs. Phospho-STAT3 induction, shown as means of fold induction ± SEM from 3 independent experiments, was quantitated as described after normalization to total STAT3 expression.
To further characterize the effect of IGF-1R DN on the IFN-γ/STAT1 signaling pathway, induction of IRF-1, an IFN-γ–inducible gene whose transcription depends on STAT1 and is implicated in IFN-γ–dependent apoptosis,7,38,39 was analyzed. Western blot analysis with an anti–IRF-1 Ab revealed that similar amounts of IRF-1 were constitutively expressed in ST4-E, ST4-WT, and ST4-DN. IFN-γ did not enhance IRF-1 expression in ST4-E and ST4-WT cells, whereas it induced a progressive increase of IRF-1 in ST4-DN cells that was already detectable after 4 hours and peaked after 24 hours (Figure 3B).
In certain conditions IFN-γ induces STAT3 phosphorylation as well, leading to proliferative effects.7 We used Western blot analysis with a specific Ab to determine the time course of STAT3 phosphorylation in ST4-E, ST4-WT, and ST4-DN cells treated with IFN-γ. IFN-γ did not induce STAT3 phosphorylation in any cell type, though it was observed in ST4-E, ST4-WT, and ST4-DN treated for 15 minutes with 2 ng/mL of IFN-α (positive control; Figure 3C).
These data together with the results on IFN-γR2 expression indicate that IGF-1R DN restores the IFN-γ/STAT1 signaling and leads to induction of IRF-1.
IGF-1R DN reinstates malignant T-cell sensitivity to IFN-γ–dependent apoptosis
As IRF-1 induces the expression of FasL, which triggers the Fas-dependent cell death program,40 the effects of IFN-γ on Fas and FasL surface expression in ST4-E, ST4-WT, and ST4-DN were investigated.
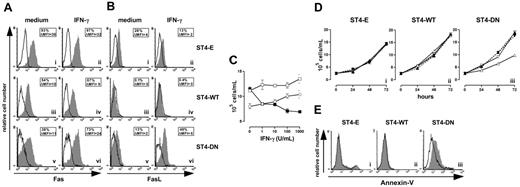
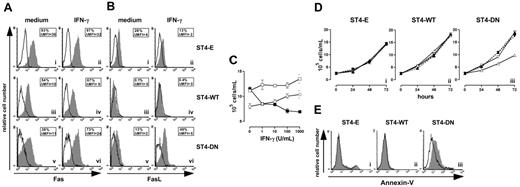
Cells were treated for 24 hours with or without IFN-γ, then Fas and FasL surface expressions were evaluated using specific mAbs. Flow cytometry showed that IFN-γ did not enhance the constitutive high expression of Fas in ST4-E and ST4-WT, but slightly enhanced that in ST4-DN (Figure 4A). Moreover, the low or absent amount of FasL membrane expression in ST4-E and ST4-WT was not increased by IFN-γ, whereas it strongly enhanced this expression in ST4-DN (Figure 4B).
IGF-1R blockade reinstates the IFN-γ/STAT1 apoptotic pathway. Flow cytometric analysis of (A) Fas and (B) FasL surface expression on ST4-E (i-ii), ST4-WT (iii-iv), and ST4-DN (v-vi) cultured for 24 hours in complete medium with or without IFN-γ. Expression of Fas or FasL (gray histograms) and the background of mouse IgG1 negative control (open histograms) are shown for 1 representative experiment out of 3 independently performed. Boxed results show the percentage of positive cells and the mean specific fluorescence. (C) ST4-E (○), ST4-WT (□), and ST4-DN (▪) cells were cultured in complete medium with different doses of IFN-γ. After 72 hours, cells were harvested, stained with trypan blue and counted. (D) ST4-E (i), ST4-WT (ii), and ST4-DN (iii) cells were cultured in the presence of complete medium (▴), IFN-γ (▵), a blocking anti-Fas mAb (1 μg/mL) (▾), and IFN-γ plus blocking anti-Fas mAb (▿), and the kinetics of their growth was evaluated. (E) ST4-E (i), ST4-WT (ii), and ST4-DN (iii) cells were cultured in complete medium in the presence (gray histogram) or absence (open histogram) of IFN-γ. After 48 hours, cells were recovered, stained with PE-conjugated annexin-V, and analyzed by flow cytometry. All experiments were performed independently at least 3 times.
