Abstract
A previous study (LMB89) of the French Society of Pediatric Oncology for childhood mature B-cell lymphoma (B-NHL) demonstrated a 92% 3-year event-free survival (EFS) for intermediate-risk group B defined as “non-resected” stage I/II and CNS-negative advanced-stage III/IV (70% of cases). We performed the FAB/LMB96 trial to assess the possibility of reducing treatment in children/adolescents with intermediate-risk B-NHL without jeopardizing survival. “Early responding” patients (tumor response > 20% at day 7) were randomized in a factorial design between 4 arms, 2 receiving half-dose of cyclophosphamide in the second induction course with cyclophosphamide, Oncovin (vincristine), prednisone, Adriamycin (doxorubicin), methotrexate (COPADM) and 2 not receiving the maintenance course M1. A total of 657 patients were randomized (May 1996 to June 2001) and 637 were analyzed. The analysis showed no significant effect of any of the treatment reductions on EFS and survival. The 4-year EFS was 93.4% and 90.9% in the groups with full-dose and half-dose of cyclophosphamide (RR = 1.3, P = .40) and 91.9% and 92.5% in the groups with and without M1 (RR = 1.01, P = .98). There was no interaction between the 2 treatment reductions or between each treatment reduction and LDH level or histologic subtypes (Burkitt/Burkitt-like or large B-cell). Children/adolescents with intermediate-risk B-NHL who have an early response and achieve a complete remission after the first consolidation course can be cured with a 4-course treatment with a total dose of only 3.3 g/m2 cyclophosphamide and 120 mg/m2 doxorubicin.
Introduction
Cure rates of children with mature B-cell lymphoma, that is, mainly Burkitt, but also diffuse large B-cell lymphoma (DLBCL), have significantly improved over the past 25 years1-15 largely due to prospective studies, including the Lymphomes Malins B (LMB) studies of the French Society of Pediatric Oncology (SFOP).1,3,11 Although the outcome initially was dismal, Burkitt lymphoma appeared chemosensitive, especially to cyclophosphamide, but also to vincristine, cytarabine, and methotrexate. Based on the characteristics of this lymphoma, specifically a high growth fraction and a short doubling time, successful treatments that were developed were intensive, delivering drugs either fractionated or by continuous infusion maintain serum drug level for at least 48 to 72 hours with the shortest delay between the courses. Because relapses were mainly seen within the first year, reduction of treatment duration was attempted. The randomized LMB84 trial (1984-1987) in children with advanced stage Burkitt without CNS involvement showed it was possible to decrease treatment to 5 intensive courses.3 Further improvements consisted in adapting treatment intensity to known or recently recognized prognostic factors. Generally, treatment was stratified by stage as defined by Murphy at St Jude Children's Research Hospital. LDH level, although recognized for a long time as prognostic, but much correlated to stage, was not taken into account for treatment stratification, except in the German Berlin-Frankfurt-Münster (BFM) 90 and 95 strategies. In the LMB89 study (1989-1996), we defined 3 risk groups receiving treatment of progressive intensity: A (resected stage I and abdominal stage II), B (not eligible for A or C), and C (stages IV and L3ALL with CNS involvement or bone marrow involvement > 70%).11 Response to chemotherapy, especially after the first week of treatment, was also considered for adapting treatment intensity. Another question concerned treatment strategy of DLBCL in children. This subentity is rare in children and the results of treatment strategies in adult DLBCL did not encourage us to treat the same way. The option taken in the European groups, mainly the BFM and the SFOP groups, was to treat them using the same protocols as the other aggressive B-cell lymphomas, namely, Burkitt lymphoma. This option was successful in the LMB89 and BFM90 studies, but also in the National Cancer Institute (NCI)/Magrath protocol, with similar results in Burkitt and DLBCL. Thus in the LMB89 study, the event-free survival (EFS) of 63 patients with DLBCL was 89% versus 92% for 420 patients with Burkitt lymphoma (P = .59). For this reason childhood DLBCL, although having a different biology, is treated in several current protocols with the same strategy as Burkitt lymphoma.
