Abstract
In a search for new genes involved in the regulation of erythropoiesis, we identified murine Penumbra cDNA from a multipotent hematopoietic cell line based on its predominant expression in erythroblasts. Subsequently, we identified the human PENUMBRA from a bone marrow cDNA library. Penumbra is a new member of the tetraspanin superfamily of membrane proteins, many of which are thought to function as organizers of supramolecular signaling complexes. Human and murine Penumbras contain 283 amino acids and are 97% identical. The human PENUMBRA gene is mapped to chromosome 7q32, a hot spot for deletions in myelodysplastic syndromes and acute myelogenous leukemias. Penumbra is targeted to the cell surface and forms disulfide-bonded homodimers. To study the effects of Penumbra deletions, we created a knockout mouse model by gene targeting. Penumbra−/− mice develop massive splenomegaly, basophilic macrocytic red blood cells, and anemia as they age. A multipotent hematopoietic cell line, EMX, was established from the bone marrow of a Penumbra−/− mouse. EMX exhibits ineffective erythropoiesis in the presence of erythropoietin, a defect that is reversed by reexpression of Penumbra. These findings indicate that Penumbra has a positive function in erythropoiesis and its deletion or mutation may result in anemia.
Introduction
Many signaling events occur in specialized membrane microdomains such as the tetraspanin microdomain and the lipid raft.1-9 Tetraspanin microdomains are supramolecular complexes of tetraspanins, cell surface receptors, and signal transducers.10,11 Members of the tetraspanin family, also known as the transmembrane 4 superfamily, are transmembrane (TM) proteins that are conserved from Dictyostelium to humans and characterized by 4 TM domains, a small extracellular domain (ECD1) between the first and second TM domains, and a large ECD2 between the third and fourth TM domains.12 Most tetraspanins also contain CCG and PXSCC motifs in ECD2. The cysteines in these motifs are thought to form intramolecular disulfide bonds.12
The second microdomain, the lipid raft, contains high concentrations of sphingolipid and cholesterol and is resistant to mild detergents.1,5,6 Lipid rafts play important roles in organizing the B-cell receptor (BCR) complex, which also contains cell membrane proteins cluster of differentiation 19 (CD19), CD21, and CD81.13-16 CD81 is also a tetraspanin.17 Evidence suggests that CD81 facilitates the partitioning of coligated BCR and CD19/CD21 complexes into lipid rafts and stabilizes the BCR signaling complexes, thereby reducing the threshold for signaling.18-20
The fact that tetraspanins are found in both microdomains suggests that they may have a fundamental role in the functional organization of cell membranes. Emerging evidence indicates that some tetraspanins serve as the organizers of supramolecular receptor complexes.11 A well-characterized association between tetraspanins and cell surface receptors is that between CD151 and β1 integrins.21,22 Similar associations have been described between CD81 and α4β1 and between CD82 and α4β1 and αLβ1.11 Interactions between tetraspanins and intracellular signal transduction molecules have been described for CD81 and γ-glutamyl transpeptidase, protein kinase C, and phosphatidylinositol 4-kinase.11
Some tetraspanins were initially identified as leukocyte differentiation antigens such as CD9, CD37, CD53, CD81, and CD151. CD63 was first identified as a protein associated with melanoma progression while CD82 was originally cloned as a suppressor of prostate cancer metastasis.23-25 A specialized tetraspanin is peripherin,26 which is expressed exclusively in the disc rim of the outer segment of rod photoreceptors and plays a crucial role in the morphogenesis of lamellar discs.27,28 Spontaneous mutations of peripherin cause retinal degeneration and blindness.29
Here, we describe the cloning and characterization of a new tetraspanin, Penumbra (Pen), with predominant expression in erythroblasts. Many Pen knockout (KO) mice develop marked splenomegaly and severe anemia as they age, indicating that Pen has an important role in normal erythropoiesis.
Materials and methods
cDNA representational difference analysis
cDNA representational difference analysis (RDA) was performed as previously described.30,31 Briefly, both EML C.1 32 and MPRO33 cDNAs were synthesized from polyA-positive RNAs using oligo(dT) as primers. Double-stranded cDNAs were digested with DpnII and ligated with R-Bgl-12 and R-Bgl-24 linker/primers and amplified by polymerase chain reaction (PCR) to generate a representation of cDNAs. The MPRO cDNA representation (the “driver”) was digested with DpnII to remove linker/primers. The EML C.1 cDNA representation (the “tester”) was digested with DpnII to remove linker/primers, gel purified, and religated with a second set of linker/primers (J-Bgl-12 and J-Bgl-24). The J-Bgl-12/24-ligated EML C.1 cDNA representation was mixed with a 100-fold excess of melted MPRO cDNA representation and hybridized at 67°C for 24 hours. Common sequences formed tester-driver duplexes that contained the linker/primer at only one end and could be amplified only in a linear fashion by PCR. Unique sequences formed tester-tester duplexes that contained linker/primer at both ends and were amplified exponentially. The resultant DNAs were used to start the next round of RDA. The ratios of tester to driver DNAs were 1:8000 and 1:40 000 for the second and third rounds of RDA, respectively. The final products were gel purified and cloned into pBluescript SK(+) (Stratagene, La Jolla, CA). To obtain full-length cDNAs, oligo(dT)-primed cDNA libraries of EML C.1 and human bone marrow (BM) were screened with P32 -labeled probes.
Northern and Western analyses
Total RNAs (5 to 10 μg) were resolved on 1% formaldehyde agarose gels, blotted onto Hybond-N (Amersham, Piscataway, NJ), and hybridized with P32-labeled probes at 65°C in Rapid-hyb (Amersham). Final washing was done in 0.1× standard sodium citrate (SSC)/0.1% sodium dodecyl sulfate (SDS) at 65°C. The multitissue Northern blot (Clontech, Palo Alto, CA) contains 1 μg poly(A)-positive RNA per lane. For Western analyses, protein lysates (5 to 10 μg) were separated by denaturing SDS–polyacrylamide gel electrophoresis (SDS-PAGE), blotted onto Immobilon-P PVDF (Millipore, Bedford, MA), and visualized by enhanced chemiluminescence.
Cell purification and quantitative real-time reverse transcription–PCR
Phycoerythrin (PE)– or fluorescein isothiocyanate (FITC)–conjugated monoclonal antibodies (MAbs) against TER119, CD3 (clone 145-2C11), B220 (clone RA3-6B2), Gr1(clone RB6-8C5), and NK1.1 (clone PK136) were purchased from Becton Dickinson (San Jose, CA). For BM or splenocyte subset isolation, mononuclear cells depleted of red blood cells (RBCs) were labeled with MAb and purified by fluorescence-activated cell sorting (FACS) using a FACS Vantage (Becton Dickinson). Total RNAs were isolated using an RNeasy Kit (Qiagen, Valencia, CA) and reverse transcribed with oligo(dT) and Superscript III (Invitrogen, Carlsbad, CA). Real-time PCR assays were performed using SYBR Green PCR Master Mix (Applied Biosystems, Foster City, CA) and the MyiQ Real-Time PCR Detection System (Bio-Rad, Hercules, CA). Each amplification mixture contained 2 μL cDNA and 0.25 μM forward and reverse primers with the exception of β-actin PCR, in which 0.5 μM primers were used. Cycling parameters were 50°C for 10 minutes, 40 cycles of 95°C for 1 second and 60 °C for 1 minute, and 72°C for 5 minutes. PCR was performed in triplicate and normalized using β-actin as internal control. Relative levels of gene expression were calculated by the comparative cT method.34 Primer sequences were as follows: β-actin, 5′-TGGGTCAGAAGGACTCCTATG (sense) and 5′-CAGGCAGCTCATAGCTCTTCT (antisense); βmajor-globin, 5′-GCTTCTGACATAGTTGTGTTG (sense) and 5′-GTGGTACTTGTGAGCCAAGGC (antisense); and erythropoietin receptor (EPO-R), 5′-GCAGGAGGGACACAAAGG (sense) and 5′-AGGTTGCTCAGAACACACTCAG (antisense).