IGF-1R blockade reinstates the IFN-γ/STAT1 apoptotic pathway. Flow cytometric analysis of (A) Fas and (B) FasL surface expression on ST4-E (i-ii), ST4-WT (iii-iv), and ST4-DN (v-vi) cultured for 24 hours in complete medium with or without IFN-γ. Expression of Fas or FasL (gray histograms) and the background of mouse IgG1 negative control (open histograms) are shown for 1 representative experiment out of 3 independently performed. Boxed results show the percentage of positive cells and the mean specific fluorescence. (C) ST4-E (○), ST4-WT (□), and ST4-DN (▪) cells were cultured in complete medium with different doses of IFN-γ. After 72 hours, cells were harvested, stained with trypan blue and counted. (D) ST4-E (i), ST4-WT (ii), and ST4-DN (iii) cells were cultured in the presence of complete medium (▴), IFN-γ (▵), a blocking anti-Fas mAb (1 μg/mL) (▾), and IFN-γ plus blocking anti-Fas mAb (▿), and the kinetics of their growth was evaluated. (E) ST4-E (i), ST4-WT (ii), and ST4-DN (iii) cells were cultured in complete medium in the presence (gray histogram) or absence (open histogram) of IFN-γ. After 48 hours, cells were recovered, stained with PE-conjugated annexin-V, and analyzed by flow cytometry. All experiments were performed independently at least 3 times.
These data suggested that IFN-γ blocks ST4-DN cell proliferation by up-regulating Fas and FasL expression. This was evaluated by culturing ST4-E, ST4-WT, and ST4-DN in complete medium in the presence of scalar doses of IFN-γ and evaluating their proliferation after 72 hours. ST4-E and ST4-WT displayed a slight dose-dependent increase of proliferation in response to IFN-γ, indicating that they were resistant to its antiproliferative effect, whereas a dose-dependent inhibition was induced in ST4-DN (Figure 4C).
To assess whether this inhibition was dependent on Fas/FasL interaction, cells were cultured in the presence of (1) complete medium, (2) IFN-γ, (3) blocking anti-Fas mAb (1 μg/mL), and (4) IFN-γ plus blocking anti-Fas mAb; the kinetics of their growth was then evaluated. Figure 4D shows that the growth of ST4-E and ST4-WT was not inhibited by the presence of IFN-γ, anti-Fas mAb, singly or together (Figure 4Di-ii), whereas that of ST4-DN was inhibited by IFN-γ; this inhibition was abolished by anti-Fas blocking mAb. The presence of anti-Fas mAb alone did not influence the growth of ST4-DN cells (Figure 4Diii).
These results suggested that IFN-γ induces Fas-dependent apoptosis in ST4-DN. To examine this possibility, cells were cultured in complete medium in the presence or absence of IFN-γ for 48 hours, and apoptosis was evaluated by annexin-V staining. In the absence of IFN-γ, there were few or absent apoptotic cells (Figure 4E; open histograms). In its presence, enhancement of apoptosis was evident in ST4-DN cells only (Figure 4E; gray histograms). These data indicate that IGF-1R DN reinstates T-cell sensitivity to IFN-γ–induced, Fas-dependent apoptosis.