In the LMB89 stratification, the intermediate-risk group B represents the majority of the patients (about two thirds). With a 5-course treatment as defined by the randomized LMB84 study, and a treatment intensification for the small subgroup of “bad responders” after 1 week or in partial remission after 3 courses, their 5-year overall survival (OS) and EFS were 94% (95% CI, 91%-96%) and 92% (95% CI, 89%-95%), respectively. However, this successful treatment was associated with a high incidence of acute toxicity including severe mucositis and infection. It was also based on high doses of cyclophosphamide, a drug known to be associated with a risk of infertility.16 This led to an attempt to further reduce treatment without jeopardizing survival, which was the aim of the French-American-British (FAB) LMB96 international study. We report the results of the randomized trial for children and adolescents with mature B-cell lymphoma of intermediate-risk disease (group B).
Patients and methods
General
The FAB/LMB96 study was an open randomized trial that investigated the reduction of treatment. It was a cooperative international study with the collaboration of SFOP (France and some centers in Belgium and The Netherlands), Children's Cancer Group (CCG of the United States, Canada, and Australia), and the United Kingdom Children's Cancer Study Group (UKCCSG). It was a planned 5-year study that opened in May 1996 and closed in June 2001. It included patients in 161 pediatric cancer centers from the 3 national groups. Parents or patients over 18 years of age signed an informed consent form before randomization, in accordance with the Declaration of Helsinki. The protocol was approved by each participating institution's institutional review board. Each national group was responsible for the scientific, ethical, and administrative approvals, the randomization, and the data collection in a national database. These data were transferred every 6 months to the international database held at Institut-Gustave-Roussy for the SFOP. SFOP was responsible for interim and final analysis of group B. Every 6-month reports and interim analyses were reviewed by an international, independent Data and Safety Monitoring Committee (DSMC) including 3 pediatric oncologists and one statistician.
Eligibility
Nonimmunosuppressed patients under 18 (SFOP, UKCCSG) or 21 years (CCG) of age with newly diagnosed de novo mature B-cell lymphoma (either Burkitt, Burkitt-like, or DLBCL) were eligible; other B-cell lymphomas, such as B-lymphoblastic or follicular, were not eligible. Slides were reviewed nationally and by an international panel of cytopathologists.
Group B was defined as in LMB89, with one exception: the upper limit of the bone marrow involvement was 25% instead of 70%.3 Eligibility included the non-resected stages I and II, all stage III, and stage IV CNS negative, according to the St Jude's or Murphy classification.17 Minimal work-up included clinical examination, chest x-ray, abdominal ultrasound or computed tomography (CT), 2 bone marrow aspirates, CSF cytology, and LDH level. CT scans and bone scintigraphy were done as clinically indicated.
Treatment and randomization
Standard treatment was similar to that of group B in LMB89 (except the elimination of vincristine on day 6 in the second induction course of cyclophosphamide, Oncovin [vincristine], prednisone, Adriamycin [doxorubicin], methotrexate [COPADM] course; Figure 1). The prephase COP consisted of low doses of cyclophosphamide, Oncovin (vincristine), and prednisone. Patients with at least a 20% response at day 7 received the first induction course, COPADM1 (cyclophosphamide 1.5 g/m2, Oncovin, prednisone, Adriamycin, high-dose methotrexate [HDMTX 3 g/m2 in 3-hour infusion with intrathecal MTX]). As soon as possible following recovery, patients received the second induction course COPADM2, in which the cyclophosphamide dose was doubled (3 g/m2 divided in 6 fractions administered every 12 hours). After 2 consolidation courses named CYM (cytarabine, HDMTX) treatment concluded with one maintenance course M1 (cyclophosphamide, Oncovin, prednisone, Adriamycin, HDMTX). Response to treatment was defined at 3 time points. The first evaluation was performed after COP at day 7. Patients with tumor reduction less than 20% (poor responder to COP) were switched to the more intensive group C regimen including higher dose methotrexate (8 g/m2), high-dose Ara-C, and VP-16, and were not eligible for randomization. The second evaluation was performed after the first COPADM course and patients were randomized if there was no disease progression. The third evaluation was performed after the first consolidation CYM course. Residual mass was recommended to be removed for histology or at least to be biopsied. Patients with the presence of malignant cells in residual mass were switched to the more intensive group C regimen.