Expression vectors and immunofluorescence microscopy
The entire coding region of Pen except the stop codon was cloned in frame into pcDNA3.1 containing the Myc-His tag (Invitrogen) or into pEGFP N1 (Clontech) that expresses proteins as fusion proteins with enhanced green fluorescent protein (EGFP) in the C termini. The sequence preceding the start codon was modified to create a Kozak consensus sequence (5′GCCGCCACC). The resultant vectors were designated as pcDNA3.1/pen-Myc and pEGFP N1/pen-EGFP, respectively. Cells were transfected with 4 to 8 μg DNA per 2.5 × 106 cells or per 100 mm dish by electroporation using Gene Pulser Xcell (Bio-Rad) or by the calcium phosphate precipitate method. For detection of Myc-tagged proteins, methanol-fixed cells were stained with rhodamine-conjugated anti-Myc MAb, 9E10 (Santa Cruz Biotechnology, Santa Cruz, CA), and embedded in Vectashield containing 4,6-diamidino-2-phenylindole (DAPI) (Vector Laboratories, Burlingame, CA).
The retroviral vector MSCV-pen-PGK-EGFP was constructed by cloning the coding sequence of mPen including the stop codon into the BglII/HpaI cloning sites of the parental vector, MSCV-PGK-EGFP,35 which expresses the transgene from the MSCV promoter and EGFP from the PGK promoter. The recombinant plasmids were transfected into the retroviral packaging cell line, PE501, along with pSV2Neo at a 20:1 ratio and selected with G418 (0.8 mg/mL) for 10 days. Stable, EGFP-expressing cells were sorted by FACS and maintained with G418 (0.5 mg/mL).
Immunoprecipitation-Western
Cell lysates were prepared in RIPA buffer and precleared with normal mouse IgG and Protein G-Plus agarose (Santa Cruz Biotechnology). Cleared lysates were incubated with anti-Myc MAb, 9E10 (Santa Cruz Biotechnology), for 2 hours at 4°C and then with Protein G-Plus agarose overnight at 4°C. Precipitates were washed 3 times with phosphate-buffered saline (PBS), once with RIPA buffer, and eluted by adding 2× Western sample loading dye with or without β-mercaptoethanol (β-ME). Equal amounts of immunoprecipitates were run on SDS-PAGE, blotted onto Immobilon-P PVDF, and probed with anti-Myc or anti-EGFP antibodies, followed by horseradish peroxidase–conjugated secondary goat antibodies (PharMingen, San Diego, CA).
Establishment of Pen KO mice
The gene targeting vector was assembled in pBluescript and contained a 4.4 kb NotI/XbaI fragment (5′ arm) and a 4.6 kb BamHI/XhoI fragment (3′ arm) of mPen genomic DNA, a self-excising Cre-Neo cassette flanked by 2 lox P sites36 and 2 thymidine kinase expression cassettes in tandem (TK1-TK2). The transfection and selection of embryonic stem (ES) cells (R1), blastocyst (C57BL/6) injection, and the generation of chimeras and F1 breeding pairs were performed as described.36 ES clones were screened by Southern blots. Genotyping of F1×F1 offspring was done by PCR using GT1 (5′-GCAGATGTTCTCCCGAAGGGATC), GT2 (5′-GCTGCTCCACTCGTGATGCTGG), and GT3 (5′-CCCCAGAAGATGGAGCAAGTG) primers. The specificity and accuracy of PCR genotyping were verified by Southern blots. Blood samples were obtained by retro-orbital bleeding, and complete blood counts were determined using a Hemavet 850 analyzer (Drew Scientific, Oxford, CT).
Cell cultures
EML C.1 was maintained in Iscove modified Dulbecco medium (IMDM; Gibco, Grand Island, NY) plus 20% horse serum (HS; Gibco) and 8% (vol/vol) BHK/MKL conditioned medium (CM) as a source of rat stem cell factor (SCF).31 To induce erythroid differentiation, the medium was supplemented with human EPO (5 U/mL; Amgen, Thousand Oaks, CA). To obtain pure populations of erythroblasts, EML C.1 cells that had been treated with 8% BHK/MKL CM plus EPO (4 U/mL) for 7 days were washed with PBS and recultured in IMDM supplemented with 20% HS and EPO (4 U/mL).
Establishment and transduction of EMX C.1
EMX was established from a culture of Pen−/− BM initiated in IMDM supplemented with 30% fetal bovine serum (FBS), 5 × 10−5 M β-ME, 50 ng/mL murine SCF (R&D Systems, Minneapolis, MN), and 50 ng/mL murine thrombopoietin (TPO; R&D Systems). The immortalization of EMX was spontaneous and occurred within 4 weeks of the initiation of the BM culture. A clonal derivative, EMX C.1, was isolated after 2 rounds of limiting dilution cloning. EMX C.1 depends on SCF (50 ng/mL) and TPO (50 ng/mL) but grows better if interleukin-3 (IL-3; 2 ng/mL) is also present. EMX C.1 was infected by cocultivation with 9 Gy (900 rad)–irradiated retroviral producers PE501/MSCV-PGK-EGFP or MSCV-pen-PGK-EGFP for 48 hours. EGFP-expressing cells (50 000-100 000 per group) were sorted by FACS.
EPO response and colony assay of EMX C.1
A total of 5 × 105 EMX C.1 transduced with MSCV-PGK-EGFP (EMX/EGFP) or MSCV-pen-PGK-EGFP (EMX/pen) were stimulated with EPO (4 U/mL) in addition to the standard SCF+TPO+IL-3 cocktail for various periods and harvested for different assays. To determine the numbers of EPO-responsive erythroid progenitors, cells that had been stimulated with EPO (4 U/mL) in addition to SCF+TPO+IL-3 for 8 days were washed with PBS 3 times and recultured in IMDM/30% FBS plus EPO alone (0 to 32 U/mL). Cells that survived 48 hours after the EPO-alone switch were counted and plated in 0.8% methylcellulose supplemented with 30% FBS, 5 × 10−5 M β-ME, 50 ng/mL SCF, 2 ng/mL IL-3, and 4 U/mL EPO for colony formation.
Microscopy
Photomicrographs were taken with a Nikon Eclipse TE300 microscope (Nikon, Tokyo, Japan) equipped with Plan Fluor (40 ×/0.60 NA) and Plan Apo (60 ×/1.40 NA) lenses and a SPOT RT Slider digital camera (Diagnostic Instruments, Sterling Heights, MI) and SPOT RT software version 3.2 (Diagnostic Instruments). Image files were edited using Photoshop version 8.0 (Adobe Systems, San Jose, CA).
Statistics
Differences in the means of RBC numbers and hematocrits were determined by 2-tailed paired or unpaired t test. All blood counts were expressed as means ± SD.
Results
Cloning a new gene differentially expressed in proerythroblasts/erythroblasts
To identify new regulators of erythropoiesis, we performed cDNA RDA on a multipotent hematopoietic cell line, EML C.1,32 using a syngeneic myeloid cell line, MPRO,33 as the subtractor. This resulted in the enrichment of genes differentially expressed in erythroid progenitors. One of the cDNAs thus identified was E6-3, which showed differential expression in FLDS-19 murine erythroleukemia (MEL) cell line and EML C.1 in initial screening (Figure 1A).