Effect of IGF-1R DN on the in vivo growth of malignant T cells
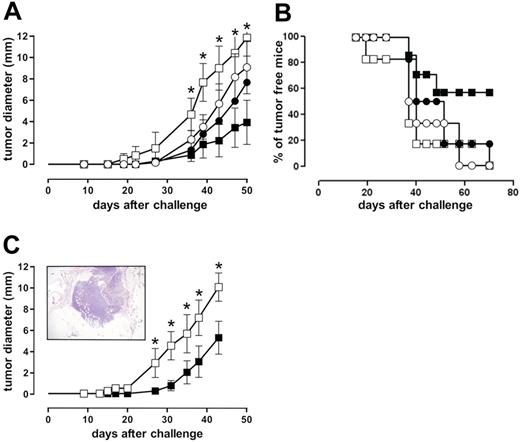
The ability of IGF-1R DN to restore IFN-γ–dependent growth inhibition of ST4 cells in a SCID mouse model was evaluated. ST4-WT and ST4-DN cells were injected subcutaneously into SCID mice that then were treated with IFN-γ or vehicle alone for 5 days. Before tumor cell challenge (24 hours), and then weekly, mice were further immunosuppressed with anti-asialo GM1 rabbit antiserum to eliminate residual NK activity.41 Growth of ST4-WT cells (evaluated as mean diameter of the tumor generated in the site of inoculum) was not significantly inhibited by IFN-γ, whereas that of ST4-DN cells was significantly inhibited (Figure 5A). After 70 days, none of the vehicle-treated mice inoculated with either ST4-WT or ST4-DN cells and 17% of IFN-γ–treated mice inoculated with ST4-WT cells were still tumor free, compared with 57% of those inoculated with ST4-DN (Figure 5B).
IGF-1R blockade reinstates IFN-γ–driven inhibition of ST4 cell growth in SCID mice. (A-B) Anti-asialo GM1-treated SCID mice were challenged subcutaneously with 10 × 106 ST4-WT (○, •) or ST4-DN (□, ▪) cells. After 2 hours, mice were injected at the site of tumor challenge with 0.4 mL vehicle solution (○, □) or 1000 U IFN-γ (•, ▪). This treatment was repeated daily for 5 days, and neoplastic masses were evaluated twice a week with calipers. Results are indicated (A) as the kinetics of the mean ± SD of the tumor diameter and (B) as percentage of tumor-free mice. (C) SCID mice were challenged with 10 × 106 ST4-DN cells as described. After 10 days, 3 mice were killed and tumor sample sections were evaluated by histologic analysis. Samples were collected, formalin fixed, and embedded in paraffin. Sections were rehydrated and stained with hematoxylin-eosin and examined under a microscope. The picture was taken with a B×51 Olympus microscope (Olympus, Hamburg, Germany) equipped with a 4×/0.10 NA Plan objective and a Nikon Coolpix 995 digital camera (Nikon, Melville, NY). The image was processed with Adobe Photoshop 7.0 software (Adobe Systems, San Jose, CA) (inset). The remaining mice were treated with vehicle (□) or IFN-γ (▪) daily for 10 days, starting from day 10 after the inoculum. The graph represents the kinetics of the mean ± SD of the tumor diameter, evaluated as described. Each group consisted of 6 mice, except the ST4-DN–challenged and IFN-γ–treated group (7 mice). *P < .05, IFN-γ–treated versus vehicle-treated ST4-DN–challenged mice.