Treatment schema and events according to the treatment arm. COP: cyclophosphamide (300 mg/m2, day 1), Oncovin (vincristine; 1 mg/m,2 day 1), prednisone (60 mg/m2/d, days 1-7), plus intrathecal methotrexate (IT MTX, day 1); COPADM: cyclophosphamide (1.5 g/m2 in COPADM1, 3 g/m2 in COPADM2), Oncovin (2 mg/m2, day 1), prednisone (60 mg/m2/d, days 1-7), Adriamycin (doxorubicin; 60 mg/m2, day 2), high-dose (HD) MTX (3 g/m2 in 3-h infusion, day 1, plus leucovorin rescue starting at hour 24 until serum MTX level is less than 1 × 10−7 M) plus IT MTX, day 2 and day 6. CYM: cytarabine (100 mg/m2 in continuous infusion, days 2-6, plus IT cytarabine, day 6), HD MTX (3 g/m2 in 3-h infusion, day 1, plus leucovorin rescue, plus IT MTX, day 2). M1: cyclophosphamide (1 g/m2), Oncovin (2 mg/m2, day 1), prednisone (60 mg/m2/d, days 1-7), Adriamycin (60 mg/m2, day 2), HD MTX (3 g/m2 in 3-h infusion, day 1, plus leucovorin rescue starting at hour 24) plus IT MTX, day2. IT: intrathecal injections: all are with hydrocortisone and age-adjusted. Oncovin: max 2 mg.
Treatment schema and events according to the treatment arm. COP: cyclophosphamide (300 mg/m2, day 1), Oncovin (vincristine; 1 mg/m,2 day 1), prednisone (60 mg/m2/d, days 1-7), plus intrathecal methotrexate (IT MTX, day 1); COPADM: cyclophosphamide (1.5 g/m2 in COPADM1, 3 g/m2 in COPADM2), Oncovin (2 mg/m2, day 1), prednisone (60 mg/m2/d, days 1-7), Adriamycin (doxorubicin; 60 mg/m2, day 2), high-dose (HD) MTX (3 g/m2 in 3-h infusion, day 1, plus leucovorin rescue starting at hour 24 until serum MTX level is less than 1 × 10−7 M) plus IT MTX, day 2 and day 6. CYM: cytarabine (100 mg/m2 in continuous infusion, days 2-6, plus IT cytarabine, day 6), HD MTX (3 g/m2 in 3-h infusion, day 1, plus leucovorin rescue, plus IT MTX, day 2). M1: cyclophosphamide (1 g/m2), Oncovin (2 mg/m2, day 1), prednisone (60 mg/m2/d, days 1-7), Adriamycin (60 mg/m2, day 2), HD MTX (3 g/m2 in 3-h infusion, day 1, plus leucovorin rescue starting at hour 24) plus IT MTX, day2. IT: intrathecal injections: all are with hydrocortisone and age-adjusted. Oncovin: max 2 mg.
The possibility of further reducing treatment by deleting M1 and giving half the dose of cyclophosphamide in the second induction COPADM course was assessed using a factorial design. Responding patients at the first and second evaluations were randomized after the first COPADM between 4 arms, 2 receiving a reduced dose (50%) of cyclophosphamide in the second COPADM (ie, induction courses were 2 COPADM1) and 2 not receiving M1 (Figure 1). The randomization was performed in blocks of 4, with equal allocation, and stratified for national group (CCG, SFOP, UKCCSG), histology (DLBCL or not), stage, and LDH level (stages I-II, stage III with LDH less than or equal to more than 2 times (2N) the upper limit of the institutional normal range, and stage III with LDH > 2N or stage IV).