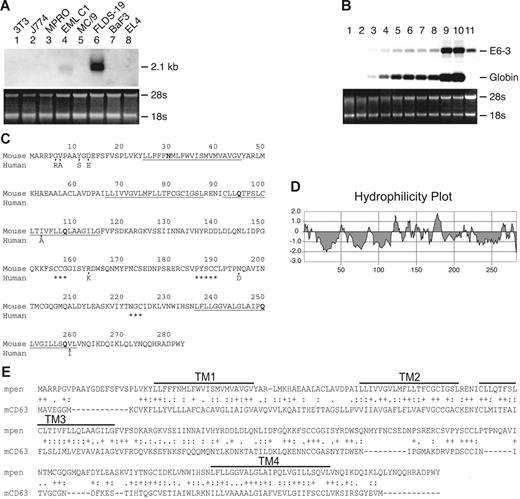
Expression and sequence of Penumbra (E6-3). (A) Expression of E6-3 in a panel of cell lines used in the initial screening: NIH3T3 (fibroblasts), J774 (macrophages), MPRO (promyelocytes), EML C.1 (multipotent), MC/9 (mast cells), FLDS-19 MEL (erythroblasts/proerythroblasts), BaF3 (B-cell–like), EL4 (T-cell–like). Expression in uninduced EML C.1 is due to spontaneously generated proerythroblasts and erythroblasts. The ethidium bromide–stained gel is shown on the bottom. (B) Expression of E6-3 during erythroid differentiation. Lanes 1 to 8: EML C.1 stimulated with SCF+EPO for 0 to 7 days; lanes 9 and 10: EML C.1 stimulated with SCF+EPO for 7 days and then EPO alone for 1 and 2 days, respectively; lane 11: uninduced FLDS-19 MEL. The blot was sequentially hybridized with the E6-3 and mouse βmajor-globin probes. (C) Amino acid sequences of mpen and hpen. Only differing aa's are shown for hpen. The 4 hydrophobic segments are underlined. Polar amino acids within the transmembrane segments are in bold. Asterisks denote conserved cysteine-containing motifs. (D) A hydrophilicity plot of mpen. (E) Amino acid sequence alignment between mpen and mCD63 by CLUSTAL W. “+” indicates identical aa; “:” indicates conserved substitution; “.” indicates semiconserved substitution.
Expression and sequence of Penumbra (E6-3). (A) Expression of E6-3 in a panel of cell lines used in the initial screening: NIH3T3 (fibroblasts), J774 (macrophages), MPRO (promyelocytes), EML C.1 (multipotent), MC/9 (mast cells), FLDS-19 MEL (erythroblasts/proerythroblasts), BaF3 (B-cell–like), EL4 (T-cell–like). Expression in uninduced EML C.1 is due to spontaneously generated proerythroblasts and erythroblasts. The ethidium bromide–stained gel is shown on the bottom. (B) Expression of E6-3 during erythroid differentiation. Lanes 1 to 8: EML C.1 stimulated with SCF+EPO for 0 to 7 days; lanes 9 and 10: EML C.1 stimulated with SCF+EPO for 7 days and then EPO alone for 1 and 2 days, respectively; lane 11: uninduced FLDS-19 MEL. The blot was sequentially hybridized with the E6-3 and mouse βmajor-globin probes. (C) Amino acid sequences of mpen and hpen. Only differing aa's are shown for hpen. The 4 hydrophobic segments are underlined. Polar amino acids within the transmembrane segments are in bold. Asterisks denote conserved cysteine-containing motifs. (D) A hydrophilicity plot of mpen. (E) Amino acid sequence alignment between mpen and mCD63 by CLUSTAL W. “+” indicates identical aa; “:” indicates conserved substitution; “.” indicates semiconserved substitution.
To examine the temporal expression of E6-3 during erythropoiesis, we used EML C.1 as a source of erythroid progenitors. In the presence of SCF and EPO, EML C.1–derived erythroid progenitors proliferate and differentiate into hemoglobinized erythroblasts in 5 to 7 days.32 Pure populations of proerythroblasts/erythroblasts can be obtained by switching EML C.1 that has been stimulated with SCF+EPO for 5 to 7 days to a medium containing EPO alone. Only proerythroblasts/erythroblasts survive 24 hours after the switch. Northern analyses of EML C.1 that had been stimulated with SCF+EPO for 0 to 7 days (Figure 1B, lanes 1 to 8) as well as pure populations of proerythroblasts/erythroblasts 1 and 2 days after the EPO-alone switch (Figure 1B, lanes 9 and 10) showed that the expression of E6-3 correlated with the appearance of βmajor-globin–expressing cells (Figure 1B).
E6-3 encodes a new tetraspanin
The E6-3 fragment contains 480 base pairs (bp). The full-length cDNA of E6-3 contains 2017 nucleotides (nt) and encodes a protein of 283 amino acids (aa) with an apparent molecular weight of 26 kDa (Figure 1C). We named this gene Penumbra (GenBank accession no. AF276890), abbreviated as Pen, for “Proerythroblast nu (new) membrane.”
Hydrophilicity analysis of murine pen (mpen) revealed the presence of 4 hydrophobic segments (Figure 1D). Further analysis indicated that mpen shares sequence homology with several members of the tetraspanin superfamily. The nearest family member that has been studied is CD63, which exhibits 26.5% identity with mpen in aa sequence (Figure 1E).24 The TM segments of mpen harbor polar aa's such as asparagine and glutamine (Asn30, Gln95, Gln108, Gln250, Gln258) that are destabilizing in the hydrophobic phospholipid bilayer. It is possible that they form intramolecular salt bridges with other aa's to maintain the tertiary structure, or they may interact with the TM segments of adjacent membrane proteins. The ECD2 of mpen contains several well-conserved cysteines that are also present in other tetraspanins (C156CG158, P186XSCC190, and X222GC224)12 (Figure 1C). Using mPEN as the probe, we identified the cDNA of hPEN (GenBank accession no. AF276891) from a human BM cDNA library. Human pen (hpen) and mpen are 97% identical in aa sequence (Figure 1C).
Pen is predominantly expressed in erythroblasts
We compared the expression levels of Pen in various hematopoietic tissues or cells by quantitative reverse transcription (RT)–PCR. Pen is highly expressed in BM and spleen (Figure 2A-B). Most of the BM expression is found in the TER119+ fraction, which includes all erythroblasts.37 Little expression is found in nonerythroid tissues or cells such as thymus, Gr1+ neutrophils, CD3+ T cells, B220+ B cells, CD11c+ monocytes, or NK1.1+ natural killer cells (Figure 2A). The expression in spleen is likely due to extramedullary hematopoiesis in this organ in mice because human spleen (which normally contains no extramedullary hematopoiesis) expresses lower levels of hPEN (not shown). Northern analyses of poly(A)-positive RNA detected some but lower levels of expression in liver, brain, and kidney (Figure 2B).