IGF-1R blockade reinstates IFN-γ–driven inhibition of ST4 cell growth in SCID mice. (A-B) Anti-asialo GM1-treated SCID mice were challenged subcutaneously with 10 × 106 ST4-WT (○, •) or ST4-DN (□, ▪) cells. After 2 hours, mice were injected at the site of tumor challenge with 0.4 mL vehicle solution (○, □) or 1000 U IFN-γ (•, ▪). This treatment was repeated daily for 5 days, and neoplastic masses were evaluated twice a week with calipers. Results are indicated (A) as the kinetics of the mean ± SD of the tumor diameter and (B) as percentage of tumor-free mice. (C) SCID mice were challenged with 10 × 106 ST4-DN cells as described. After 10 days, 3 mice were killed and tumor sample sections were evaluated by histologic analysis. Samples were collected, formalin fixed, and embedded in paraffin. Sections were rehydrated and stained with hematoxylin-eosin and examined under a microscope. The picture was taken with a B×51 Olympus microscope (Olympus, Hamburg, Germany) equipped with a 4×/0.10 NA Plan objective and a Nikon Coolpix 995 digital camera (Nikon, Melville, NY). The image was processed with Adobe Photoshop 7.0 software (Adobe Systems, San Jose, CA) (inset). The remaining mice were treated with vehicle (□) or IFN-γ (▪) daily for 10 days, starting from day 10 after the inoculum. The graph represents the kinetics of the mean ± SD of the tumor diameter, evaluated as described. Each group consisted of 6 mice, except the ST4-DN–challenged and IFN-γ–treated group (7 mice). *P < .05, IFN-γ–treated versus vehicle-treated ST4-DN–challenged mice.
To rule out the possibility that the efficacy of IFN-γ in inhibiting the growth of ST4-DN cells in vivo was due to an effect on the take rate of the xenograft, they were injected subcutaneously into SCID mice and allowed to grow for 10 days. Mice were then treated with IFN-γ or vehicle for 10 days. After 10 days from tumor challenge, histologic analysis revealed that the xenograft had reached 2 to 3 mm in diameter and was stabilized and growing by means of newly formed stromal and vascular structures (Figure 5C; insert) as well as the presence of mitoses and the absence of necrotic areas (not shown). Once again, IFN-γ significantly hampered the growth of ST4-DN cells (Figure 5C), showing that the combination of IGF-1R genetic blockade and IFN-γ administration strongly inhibits ST4 tumorigenicity.
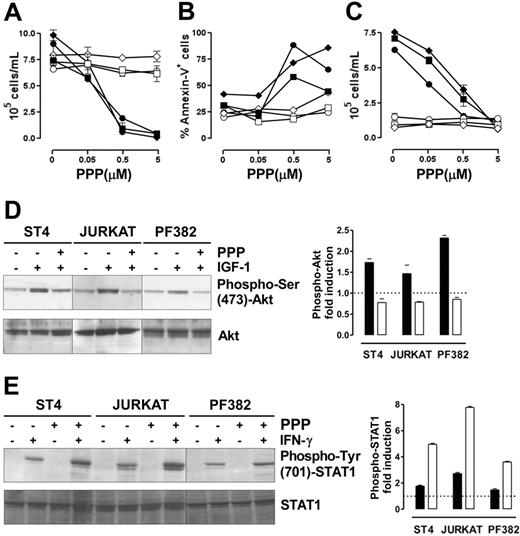
The IGF-1R inhibitor PPP restores IFN-γ/STAT1–dependent apoptosis and suppression of proliferation in human malignant T cells
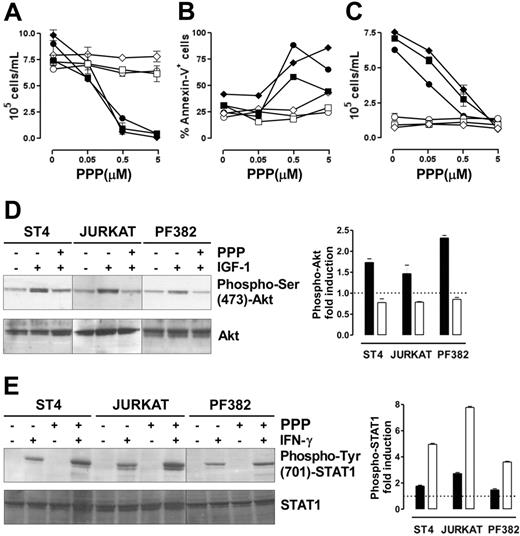