Statistical methods
The primary end point of the trial was EFS, defined as the minimum time between randomization and progressive disease or relapse or second malignancy or death from any cause or the last follow-up contact for patients who did not experience any event. Secondary end points were survival and failure-free survival (FFS). Survival was defined as the time between randomization and death from any cause or the last follow-up contact for patients who were alive. The FFS was defined as the minimum time between randomization and biopsy-positive residual disease following the first CYM (no complete response at third evaluation) or any other event as defined in the EFS (ie, progressive disease or relapse or second malignancy or death from any cause) or the last follow-up contact for patients who did not experience any failure. The term FFS was applied to account for patients with residual biopsy-proven disease after CYM1 who may have achieved and remained in remission after intensified therapy. Therefore, the FFS was analyzed and compared between reduced dose of cyclophosphamide in the second COPADM induction course and full dose of cyclophosphamide but not between no M1 versus M1.
The comparisons between treatments (first comparison: reduced dose of cyclophosphamide in the second COPADM induction course versus full dose of cyclophosphamide and second comparison: no M1 versus M1) were based primarily on the profile Cox-likelihood confidence bounds for the log hazard ratio β, where h1(t) and h0(t) are the hazard function in the reduced and standard therapy groups, respectively, and h1(t) = h0(t)eβ. The criterion for detecting a reduction in treatment efficacy was that the lower 80% profile-likelihood confidence for β exceeded zero. Three interim analyses were performed, with nominal confidence levels adjusted according to the spending function approach of Lan and DeMets.18 Two-sided 90% bounds are also reported, the higher number of which provides a one-sided 95% bound on the possible reduction in efficacy.
The trial was planned with a 5-year accrual to include at least 460 evaluable patients for the randomized comparisons. In the event of a 7% reduction in EFS, from 90% to 83% observed in the 460 patients, the probability that the lower one-sided 80% confidence bound exceeded 0 (ie, that the reduced treatment was declared insufficient) was 90% at the final analysis using the methods of Rubinstein19 adapted for survival functions that exhibit a cured fraction.20
Survival functions for the time-to-event end points were estimated with the Kaplan-Meier method. The 95% CIs of the actuarial rates were calculated with the Rothman method. Cox models that included the treatment factors (reduced dose of cyclophosphamide in the second COPADM course and deleting M1) and the stratification factors (national group, histology, stage, and LDH level) were used to analyze each end point. Because there was no significant interaction between the 2 treatment reductions, the interaction was not included in the final models. The interaction between major clinical characteristics (stage, LDH level, histology, primary mediastinal DLBCL) and treatment reductions were tested in the Cox models (Wald test). Analyses were carried out according to the intention-to-treat principle on eligible patients (exclusion of 20 ineligible randomized patients). Analysis was also performed on all the randomized patients and gave similar results (data not shown). Reported P values are 2-sided.