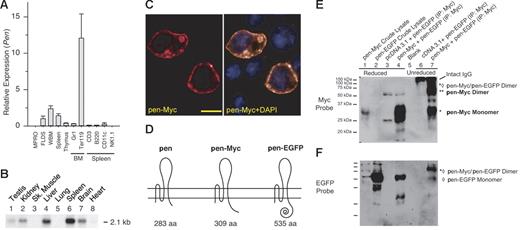
Tissue expression, subcellular localization, and dimer formation of Pen. (A) Relative expression of Pen in various murine hematopoietic tissues and lineages. BM and spleen cell subsets were purified by FACS. Quantitative real-time RT-PCR was performed in triplicate and normalized using β-actin as the internal control. The level of Pen expression in FLDS-19 is taken as 1.0. Means ± SD. (B) A multitissue (murine) Northern blot of Pen expression. Each lane contained 1 μg poly(A)-positive RNA. (C) Subcellular localization of pen. BaF3 was transfected with pcDNA3.1/pen-Myc. pen-Myc fusion protein was detected using rhodamine-conjugated anti-Myc MAb (left). (Right) Double staining with DAPI to reveal nuclei. Bar = 20 μM. (D) A schematic representation of pen, pen-Myc, and pen-EGFP fusion proteins. (E) Pen forms disulfide-bonded homodimers. NIH3T3 cells were transfected with expression vectors pcDNA3.1/pen-Myc (labeled as pen-Myc), pEGFP N1/pen (labeled as pen-EGFP), and pcDNA3.1 (negative control) alone or in combination. Cell lysates were prepared 24 hours after transfection and immunoprecipitated with anti-Myc antibodies. The immunoprecipitates were electrophoresed after reduction with β-mercaptoethanol (β-ME) (lanes 1 to 4) or without reduction (lanes 6 and 7), transferred to a PVDF membrane, and probed with anti-Myc antibodies. Under nonreducing conditions, about half of pen-Myc proteins existed as dimers (lane 7, **) and about half as monomers (lane 7, *). Some pen-Myc/pen-EGFP dimers can also be detected (lane 7, *⋄). After reduction, all pen-Myc proteins became monomers (lane 4). The 2 faint bands at about 50 and about 25 kDa positions in lanes 3 and 4 are reduced heavy and light chains of immunoglobulins, respectively. (F) The blot in panel E was stripped and reprobed with anti-EGFP antibodies to reveal pen-EGFP monomers (lanes 2, 4, and 7; ⋄) and pen-Myc/pen-EGFP dimers (lane 7, *⋄). The pen-EGFP monomer in lane 7 represented protein that was noncovalently associated with pen-Myc and therefore coimmunoprecipitated during anti-Myc immunoprecipitation. The lower, 29 kDa band in lane 4 is the unstripped pen-Myc monomer signal.
Tissue expression, subcellular localization, and dimer formation of Pen. (A) Relative expression of Pen in various murine hematopoietic tissues and lineages. BM and spleen cell subsets were purified by FACS. Quantitative real-time RT-PCR was performed in triplicate and normalized using β-actin as the internal control. The level of Pen expression in FLDS-19 is taken as 1.0. Means ± SD. (B) A multitissue (murine) Northern blot of Pen expression. Each lane contained 1 μg poly(A)-positive RNA. (C) Subcellular localization of pen. BaF3 was transfected with pcDNA3.1/pen-Myc. pen-Myc fusion protein was detected using rhodamine-conjugated anti-Myc MAb (left). (Right) Double staining with DAPI to reveal nuclei. Bar = 20 μM. (D) A schematic representation of pen, pen-Myc, and pen-EGFP fusion proteins. (E) Pen forms disulfide-bonded homodimers. NIH3T3 cells were transfected with expression vectors pcDNA3.1/pen-Myc (labeled as pen-Myc), pEGFP N1/pen (labeled as pen-EGFP), and pcDNA3.1 (negative control) alone or in combination. Cell lysates were prepared 24 hours after transfection and immunoprecipitated with anti-Myc antibodies. The immunoprecipitates were electrophoresed after reduction with β-mercaptoethanol (β-ME) (lanes 1 to 4) or without reduction (lanes 6 and 7), transferred to a PVDF membrane, and probed with anti-Myc antibodies. Under nonreducing conditions, about half of pen-Myc proteins existed as dimers (lane 7, **) and about half as monomers (lane 7, *). Some pen-Myc/pen-EGFP dimers can also be detected (lane 7, *⋄). After reduction, all pen-Myc proteins became monomers (lane 4). The 2 faint bands at about 50 and about 25 kDa positions in lanes 3 and 4 are reduced heavy and light chains of immunoglobulins, respectively. (F) The blot in panel E was stripped and reprobed with anti-EGFP antibodies to reveal pen-EGFP monomers (lanes 2, 4, and 7; ⋄) and pen-Myc/pen-EGFP dimers (lane 7, *⋄). The pen-EGFP monomer in lane 7 represented protein that was noncovalently associated with pen-Myc and therefore coimmunoprecipitated during anti-Myc immunoprecipitation. The lower, 29 kDa band in lane 4 is the unstripped pen-Myc monomer signal.
Pen is targeted to cell surface and forms disulfide-bonded homodimers
To map the subcellular location of pen, we constructed an expression vector, pcDNA3.1/pen-Myc, to express mpen as a fusion protein with the 11 aa Myc and 6 aa His tags in C terminus. In vitro translation of pcDNA3.1/pen-Myc in the presence of canine pancreatic microsomal membranes yielded a 29 kDa fusion protein (not shown). pcDNA3.1/pen-Myc was transfected into the BaF3 and FLDS-19 cells followed by staining with a rhodamine-conjugated anti-Myc MAb, 9E10. Most of the pen-Myc fusion protein was found on the surface of cells. Some was found in the membranes of cytoplasmic vesicles, especially if the level of expression was very high (Figure 2C).
To determine if pen can form disulfide-bonded homodimers, we cotransfected NIH3T3 fibroblasts with pcDNA3.1/pen-Myc (abbreviated as pen-Myc in Figure 2D) and pEGFP N1/pen. The latter expressed mpen as a fusion protein with enhanced green fluorescent protein (EGFP) in its C terminus (abbreviated as pen-EGFP in Figure 2D). The Pen-Myc fusion protein was first immunoprecipitated from cell lysates using anti-Myc MAb, Western blotted, and sequentially probed with anti-Myc and anti-EGFP antibodies. If pen formed homodimers via disulfide bonds, we expected to see both pen-Myc/pen-Myc and pen-Myc/pen-EGFP dimers in the immunoprecipitates under nonreducing conditions. Furthermore, after reduction these dimers would dissociate into monomers. This was indeed the case. Figure 2E-F demonstrates that under reducing conditions, all pen-Myc fusion proteins existed as 29 kDa monomers (Figure 2E, lanes 1 and 4). Under nonreducing conditions, about half of pen-Myc protein existed as 58 kDa homodimers (Figure 2E, lane 7, **), while the remaining pen-Myc protein existed either as 29 kDa monomers (Figure 2E, lane 7, *) or 89 kDa dimers with pen-EGFP (Figure 2E, lane 7, *⋄). The same blot was stripped and reprobed with anti-EGFP antibodies to reveal the approximately 60 kDa pen-EGFP monomer (Figure 2F, lanes 2 and 4, ⋄) and the 89 kDa pen-Myc/pen-EGFP dimer (Figure 2F, lane 7, *⋄). The small mount of pen-EGFP monomer in lane 7 of Figure 2F probably represented pen-EGFP that was coimmunoprecipitated with pen-Myc (by anti-Myc MAb) through noncovalent association. These results indicate that nearly half of pen-Myc protein exists as disulfide-bonded homodimers in this assay.
Establishment of a Pen KO mouse model
To investigate the effects of Pen deletion, we created a KO mouse model by homologous recombination. To avoid any pathology caused by Neo, we used a targeting vector containing a self-excising Cre-Neo cassette flanked by 2 lox P sites (Figure 3A).36 Cre expression was controlled by a testis-specific promoter. As the Pen+/− ES cells passed through the testes of male chimeras, Cre was induced and mediated excision of the Cre-Neo cassette (Figure 3A). Successful targeting resulted in the deletion of part of TM1, ECD1, TM2, and part of TM3 of pen and the production of a shortened, aberrantly spliced mRNA (confirmed by sequencing corresponding cDNA). Genotyping of ES cells was done by Southern blots using diagnostic restriction enzymes and DNA probes located outside the targeted segment. Genotyping of mice was done by PCR using GT1, GT2, and GT3 primers (Figure 3A-B).