We assessed whether the ability of IGF-1 signaling blockade to restore IFN-γ/STAT1–dependent apoptosis is a general effect that could be translated into a therapeutic strategy to inhibit the growth of human malignant T cells. Therefore, 3 human malignant T-cell lines (ST4, Jurkat, and PF382) were cultured in complete medium in the presence of scalar doses of PPP, a specific inhibitor of IGF-1R tyrosine kinase activity,42 with or without IFN-γ. After 48 hours, cell proliferation and apoptosis were evaluated. Neither PPP nor IFN-γ alone inhibited the proliferation of these lines, whereas the combination of IFN-γ and PPP hindered their proliferation in a dose-dependent manner (Figure 6A). Similarly, annexin-V staining confirmed that only this combination of IFN-γ and PPP (0.5 or 5 μM) induced their apoptosis (Figure 6B). These 2 PPP doses inhibited IGF-1 signaling. They blocked the proliferative response to IGF-1 in cells maintained 48 hours in serum-free medium, but did not alter the growth of cells cultured in serum-free medium without IGF-1 (Figure 6C). Moreover, IGF-1–induced Akt phosphorylation was completely inhibited in all lines after 6 hours of preincubation with PPP (1 μM), whereas total Akt was unaltered (Figure 6D). IGF-1–dependent MAPK phosphorylation, however, was only slightly reduced even after 24 hours of incubation with PPP (data not shown).
The combined administration of PPP and IFN-γ blocks malignant T-cell growth. (A-B) ST4 (○, •), PF382 (⋄, ♦) and Jurkat (□, ▪) T cells were cultured in complete medium with scalar doses of PPP in the absence (white symbols) or presence (black symbols) of IFN-γ. After 48 hours, cells were harvested and (A) stained with trypan blue and counted or (B) stained with PE-conjugated annexin-V and analyzed by flow cytometry. (C) To verify that the doses of PPP used were effective in inhibiting IGF-1 signaling, ST4 (○, •), PF382 (⋄, ♦) and Jurkat (□, ▪) cells were cultured for 48 hours with PPP (from 0 to 5 μM) in serum-free medium with (black symbols) or without (white symbols) IGF-1 (100 ng/mL), then cells were harvested and counted. All the experiments were performed independently at least 3 times. (D) ST4, Jurkat, and PF382 T cells were preincubated with or without PPP (1 μM) for 6 hours before stimulation with or without IGF-1 for 15 minutes. Akt activation was evaluated by Western blot analysis of protein cell extracts with anti–phospho-Ser (473)-Akt Ab. Membranes were subsequently probed with an anti-Akt Ab. Phospho-Akt fold induction in cells treated with IGF-1 (▪) or IGF-1 + PPP (□), shown as means of fold induction ± SEM from 3 independent experiments, was quantified after normalization to Akt. (E) ST4, Jurkat, and PF382 T cells were treated with or without PPP (1 μM) for 6 hours and then stimulated with or without IFN-γ for 15 minutes. STAT1 activation was evaluated by Western blot analysis with anti–phospho-Tyr (701)-STAT1 Ab. Phospho-STAT1 fold induction in cells treated with IFN-γ (▪) or IFN-γ + PPP (□), shown as means of fold induction ± SEM from 3 experiments, was measured after normalization to total STAT1.