Results
Patient demographics and randomization
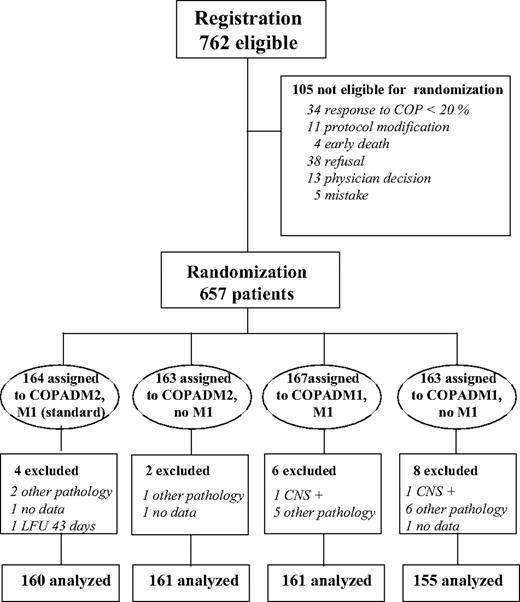
As indicated in Figure 2, during the planned 5-year period of inclusion, 762 patients were registered in group B (349 CCG, 260 SFOP, 153 UKCCSG), of which 105 (14%) were not randomized: 49 (6.5%) were not eligible for randomization (no response to COP [4.5%], protocol modifications, or death) and 56 for various reasons, mainly parental or physician refusals. Histopathologic slides of 606 of the 657 randomized patients (92%) were reviewed by the international pathology review panel. Final diagnosis was the international panel diagnosis and, if not available, that of the national or local pathologist. Among the 657 randomized patients, 16 were declared ineligible after pathologic review (7 B-lymphoblastic, 1 follicular, 1 other B-NHL, 2 T-NHL, 2 other NHL, 2 Hodgkin lymphoma, 1 malignancy other than lymphoma; among them, 3 had an event, but are alive) and 4 have no available clinical data. Thirty-five were considered eligible although not subclassified because of poor technical quality of slides. The analysis of the trial is based on 637 patients. Their baseline characteristics are shown in Table 1. They are not significantly different in the 4 randomized arms.
There were very few protocol deviations: 3 patients in the reference arm did not receive M1 because of “toxicity,” one patient in each of the 3 reduced arms received the reference regimen, one by error and 2 after parental consent was withdrawn.
Treatment failures and events of randomized patients
There were 68 failures. Twenty-three were related to no complete remission (CR) at the third evaluation (10 with full dose and 13with reduced dose of cyclophosphamide in second COPADM). As recommended by the protocol, these 23 patients were switched to the group C regimen. Among them, 16 patients did not progress and were cured (these patients were considered as having failure but not an event). The other 7 patients had an event (5 no further CR and 2 relapses) included in those described below.
There were 52 events after randomization, equally distributed among arms: 12 progressions during treatment (7 before or at third evaluation, 5 after third evaluation; all died despite switching to the group C regimen or another treatment), 38 relapses, 1 late death unrelated to lymphoma (4.9 years after lymphoma), and 1 second Burkitt lymphoma (4.4 years after the first one) who was cured after receiving the group C regimen.
Among the 38 relapses, 21 occurred in Burkitt/Burkitt-like, 15 in DLBCL (8 mediastinum and 2 T-cell rich), and 2 in non-subclassified lymphoma. Twenty-three were in only one site (16 local, 1 CNS, 6 in another site than primary, none in bone marrow) and 15 in several sites (13 involving primary site and 2 CNS). Most of the relapses occurred during the first year after diagnosis, except one Burkitt at 1.7 years and 3 DLBCLs at 1.6, 4, and 4.6 years, respectively. Sixteen of the 38 patients who relapsed are alive.
EFS, FFS, and OS
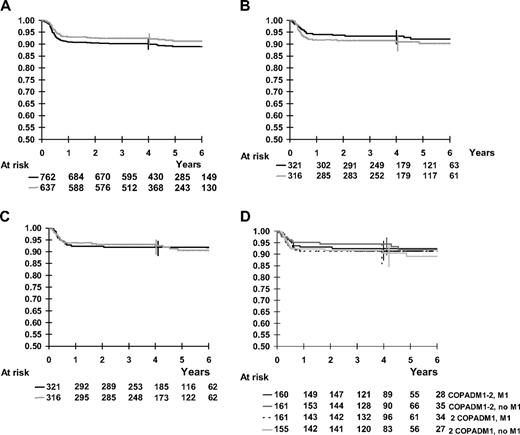
The median follow-up was 54 months (range, 6 months to 8.3 years). For all 762 patients registered in group B, 4-year survival was 92.7% and 4-year EFS was 90.2%. Among the randomized patients, 4-year OS, EFS, and FFS were 94.6% (95% CI, 93%-96%), 92.2% (95% CI, 90%-94%), and 89.9% (95% CI, 87%-92%), respectively (Figure 3A). By stage, 4-year EFS was 98.4% in stages I and II, 89.8% in stage III, and 85.6% in stage IV CNS negative. The 4-year EFS was 95.8% and 85.7%, respectively, for patients with LDH below or above 2-fold the upper limit of the institutional normal value. The 4-year EFS was 93.3%, 92.7%, and 71.5%, respectively, for patients with Burkitt, DLBCL not primary mediastinal, and primary mediastinal DLBCL.