Establishment of Pen KO mice and comparison of RBC numbers. (A) Creation of a KO allele by homologous recombination. The exon/intron structure of mPen is shown at the top. The targeting vector employs a self-excising Cre-Neo cassette in which the expression of Cre is under the control of a testis-specific promoter. The locations of the genotyping primers are also shown. (B) Genotyping of the KO littermates. GT1/GT3 amplify the KO but not the WT allele due the long distance between the primer sites in the WT allele. (C) A scatter plot of RBC numbers of young mice matched in age (3 months) and sex. No significant difference is noted between Pen+/+ and Pen−/− mice at this age. Bars indicate means. (D) A scatter plot of RBC numbers of older mice matched in age (6 to 17 months) and sex. Significant difference exists between Pen+/+ (○) and randomly selected Pen−/− (▴) groups (P = .004 by paired t test). Mice with increased (more than 4%) basophilic macrocytes (▵) in prescreening were plotted separately. Significant difference is again noted between Pen+/+ (○) and the prescreened Pen−/− mice (▵) (P < .001 by unpaired t test). Pen−/− mice with very severe anemia (hematocrit less than 0.20 [20%]) are not included in this analysis. Bars indicate means.
Establishment of Pen KO mice and comparison of RBC numbers. (A) Creation of a KO allele by homologous recombination. The exon/intron structure of mPen is shown at the top. The targeting vector employs a self-excising Cre-Neo cassette in which the expression of Cre is under the control of a testis-specific promoter. The locations of the genotyping primers are also shown. (B) Genotyping of the KO littermates. GT1/GT3 amplify the KO but not the WT allele due the long distance between the primer sites in the WT allele. (C) A scatter plot of RBC numbers of young mice matched in age (3 months) and sex. No significant difference is noted between Pen+/+ and Pen−/− mice at this age. Bars indicate means. (D) A scatter plot of RBC numbers of older mice matched in age (6 to 17 months) and sex. Significant difference exists between Pen+/+ (○) and randomly selected Pen−/− (▴) groups (P = .004 by paired t test). Mice with increased (more than 4%) basophilic macrocytes (▵) in prescreening were plotted separately. Significant difference is again noted between Pen+/+ (○) and the prescreened Pen−/− mice (▵) (P < .001 by unpaired t test). Pen−/− mice with very severe anemia (hematocrit less than 0.20 [20%]) are not included in this analysis. Bars indicate means.
Pen−/− mice develop anemia and splenomegaly
Pen−/− mice are viable and fertile, although low fertility was observed in rare cases after inbreeding. When examined at 3 months of age, Pen−/− mice had similar numbers of RBCs (9.32 × 1012 ± 1.05 × 1012/L versus 9.51 × 1012 ± 0.85 × 1012/L in wild type [WT]) (Figure 3C), hematocrits (0.47 ± 0.04 versus 0.47 ± 0.04 in WT), white blood cells (WBCs) (8.60 × 109 ± 2.67 × 109/L versus 7.70 × 109 ± 2.54 × 109/L in WT), and platelets (826.46 × 109 ± 168.57 × 109/L versus 919.78 × 109 ± 151.19 × 109/L in WT) as WT littermates. However, the blood smears of about 30% of young Pen−/− mice contained RBCs that were basophilic and larger. Many of these basophilic RBCs had the “target cell” appearance reflecting a decreased cytoplasm-to-cell surface ratio. These abnormal RBCs are henceforth referred to as basophilic macrocytes (Figure 4A). The percentages of basophilic macrocytes increased with age in the same mice. When examined at about 1 year (range, 6-17 months) of age, the Pen−/− mice as a group had lower RBC numbers (7.71 × 1012 ± 0.88 × 1012/L versus 8.88 × 1012 ± 0.51 × 1012/L in WT; P = .004 by paired t test) (Figure 3D) and hematocrits (0.39 ± 0.06 versus 0.46 ± 0.04 in WT; P = .003). Many of the 1-year-old or older Pen−/− mice had very high percentages of basophilic macrocytes in blood smears. In the most severe cases, about 60% of the RBCs were basophilic macrocytes. Most Pen−/− mice with severe anemia also had mild to moderate monocytosis and thrombocytopenia (not shown). When mice with increased percentages of basophilic macrocytes were examined, they were found to have even lower numbers of RBCs (6.24 × 1012 ± 1.87 × 1012/L versus 8.88 × 1012 ± 0.51 × 1012/L in WT; P < .001 by unpaired t test) and hematocrits (0.37 ± 0.08 versus 0.46 ± 0.04 in WT; P = .002). The ranges of RBC numbers are better appreciated in scatter plots (Figure 3D). Many Pen−/− mice had hematocrits as low as 0.12 to 0.15 (12% to 15%) (not included in the statistics above). All Pen−/− mice with high percentages of basophilic macrocytes and anemia had markedly (about 10-fold) enlarged spleens on autopsy (1.023 ± 0.223 versus 0.108 ± 0.029 g in WT; P = .003) (Figure 4D,G). Some Pen−/− mice had spleens weighing more than 5 g (not included in the statistics above), and some Pen−/− spleens showed evidence of infarctions (Figure 4G). Morphologic and flow cytometric examination of the spleens of anemic mice revealed replacement of splenic T and B lymphocytes by intensely basophilic and sometimes dysplastic erythroblasts and proerythroblasts (Figure 4E,J). In 2 cases, extrameduallary erythropoiesis in the liver resulted in the formation of pedunculated hepatic tumors (Figure 4H-I). The abnormalities described above are also seen in some Pen+/− mice (not shown). These findings indicate that Pen plays an important role in normal erythropoiesis.
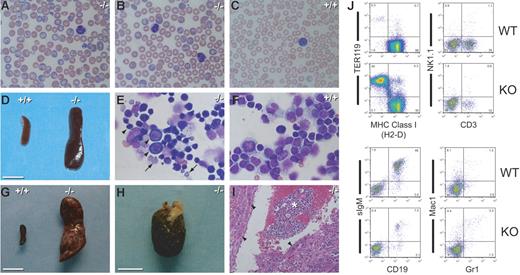
Basophilic macrocytes, splenomegaly, and extramedullary hematopoiesis in Pen KO mice. (A-B) Wright-Giemsa–stained blood smears of 2 representative Pen−/− mice. Note the basophilic macrocytes, target cells, overall larger RBCs, and the absence of microspherocytes. (C) A Wright-Giemsa–stained blood smear of a Pen WT mouse. Note the smaller RBC size, the absence of basophilic macrocytes, and a pale blue “shift cell” in the right lower corner. (D) The spleen of the Pen−/− mouse seen in panel A. A WT spleen is included for comparison. Bar = 1 cm. (E) A Wright-Giemsa–stained cytospin preparation of the spleen cells of the Pen−/− mouse seen in panel D. Most cells are intensely basophilic proerythroblasts or erythroblasts (arrowheads) or enucleated, basophilic RBCs (arrows). (F) A Wright-Giemsa–stained cytospin preparation of the spleen cells of a Pen WT mouse. Most cells are lymphocytes. Panels A, B, C, E, and F have the same magnification (300×). (G) The spleen of a Pen−/− mouse with multiple infarcts (pale scars). Bar = 1 cm. (H) The hepatic tumor of the same Pen−/− mouse seen in panel G. Bar = 1 cm. (I) A hematoxylin and eosin–stained thin section of panel H. The asterisk denotes extramedullary hematopoiesis; arrowheads, hepatocytes. (J) Flow cytometric analyses of the spleen cells seen in panels D-F. RBCs were removed by hypotonic lysis before staining with MAb. The percentage of cells in each quadrant/gate is shown. CD3 is a marker for T cells while CD19 is a marker for B cells. The frequency of TER119+ erythroblasts in Pen−/− spleen is increased by 150-fold.