The combined administration of PPP and IFN-γ blocks malignant T-cell growth. (A-B) ST4 (○, •), PF382 (⋄, ♦) and Jurkat (□, ▪) T cells were cultured in complete medium with scalar doses of PPP in the absence (white symbols) or presence (black symbols) of IFN-γ. After 48 hours, cells were harvested and (A) stained with trypan blue and counted or (B) stained with PE-conjugated annexin-V and analyzed by flow cytometry. (C) To verify that the doses of PPP used were effective in inhibiting IGF-1 signaling, ST4 (○, •), PF382 (⋄, ♦) and Jurkat (□, ▪) cells were cultured for 48 hours with PPP (from 0 to 5 μM) in serum-free medium with (black symbols) or without (white symbols) IGF-1 (100 ng/mL), then cells were harvested and counted. All the experiments were performed independently at least 3 times. (D) ST4, Jurkat, and PF382 T cells were preincubated with or without PPP (1 μM) for 6 hours before stimulation with or without IGF-1 for 15 minutes. Akt activation was evaluated by Western blot analysis of protein cell extracts with anti–phospho-Ser (473)-Akt Ab. Membranes were subsequently probed with an anti-Akt Ab. Phospho-Akt fold induction in cells treated with IGF-1 (▪) or IGF-1 + PPP (□), shown as means of fold induction ± SEM from 3 independent experiments, was quantified after normalization to Akt. (E) ST4, Jurkat, and PF382 T cells were treated with or without PPP (1 μM) for 6 hours and then stimulated with or without IFN-γ for 15 minutes. STAT1 activation was evaluated by Western blot analysis with anti–phospho-Tyr (701)-STAT1 Ab. Phospho-STAT1 fold induction in cells treated with IFN-γ (▪) or IFN-γ + PPP (□), shown as means of fold induction ± SEM from 3 experiments, was measured after normalization to total STAT1.
Analysis of IFN-γ–induced STAT1 phosphorylation revealed that in the absence of PPP, IFN-γ weakly induced STAT1 phosphorylation in all 3 lines. By contrast, in cells pretreated with PPP for 6 hours, IFN-γ induced a 2- to 3-fold higher STAT1 phosphorylation (Figure 6E).
These data clearly indicate that the pharmacologic blockade of IGF-1 signaling alone is not enough to suppress the proliferation of malignant T cells. However, it effectively reinstates IFN-γ/STAT1–dependent apoptosis in human malignant T cells.
Discussion
This study highlights the critical role played by IGF-1 in down-regulating IFN-γR2 expression and provides the proof-of-concept that either pharmacologic or genetic targeting of IGF-1 signaling in malignant T cells reinstate their sensitivity to IFN-γ/STAT1 apoptotic signaling in vitro and in vivo. This is consistent with our previous data indicating that a mAb directed to IGF-1R up-regulates IFN-γR2 expression and IFN-γ–dependent apoptosis in human T lymphocytes.21
Our data clearly indicate that the expression of a truncated dominant negative form of the IGF-1R in malignant T cells not only hampers IGF-1–induced activation of MAPK and PI3K/Akt pathways and IGF-1–dependent proliferation, but also reinstates the IFN-γ/STAT1 signaling pathway in T lymphocytes. Similarly, treatment with the IGF-1R inhibitor PPP restores their sensitivity to IFN-γ–induced apoptosis. These results shed some lights on how the responsiveness of autoreactive or malignant T lymphocytes could be reverted by gene therapy or pharmacologic approaches that hinder IGF-1 signaling, combined with IFN-γ administration.
Hampering of IGF-1 signaling perturbs the intracellular trafficking of IFN-γR2 and results in its cell-surface accumulation. Compared with T cells in which IGF-1R is intact, T cells with impaired IGF-1 signaling express many more functional IFN-γR complexes that might be engaged by IFN-γ. This induces a strong and sustained STAT1 activation that leads to up-regulation of Fas and FasL and terminates with a triggering of the apoptotic program. This observation fits in well with our previous indications that IFN-γ–dependent apoptotic response in T lymphocytes requires IRF-1, Fas, and FasL induction.8,43 In this respect, others have also demonstrated that IRF-1 induces the expression of caspase-1, caspase-7, and FasL.40,43–45
How IGF-1 could regulate IFN-γR2 intracellular trafficking is still an open question. Crosstalk with TfR, whose trafficking is also clathrin dependent,46 is probably involved. We have previously reported that in T cells iron, through TfR, induces internalization of IFN-γR2.22 Interestingly, IGF-1 enhances iron uptake by inducing an increase of TfR at the cell surface.23 In effect, ST4-DN displayed a lower TfR surface expression than ST4-E and ST4-WT cells (data not shown). IGF-1R blockade may thus be supposed to decrease iron uptake and so reduce iron-dependent IFN-γR2 internalization. This would contribute to the accumulation of IFN-γR2 on the membrane of T cells in which IGF-1R is blocked. This possibility is currently under investigation in our laboratory.