Kaplan-Meier curves for survival, EFS, and FFS. Vertical bars denote 95% CI of the actuarial rates. (A) Kaplan-Meier estimates of EFS of all patients and of randomized patients. Solid black line indicates all patients, 4-year rate, 90.2%; dotted line, randomized patients, 4-year rate from start of treatment, 92.4% (92.2% from time of randomization). (B) Kaplan-Meier estimates of EFS according to cyclophosphamide dose randomization in the second COPADM course. Black line indicates full-dose CPM in second COPADM, 4-year rate, 93.4%; gray line, half-dose CPM in second COPADM, 4-year rate, 90.9% (C) Kaplan-Meier estimates of EFS according to maintenance course M1 randomization. Black line indicates with M1, 4-year rate, 91.9%; gray line, without M1, 4-year rate, 92.5%. (D) Kaplan-Meier estimates of EFS according to the 4 arms of randomization. COPADM1-2, M1 (standard), 4-year rate, 92.5%; COPADM1-2, no M1, 4-year rate, 94.4%; 2 COPADM1, M1, 4-year rate, 91.3%; 2 COPADM1, no M1, 4-year rate, 90.5.
Kaplan-Meier curves for survival, EFS, and FFS. Vertical bars denote 95% CI of the actuarial rates. (A) Kaplan-Meier estimates of EFS of all patients and of randomized patients. Solid black line indicates all patients, 4-year rate, 90.2%; dotted line, randomized patients, 4-year rate from start of treatment, 92.4% (92.2% from time of randomization). (B) Kaplan-Meier estimates of EFS according to cyclophosphamide dose randomization in the second COPADM course. Black line indicates full-dose CPM in second COPADM, 4-year rate, 93.4%; gray line, half-dose CPM in second COPADM, 4-year rate, 90.9% (C) Kaplan-Meier estimates of EFS according to maintenance course M1 randomization. Black line indicates with M1, 4-year rate, 91.9%; gray line, without M1, 4-year rate, 92.5%. (D) Kaplan-Meier estimates of EFS according to the 4 arms of randomization. COPADM1-2, M1 (standard), 4-year rate, 92.5%; COPADM1-2, no M1, 4-year rate, 94.4%; 2 COPADM1, M1, 4-year rate, 91.3%; 2 COPADM1, no M1, 4-year rate, 90.5.
In the first comparison (Table 2), the 4-year EFS (95% CI) was 93.4% (90%-96%) and 90.9% (87%-94%) in the groups with the full versus half dose of cyclophosphamide in second COPADM, respectively (Figure 3B). The 4-year OS rates (95% CI) were 95.6% (93%-97%) and 93.6% (90%-96%) and the 4-year FFS rates (95% CI) were 90.6% (87%-93%) and 88.7% (85%-92%), respectively. The hazard ratio of event in the group randomized to half-dose cyclophosphamide compared to the group randomized to full dose was 1.27 (P = .40). The hazard ratio of death was 1.56 (P = .20) and the hazard ratio of failure was 1.10 (P = .70). The upper one-sided 95% confidence bounds for these hazard ratios were 2.0, 2.8, and 1.6, respectively.
In the second comparison, the 4-year EFS rates (95% CI) were 91.9% (88%-94%) and 92.5% (89%-95%) in the groups with and without M1, respectively (Figure 3C). The 4-year OS rates (95% CI) were 94.0% (91%-96%) and 95.2% (92%-97%) respectively. The hazard ratios of event and death were, respectively, 1.01 (P = .98) and 0.86 (P = .66) in the group randomized to no M1 as compared to the group randomized to M1. The upper one-sided 95% confidence bounds for these hazard ratios were 1.6 and 1.5, respectively.