Basophilic macrocytes, splenomegaly, and extramedullary hematopoiesis in Pen KO mice. (A-B) Wright-Giemsa–stained blood smears of 2 representative Pen−/− mice. Note the basophilic macrocytes, target cells, overall larger RBCs, and the absence of microspherocytes. (C) A Wright-Giemsa–stained blood smear of a Pen WT mouse. Note the smaller RBC size, the absence of basophilic macrocytes, and a pale blue “shift cell” in the right lower corner. (D) The spleen of the Pen−/− mouse seen in panel A. A WT spleen is included for comparison. Bar = 1 cm. (E) A Wright-Giemsa–stained cytospin preparation of the spleen cells of the Pen−/− mouse seen in panel D. Most cells are intensely basophilic proerythroblasts or erythroblasts (arrowheads) or enucleated, basophilic RBCs (arrows). (F) A Wright-Giemsa–stained cytospin preparation of the spleen cells of a Pen WT mouse. Most cells are lymphocytes. Panels A, B, C, E, and F have the same magnification (300×). (G) The spleen of a Pen−/− mouse with multiple infarcts (pale scars). Bar = 1 cm. (H) The hepatic tumor of the same Pen−/− mouse seen in panel G. Bar = 1 cm. (I) A hematoxylin and eosin–stained thin section of panel H. The asterisk denotes extramedullary hematopoiesis; arrowheads, hepatocytes. (J) Flow cytometric analyses of the spleen cells seen in panels D-F. RBCs were removed by hypotonic lysis before staining with MAb. The percentage of cells in each quadrant/gate is shown. CD3 is a marker for T cells while CD19 is a marker for B cells. The frequency of TER119+ erythroblasts in Pen−/− spleen is increased by 150-fold.
Pen promotes effective erythropoiesis
A continuous multipotent hematopoietic cell line, EMX (for “erythroid-myeloid-and-unknown”), was established from the BM of a Pen−/− mouse. A clonal derivative, EMX C.1, was used as the prototytpe and maintained with SCF, TPO, and IL-3. In the presence of SCF+TPO+IL-3 plus EPO, EMX C.1 differentiates into erythrocytes, monocytes, neutrophils, mast cells, and megakaryocytes (Figure 5A). Genotyping confirmed that EMX C.1 is Pen−/− (Figure 5A). Although EMX C.1 was capable of differentiating into erythrocytes, the efficiency was low as reflected in the low levels of GATA-1 and βmajor-globin mRNAs (Figure 5B, lane 3). Similar findings were seen in EMX C.1 transduced with the negative control retroviral vector MSCV-PGK-EGFP (EMX/EGFP) without or with EPO stimulation (Figure 5B, lanes 4 and 6). In contrast, EMX C.1 transduced with MSCV-pen-PGK-EGFP (EMX/pen, expressing mpen) exhibited robust erythropoiesis in response to EPO as evidenced by higher levels of GATA-1 and βmajor-globin mRNAs (Figure 5B, lanes 5 and 7). These findings indicate that pen enhanced erythropoiesis in EMX C.1. Examination of Wright-Giemsa–stained cytospins of EMX/EGFP and EMX/pen confirmed that after 12 days' stimulation with EPO (4 U/mL), about 45% of the EMX/pen cells were erythroblasts compared with only 5% in the control EMX/EGFP cultures (Figure 5F-G).
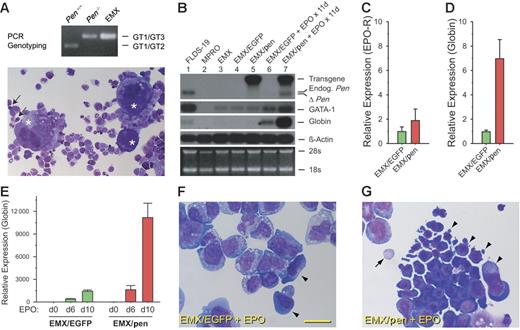
Pen enhances erythropoiesis in EMX C.1. (A) A Wright-Giemsa–stained cytospin preparation of EMX C.1. The asterisks denote megakaryocytes. Arrows point to 2 basophilic erythroblasts. Also visible are neutrophils with donut-shaped nuclei, monocytes, and mast cells. The genotyping result is at the top. (B) Northern analyses of the expression of Pen, GATA-1, βmajor-globin, and β-actin in EMX C.1, EMX/EGFP, and EMX/Pen without and with EPO stimulation. FLDS-19 serves as a positive control and MPRO as a negative control for GATA-1 and βmajor-globin. The same blot was sequentially hybridized to different probes. EMX C. 1 (lane 3) and EMX/EGFP (lane 4) did not express the 4.0 kb retroviral message harboring the mPen coding sequence (labeled “Transgene”) while EMX/pen did (lane 5, top panel). The 2.1 kb endogenous mPen message in FLDS-19 (lane 1) is indicated. ΔPen is a shortened Pen message transcribed from the KO allele. The level of βmajor-globin message roughly correlated with the numbers of erythroblasts. (C) Relative EPO-R expression in EMX/EGFP versus EMX/pen without exogenous EPO. Quantitative real-time RT-PCR was performed in triplicate as described in “Materials and methods,” and the data were normalized according to the levels of β-actin expression. The level of EPO-R expression in EMX/EGFP was taken as 1.0. Data represent means ± SD. (D) Relative βmajor-globin expression in EMX/EGFP versus EMX/pen without exogenous EPO. The data were normalized according to the levels of β-actin expression. The level of βmajor-globin expression in EMX/EGFP was taken as 1.0. The expression of βmajor-globin was likely stimulated by the small amount of EPO present in FBS. Means ± SD. (E) Relative βmajor-globin expression in EMX/EGFP versus EMX/pen stimulated with EPO (4 U/mL) for 0, 6, and 10 days. The data were normalized according to the levels of β-actin expression. The level of βmajor-globin expression in EMX/EGFP on day 0 was taken as 1.0. Data represent means ± SD. (F) A representative field of Wright-Giemsa–stained cytospin preparation of EMX/EGFP stimulated with EPO (4 U/mL) for 8 days. Arrowheads indicate 2 large erythroid progenitors. The rest are myeloid cells. Bar = 40 μM. (G) A representative field of Wright-Giemsa–stained cytospin preparation of EMX/pen stimulated with EPO (4 U/mL) for 8 days. Arrowheads indicate a large cluster of basophilic erythroblasts; arrow, an enucleated RBC.