JAK proteins, as well as their kinase activity, play important roles in regulating the cellular localization and trafficking of their cognate receptors, particularly by favoring their surface export and/or stability.47–49 Since JAK2 associates with either IFN-γR2 or IGF-1R,3–5,50 binding of IGF-1 with its receptor may decrease the amount of JAK2 that interacts with IFN-γR2 and thus decrease its cell-surface expression.
Because IGF-1 is known to activate STAT3,37 which counteracts the proapoptotic actions of STAT1,7,51 increased IFN-γ–dependent STAT1 activation may reflect decreased activation of STAT3 in T cells expressing IGF-1R DN. However, Western blot analysis revealed an equally low constitutive STAT3 phosphorylation in ST4-E, ST4-WT, and ST4-DN cells, which was not enhanced by either IGF-1 or IFN-γ (Figures 1C, 3C). This implies that the effect of IGF-1 blockade on the reinstatement of IFN-γ/STAT1 signaling in T cells is entirely due to its effects on IFN-γR2 trafficking.
Many data show that IGF-1 and IGF-1R are good targets for cancer therapy, as they help to establish and maintain the transformed phenotype.24–26,30 Inhibition of IGF-1R causes apoptosis and growth arrest of many cancer cells cultured in vitro or xenotransplanted into immunodeficient mice.52 Several ways of blocking IGF-1R have been experimented, including antisense oligonucleotides,53 blocking Abs,54–56 peptides that mimic IGF-1,57 truncated DN forms of IGF-1R,27,31,34,58,59 small molecules that specifically inhibit IGF-1R kinase activity,42,60,61 and specific peptide aptamers,52 in a wide range of tumors, including hematologic tumors such as multiple myeloma, myeloid leukemias, and B-cell lymphomas.60–62 This is the first assessment of the effects of specific IGF-1 inhibition on malignant T cells. Surprisingly, their growth and survival were not inhibited by IGF-1R blockade, indicating that other factors were sustaining their proliferation. Our data, however, also show that the methods used by others and in part in this work to hinder IGF-1 signaling do inhibit their growth when combined with IFN-γ administration. Thus, cells insensitive to IFN-γ treatment and to IGF-1 signaling blockade alone are sensitive to their combination.
Authorship
Author contributions: L.C. performed most experiments of the research, analyzed the data, and wrote the manuscript; G.R. performed biochemistry experiments and participated in analyzing the data; A.L. assisted all molecular, biochemistry, and cell biology studies; P.B. performed cell sorting to obtain cell lines stably expressing high levels of the mutant IGF-1 receptor; R.C. prepared retroviral vectors and assisted generation and characterization of the transduced cell lines; M.G. performed and analyzed the in vivo experiments; and F.N. designed all aspects of the research, coordinated all experimental approaches, recorded and analyzed data, and wrote the manuscript.
Conflict-of-interest statement: The authors declare no competing financial interests.
Correspondence: Francesco Novelli, Laboratory of Tumor Immunology, CERMS, San Giovanni Battista Hospital–Molinette, Via Santena 5, 10126 Turin, Italy; e-mail: franco.novelli@unito.it.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We thank Dr J. Iliffe for critically reading the manuscript.
Supported by grants from Fondazione Piemontese Studi e Ricerche sulle Ustioni (FPSRU), Associazione Italiana per la Ricerca sul Cancro (AIRC), Compagnia di San Paolo (special project Oncology) and Ministero dell'Istruzione, dell'Università e della Ricerca (MIUR), ex 40%, and Fondo per l'Innovazione e la Ricerca di Base (FIRB). P.B. was supported by a fellowship from Fondazione Internazionale di Ricerca in Medicina Sperimentale (FIRMS).