There was no significant interaction between the 2 therapy reductions on EFS (P = .55) or survival (P = .50). EFS of the 4 randomization arms is presented in Figure 3D. Furthermore, there was no significant interaction between the therapy reductions and prognostic factors, especially LDH level, stages, and histology (Table 3).
Toxicity
Comparative acute toxicity of the COPADM courses is shown in Table 4. The only differences between them were the dose and the fractioning of cyclophosphamide. The first COPADM course and the second COPADM course with the full dose of cyclophosphamide had a similar toxicity profile, but there were significant differences in the second COPADM course between the full and half dose of cyclophosphamide, with lower toxicity in the latter. However, the rates of grade 4 infections were not significantly different between these 2 courses. Toxicity of M1 was avoided in B2 and B4 arms. Toxicity of CYM was much lower than that of the COPADM courses with 5% of any grade 4 toxicity, 3% grade 4 infections, and 1% grade 3-4 stomatitis. No toxic death occurred during treatment after the first COPADM course.
Discussion
This trial is the largest ever conducted in mature B-cell lymphoma of childhood and adolescence, including 637 eligible randomized patients. Conducting a trial investigating the reduction of treatment in a rare disease, which only in recent years has become highly curable but where salvage therapy is relatively ineffective, was a challenge that necessitated the collaboration of 3 national cooperative groups and rigorous management and monitoring by an independent DSMC. This international trial demonstrated that the reduction of the cyclophosphamide dose in the second COPADM (1.5 instead of 3 g/m2) and the deletion of the maintenance course did not significantly modify the EFS and OS. Moreover, the reduction of the cyclophosphamide dose in the second COPADM did not significantly reduce the FFS.
In the past, treatment duration has arbitrarily been reduced in several other studies for childhood mature B-cell lymphoma2,9,11,21,22 but has been demonstrated as possible in only 2 randomized studies, the previous LMB843 and the current FAB/LMB96 study. This successful reduction in therapy occurred not only in patients with less advanced stages, but also in the patients with CNS-negative advanced stage and with high LDH levels.
The intermediate-risk group B represents 70% of the children and adolescents with B-NHL. Among them, fewer than 10%, the poor responders, either at day 7 or after the third treatment course, could not benefit from the reduced treatment. Therefore, with 90% 4-year EFS, the conclusion of the trial applies to a majority of patients with mature B-cell lymphoma.
In this trial, both Burkitt lymphoma and DLBCL were included. Biology of these 2 entities is different, and this is reflected by the occurrence of later relapses in DLBCL: 3 between 21 and 32 months in the LMB89 study and 2 at 48 and 54 months in this study. However, none occurred after 5 years, especially in the LMB89, which now has a long-term follow-up. Despite their different biology, Burkitt lymphoma and childhood DLBCL had similar outcomes when treated with the LMB89 regimen,11 which was also reported with the B-BFM strategy.9 It is for this reason that both histologic entities were included in the present randomized trial. Although the numbers of DLBCL are smaller than that of Burkitt lymphoma, survival and EFS were not significantly different and there was no significant interaction between therapy reduction and histology. There appear to be biologic features indicating that DLBCL in children is less heterogeneous than in adults and is mainly of the germinal center type.23,24 This might explain the better prognosis in children and the good outcome with an intensive and short therapeutic strategy such as the LMB and the BFM regimens. The exception is the primary mediastinal large B-cell lymphoma, whose overall results are clearly inferior, indicating the need for a different treatment strategy.25
Evaluation of residual masses and the recommendation to document them histologically before intensifying treatment in cases of incomplete remission was one of the difficulties of this study. Some patients had potentially unnecessary intensification, whereas other patients with residual masses, which progressed, ultimately might have benefited from earlier intensification. Usefulness of positron emission tomography (PET) scanning to assess viability of a residual mass is under evaluation in childhood lymphoma with the hope that it might replace histologic documentation, but this evaluation is less advanced than in adult lymphoma.26