Pen enhances erythropoiesis in EMX C.1. (A) A Wright-Giemsa–stained cytospin preparation of EMX C.1. The asterisks denote megakaryocytes. Arrows point to 2 basophilic erythroblasts. Also visible are neutrophils with donut-shaped nuclei, monocytes, and mast cells. The genotyping result is at the top. (B) Northern analyses of the expression of Pen, GATA-1, βmajor-globin, and β-actin in EMX C.1, EMX/EGFP, and EMX/Pen without and with EPO stimulation. FLDS-19 serves as a positive control and MPRO as a negative control for GATA-1 and βmajor-globin. The same blot was sequentially hybridized to different probes. EMX C. 1 (lane 3) and EMX/EGFP (lane 4) did not express the 4.0 kb retroviral message harboring the mPen coding sequence (labeled “Transgene”) while EMX/pen did (lane 5, top panel). The 2.1 kb endogenous mPen message in FLDS-19 (lane 1) is indicated. ΔPen is a shortened Pen message transcribed from the KO allele. The level of βmajor-globin message roughly correlated with the numbers of erythroblasts. (C) Relative EPO-R expression in EMX/EGFP versus EMX/pen without exogenous EPO. Quantitative real-time RT-PCR was performed in triplicate as described in “Materials and methods,” and the data were normalized according to the levels of β-actin expression. The level of EPO-R expression in EMX/EGFP was taken as 1.0. Data represent means ± SD. (D) Relative βmajor-globin expression in EMX/EGFP versus EMX/pen without exogenous EPO. The data were normalized according to the levels of β-actin expression. The level of βmajor-globin expression in EMX/EGFP was taken as 1.0. The expression of βmajor-globin was likely stimulated by the small amount of EPO present in FBS. Means ± SD. (E) Relative βmajor-globin expression in EMX/EGFP versus EMX/pen stimulated with EPO (4 U/mL) for 0, 6, and 10 days. The data were normalized according to the levels of β-actin expression. The level of βmajor-globin expression in EMX/EGFP on day 0 was taken as 1.0. Data represent means ± SD. (F) A representative field of Wright-Giemsa–stained cytospin preparation of EMX/EGFP stimulated with EPO (4 U/mL) for 8 days. Arrowheads indicate 2 large erythroid progenitors. The rest are myeloid cells. Bar = 40 μM. (G) A representative field of Wright-Giemsa–stained cytospin preparation of EMX/pen stimulated with EPO (4 U/mL) for 8 days. Arrowheads indicate a large cluster of basophilic erythroblasts; arrow, an enucleated RBC.
To compare the levels of erythropoiesis precisely, we performed quantitative RT-PCR of βmajor-globin. Without added EPO, EMX/EGFP and EMX/pen expressed similar levels of EPO-R mRNA (Figure 5C). However, the expression of βmajor-globin mRNA in EMX/pen was 7-fold higher than in EMX/EGFP (Figure 5D). The expression of βmajor-globin mRNA by EMX/pen in the absence of added EPO was likely stimulated by the trace EPO in FBS. Addition of EPO (4 U/mL) for 6 to 10 days greatly stimulated βmajor-globin expression in EMX/pen but had only modest effect on EMX/EGFP (Figure 5E). These results indicate that pen plays an important role in the differentiation of erythroid progenitors.
To identify the developmental stage(s) affected by Pen KO, EMX/EGFP or EMX/pen that had been stimulated with EPO (4 U/mL) in addition to SCF+TPO+IL-3 for 14 days were washed free of cytokines and recultured in a medium containing EPO alone at various concentrations. All nonerythroid cells died due to cytokine withdrawal. Only EPO-responsive progenitors survived the switch. Cells surviving the EPO switch were counted and subjected to colony assays in the presence of SCF+IL-3+EPO (Figure 6A). While very few EMX C.1 or EMX/EGFP cells survived the EPO switch, many EMX/pen cells did (Figure 6B). These EPO-rescued EMX/pen cells consisted of erythroblasts at all stages of differentiation as well as less differentiated blasts. Colony assays of EPO-rescued cells from the EMX/pen cultures revealed many large, medium, and small erythroid burst-forming unit (BFU-E)–derived colonies (Figure 6C). In contrast, very few BFU-E–derived colonies were found in the EMX/EGFP cultures (Figure 6C). Similar differences were seen in erythroid colony-forming unit (CFU-E)–derived colonies, which degenerated quickly (not shown).
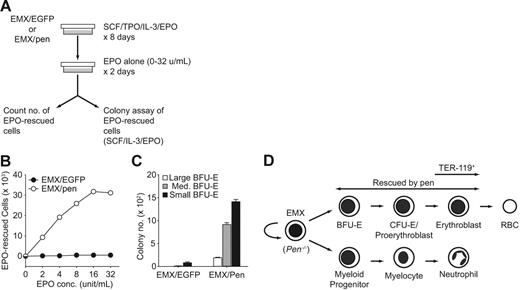
The survival or development of Pen−/− erythroid progenitors is rescued by pen. (A) An outline of the experimental design. (B) Effect of pen on the survival of EPO-responsive cells at different concentrations of EPO. A total of 5 × 105 EMX/EGFP or EMX/pen cells were stimulated with SCF+TPO+IL3 plus EPO (4 U/mL) for 8 days. Cell numbers after 8 days were 1.27 × 107 for EMX/GFP and 1.81 × 107 for EMX/pen. A total of 3 × 105 EMX/GFP or EMX/Pen cells that had been stimulated with SCF+TPO+IL-3 plus EPO for 8 days were washed with PBS and restimulated with EPO alone (0 to 32 U/mL) for 2 days. Most surviving cells were erythroblasts. Some were undifferentiated blasts. Cells rescued with EPO alone were counted. Data represent the means of triplicates. For reference, 6.1% of EMX/Pen cells were rescued by EPO at 4 U/mL. (C) Effect of Pen on the numbers of BFU-Es rescued by EPO. A total of 3 × 105 EMX/EGFP or EMX/Pen calls that had been stimulated with SCF+TPO+IL3 plus EPO (4 U/mL) for 8 days were washed with PBS and restimulated with EPO alone (4 U/mL) for 2 days and then plated in methylcellulose culture medium supplemented with SCF, IL-3, and EPO (4 U/mL). Each 35 mm dish contained the equivalent of 2 × 104 starting cells (or 1224 EPO-rescued cells in the case of EMX/pen). The graph shows the numbers of erythroid progenitors per 3 × 105 starting cells. Data represent means ± SD; n = 3. For reference, the frequencies of large, medium, and small BFU-Es among EPO-rescued cells were 1.0%, 5.0%, and 7.7%, respectively. (D) A schematic summary of the differentiation of Pen−/− EMX C.1. The TER119 MAb recognizes erythroblasts,37 which exhibit the highest level of Pen expression in BM. The survival or development of EMX-derived BFU-Es, CFU-Es/proerythroblasts, and erythroblasts is rescued by pen in the presence of EPO.
The survival or development of Pen−/− erythroid progenitors is rescued by pen. (A) An outline of the experimental design. (B) Effect of pen on the survival of EPO-responsive cells at different concentrations of EPO. A total of 5 × 105 EMX/EGFP or EMX/pen cells were stimulated with SCF+TPO+IL3 plus EPO (4 U/mL) for 8 days. Cell numbers after 8 days were 1.27 × 107 for EMX/GFP and 1.81 × 107 for EMX/pen. A total of 3 × 105 EMX/GFP or EMX/Pen cells that had been stimulated with SCF+TPO+IL-3 plus EPO for 8 days were washed with PBS and restimulated with EPO alone (0 to 32 U/mL) for 2 days. Most surviving cells were erythroblasts. Some were undifferentiated blasts. Cells rescued with EPO alone were counted. Data represent the means of triplicates. For reference, 6.1% of EMX/Pen cells were rescued by EPO at 4 U/mL. (C) Effect of Pen on the numbers of BFU-Es rescued by EPO. A total of 3 × 105 EMX/EGFP or EMX/Pen calls that had been stimulated with SCF+TPO+IL3 plus EPO (4 U/mL) for 8 days were washed with PBS and restimulated with EPO alone (4 U/mL) for 2 days and then plated in methylcellulose culture medium supplemented with SCF, IL-3, and EPO (4 U/mL). Each 35 mm dish contained the equivalent of 2 × 104 starting cells (or 1224 EPO-rescued cells in the case of EMX/pen). The graph shows the numbers of erythroid progenitors per 3 × 105 starting cells. Data represent means ± SD; n = 3. For reference, the frequencies of large, medium, and small BFU-Es among EPO-rescued cells were 1.0%, 5.0%, and 7.7%, respectively. (D) A schematic summary of the differentiation of Pen−/− EMX C.1. The TER119 MAb recognizes erythroblasts,37 which exhibit the highest level of Pen expression in BM. The survival or development of EMX-derived BFU-Es, CFU-Es/proerythroblasts, and erythroblasts is rescued by pen in the presence of EPO.
