In this issue, Johnson and colleagues provide a conformational basis for hereditary elliptocytosis (HE) due to proline substitutions in linkers between spectrin repeats.
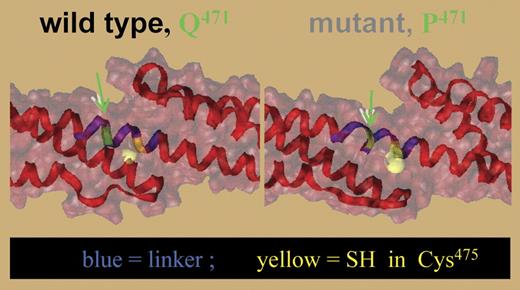
Molecular model showing destabilization of the linker (blue) between repeats 4 and 5 as a result of the Q471P substitution. The mutated site and nearby Cys475 are shown in green and yellow, respectively. Disruption in the helix in the linker increases exposure of the Cys475. See the complete figure in the article beginning on page 3538.
Molecular model showing destabilization of the linker (blue) between repeats 4 and 5 as a result of the Q471P substitution. The mutated site and nearby Cys475 are shown in green and yellow, respectively. Disruption in the helix in the linker increases exposure of the Cys475. See the complete figure in the article beginning on page 3538.
In red cells, tetramers of α- and β-spectrin impart deformability and flexibility to the membrane. Both α- and β-spectrin consist of a series of 106 amino acid repeats separated by short linker regions. Each repeat consists of 3 bundled α-helices (A, B, and C). α- and β-spectrin align side-to-side to form antiparallel heterodimers, which then self-associate into tetramers by the joining of 2 partial repeats—one at the N-terminus of α-spectrin that contributes helix C, and the other at the C-terminus of β-spectrin contributing helices A and B. The dimer-to-tetramer conversion is critical to membrane stability. Suboptimal tetramerization weakens spectrin-spectrin interactions leading to mechanically unstable red cells and hereditary elliptocytosis (HE).1 Molecular modeling predictions and nuclear magnetic resonance (NMR) studies reveal that even conservative amino acid substitutions in the self-association site cause conformational changes that destabilize tetramers and produce HE.2
Mutations in α-spectrin distant from the self-association site also occur in HE. Notable among these are mutations within linker regions between repeats, many of which result in proline substitution. One such mutation, Q471P between repeats (Rs) 4 and 5, is the focus of the current study. Johnson and colleagues show that the Q471P mutation has no effect on the association of a recombinant N-terminal R1 to R5 α-spectrin protein with recombinant C-terminal R16 to R17 β-spectrin. Despite this, nondenaturing gels show increased dimers in the patient. The authors hypothesize that proline substitutions result in cooperative unfolding of spectrin, and that these conformational changes destabilize tetramers.
Using multiple measures of thermal denaturation, data supporting this hypothesis are presented. Circular dichroism (CD) spectra reveal that the helical content of the mutant construct is decreased at body temperature compared with wild type, indicating unfolding of the mutant construct. Furthermore, the helicity in the wild-type construct decreases at temperatures higher than 40°C in 2 stages, one corresponding to an initial unfolding of the R4 to R5 domain (transition I) followed at higher temperatures by cooperative unfolding of R1 to R3 (transition II). In the mutant construct, transition I is clearly abnormal. Molecular modeling also shows that the helical structure of R4 to R5 is disrupted near the proline residue. Unfolding is predicted to increase exposure of hydrophobic cysteines, thereby increasing reactivity in the mutant to the fluorescent dye IAEDANS (see figure). Indeed, at 37°C or higher, increased labeling is seen in the mutant construct. Mass spectrometry specifically detects IAEDANS labeling of Cys475, indicating disruption of linker helicity near the mutant proline residue. Finally, single-molecule unfolding by atomic force microscopy indicates that the mutant construct has lost mechanical resiliency, consistent with destabilization of spectrin repeats.
The results illustrate that loss of linker helicity limits the stabilizing effects between repeats 4 and 5 resulting in unfolding. How does this unfolding disrupt dimer-tetramer interactions? The ends of spectrin heterodimers are staggered. In the tetramer, repeats 4-5 of α-spectrin are physically close to the C-terminus of β-spectrin, suggesting that a physical mechanism such as steric hindrance may shift the distribution toward dimers. It seems likely that similar conformation-location effects form the basis of other HE-producing linker mutations.
The author declares no competing financial interests. ▪