The experimental arm with the least therapy included 4 courses of treatment delivering 4 high doses of MTX (3 g/m2 in 3-hour infusions with leucovorin rescue), 2 courses of fractionated cyclophosphamide giving a total dose of only 3.3 g/m2, 2 courses of doxorubicin (120 mg/m2), and 3 doses of vincristine, 2 courses of 5-day continuous infusion of Ara-C, 9 intrathecal injections of MTX or Ara-C, and 3 courses of corticosteroids. In this reduced arm, patients still experienced induced acute toxicity of the 2 induction courses, mainly febrile neutropenia and mucositis, which were not modified by the addition of G-CSF as shown in a previous randomized study. 27 However, toxicity was lessened in the second course. In addition, with the omission of one course (M1), patients benefited from reduced days of hospitalization and avoided complications of this course in which grade III-IV toxicities occur in half the cases. Besides general costs, which were decreased, late toxicity should be minimal with a total dose of each drug still being rather low. In particular, it is expected that 3.3 g/m2 cyclophosphamide and 120 mg/m2 doxorubicin should not lead to infertility or cardiotoxicity and are unlikely to lead to second malignancies. A longer follow-up of these patients will be required to determine the long-term effects of this treatment. The only second malignancy observed at present in this large series is a second lymphoma of the same histology but with different biologic features, occurring at 4.4 years, raising the question of an inherent predisposition.
In conclusion, in non-resected mature B-cell lymphoma of childhood and adolescence with no CNS involvement, a response after prephase COP and a CR obtained after the first consolidation course, a 90% cure rate can be achieved with an intensive but shortened treatment delivering low doses of cyclophosphamide (3.3 g/m2) and doxorubicin (120 mg/m2), thus reducing general treatment costs.
Authorship
Contribution: M.S.C. and C.P. designed and performed the research, analyzed data, and wrote the manuscript; A.A. designed and performed research, analyzed data, and wrote the manuscript; M.G. designed and performed research and analyzed data; J.M. and R.P. designed and performed research; R.S. designed research and analyzed data; C.W. analyzed data; and M.R., S.L.P., and K.M. performed research and analyzed data.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
A complete list of the participants in the FAB LMB 96 International Study Committee appears in Appendix S1, available on the Blood website; click on the Supplemental Appendix link at the top of the online article.
Correspondence: Catherine Patte, Pediatrics Department, Institut Gustave Roussy, Rue Camille Desmoulins, 94805 Villejuif Cedex, France; e-mail: patte@igr.fr.
Presented in part at the American Society of Clinical Oncology (ASCO), June 2003, Chicago, IL, and the American Society of Hematology (ASH), December 2003, San Diego, CA.
An Inside Blood analysis of this article appears at the front of this issue.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
This work was supported by grants from the Division of Cancer Treatment, National Cancer Institute, National Institutes of Health, Department of Health and Human Services (COG); Cancer Research Campaign (UKCSSG); and Association pour la Recherche contre le Cancer, La Ligue Nationale Contre le Cancer, Institut-Gustave-Roussy (SFOP).
The authors are indebted to the members of the national and international review panels (M. Raphael, M. J. Terrier-Lacombe, C. Bayle, P. Felman, M. Lones, S. Perkins, K. McCarthy, A. Wotherspoon), and to all the CCG, SFOP, and UKCCSG committee members and investigators who participated in the trial, listed in the Supplemental Appendix (available on the Blood website; see the Supplemental Appendix link at the top of the online article), to Noelle Dupouy for the data management of the SFOP patients, to Rachel Hobson and Kath Robinson for the data management of the UKCCSG patients, to Andre Dalton for the data management of the CCG patients, to Pascale Jan as computer officer of the international database, to Ariane Dunant for statistical advice in protocol design, and to the members of the DSMC (M. Link, A. Reiter, D. Harrington, and R. Souhami) for their active work. The authors are also grateful to Linda Rahl, Virginia Davenport, and Donna Correia for editorial assistance.