Discussion
Pen is a new member of the tetraspanin family of membrane proteins that often serve as the organizers of signaling complexes in cell membranes.11 In mice, the highest level of Pen expression is seen in the BM. Most of the Pen expression in BM is restricted to the TER119+ erythroblasts (Figure 2A), suggesting that Pen may have a particular function in erythroblasts. Indeed, our KO study demonstrates that homozygous deletion of Pen frequently results in the development of basophilic macrocytic RBCs (Figure 4A-B), splenomegaly (Figure 4D,G), and anemia (Figure 3D).
One of the most consistent abnormalities in the Pen−/− mice is splenomegaly. It occurs not only in mice with anemia but also in some mice without anemia. Some Pen−/− spleens weigh more than 5 g, the equivalent of 17% of body weight (normal spleens weigh about 0.3% of body weight). Anemia may occur when there is decreased production of erythroblasts/RBCs, or increased destruction of erythroblasts/RBCs that is not compensated by increased erythropoiesis, or both. The frequent occurrence of splenomegaly in Pen−/− mice raises the possibility that increased erythroblast/RBC destruction may be a contributing factor in the development of anemia in these mice. Given the known roles of prototypical tetraspanins in cell membranes,9-11 it is possible that Pen−/− erythroblasts/RBCs have accumulated intrinsic defects in their cell membranes or cytoskeletons or cytoplasm as a result of abnormal differentiation in the absence of Pen. The frequent finding of target cells (Figure 4A) may be an indication of this problem. These intrinsic defects may increase the rate of destruction of erythroblasts/RBCs in the spleen, leading to splenomegaly and compensatory expansion of the erythroid compartment. As the mice age, these abnormalities cause progressive splenomegaly, which in turn increases the rate of destruction of erythroblasts/RBCs in a vicious cycle. Beyond a critical point, the destruction of erythroblasts/RBCs cannot be compensated by expanded erythropoiesis and anemia becomes apparent.
Another recurring abnormality in the Pen−/− mice is the basophilic macrocyte (Figure 4A-B). The basophilic macrocytes of Pen−/− mice resemble the so-called shift reticulocytes, which are immature reticulocytes released into the circulation prematurely during severe hypoxic stress. Supravital staining with new methylene blue confirmed that the basophilic macrocytes of Pen−/− mice stained intensely with this dye. While the reticulocyte counts of normal mice average less than 1%, those of the anemic Pen KO mice range from 5% to 60%. Judging from the intense basophilia and the larger cell size, the basophilic macrocytes of Pen−/− mice may be even more immature than shift reticulocytes. This is supported by the observation that it takes longer for the basophilic macrocytes of Pen−/− mice to mature in culture than the typical shift reticulocytes. The marked immaturity of basophilic macrocytes in the Pen−/− mice may reflect the severity of anemia but may also be the result of abnormal differentiation.
Studies in the multipotent EMX C.1 cell line revealed a differentiation defect in Pen−/− erythroid progenitors. As shown in Figures 5 and 6, EMX C.1–derived erythroid progenitors differentiate poorly in the presence of EPO as evidenced by the low levels of βmajor-globin expression and survival in response to EPO alone. These defects are corrected to a certain extent by Pen (Figures 5B-E and 6B-C). These findings are summarized in Figure 6D. Notably, the expression of Pen in EMX/pen cells is driven by the constitutively active long-terminal repeat of MSCV. Normal BFU-Es, which have been shown to express EPO-R in at least a subset,38 may or may not express Pen. Regardless, the ability of ectopic Pen to rescue EMX-derived BFU-Es in the presence of EPO suggests that pen may directly or indirectly influence the survival or differentiation of erythroid progenitors in the presence of EPO. Several a priori mechanisms may explain the possible effect of pen on EMX-derived erythroid progenitors, including direct or indirect physical interaction between pen and EPO-R leading to a conformational change of EPO-R or the stability of EPO-R dimers, direct or indirect interaction between pen and another cell surface receptor(s) (eg, c-kit, c-mpl, platelet-derived growth factor receptor, transferrin receptor, and β integrins) or molecules whose expression is contemporaneous, overlaps, or dovetails with that of EPO-R, and signal transduction by pen per se. The impaired survival or differentiation of Pen−/− erythroid progenitors in the presence of high concentrations of EPO suggests that they may have a reduced capacity to expand in response to hypoxia. This abnormality may hasten the development of anemia.
While older Pen−/− mice develop anemia, most young Pen−/− mice do not. The delayed onset of anemia may be related to the progressive nature of splenomegaly, or it may be linked to age-related differences in certain hormones, oxygen consumption, expression of other tetraspanins that may provide functional redundancy (such as CD151),39 phagocytic activity of macrophages, the proliferative vigor of hematopoietic stem cells or progenitors, and the number of somatic mutations. As indicated, only about 30% of the Pen−/− mice develop anemia. The cause of this phenotypic variability may lie in the outbred nature of the Pen−/− mice. Like many KO models, our Pen−/− mice have both C57BL/6 and 129 parentage and are random recombinants with respect to these 2 genetic backgrounds. Depending on the genetic composition and the relative strengths of allele-specific genetic modifiers, the phenotype may vary. Finally, we have shown that hPEN maps to 7q32,40 one of the minimally deleted regions in myelodysplastic syndrome (MDS) and acute myelogenous leukemia (AML).41-45 Given the erythroblast-restricted expression of Pen in BM, it is unlikely that hPEN deletion can account for all cytologic abnormalities of MDS such as nuclear hyposegmentation and hypogranularity. However, the findings in Pen−/− mice and EMX C.1 so far suggest that Pen provides a positive function in erythropoiesis. Thus, further investigation is warranted to determine if Pen deletion contributes to the anemia of some MDSs or AML.
Authorship
Contribution: S.T. and T.M.C. designed research; all authors performed research; and S.T., T.M.C., and M.J.H. analyzed data and wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Schickwann Tsai, Wintrobe Bldg, Rm 621, University of Utah School of Medicine, 26 North 1900 East, Salt Lake City, UT 84132-4601; e-mail: schickwann.tsai@hsc.utah.edu.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This work was supported in part by a grant from the American Cancer Society (RSG-01-165-01-LIB) (S.T.). The authors thank Suzanne Mansour, Kirk Thomas, and Mario Capecchi for the Cre-Neo and TK1-TK2 cassettes and AnneMarie Y.-J. Yang and Christopher Leukel for technical assistance.

![Figure 3. Establishment of Pen KO mice and comparison of RBC numbers. (A) Creation of a KO allele by homologous recombination. The exon/intron structure of mPen is shown at the top. The targeting vector employs a self-excising Cre-Neo cassette in which the expression of Cre is under the control of a testis-specific promoter. The locations of the genotyping primers are also shown. (B) Genotyping of the KO littermates. GT1/GT3 amplify the KO but not the WT allele due the long distance between the primer sites in the WT allele. (C) A scatter plot of RBC numbers of young mice matched in age (3 months) and sex. No significant difference is noted between Pen+/+ and Pen−/− mice at this age. Bars indicate means. (D) A scatter plot of RBC numbers of older mice matched in age (6 to 17 months) and sex. Significant difference exists between Pen+/+ (○) and randomly selected Pen−/− (▴) groups (P = .004 by paired t test). Mice with increased (more than 4%) basophilic macrocytes (▵) in prescreening were plotted separately. Significant difference is again noted between Pen+/+ (○) and the prescreened Pen−/− mice (▵) (P < .001 by unpaired t test). Pen−/− mice with very severe anemia (hematocrit less than 0.20 [20%]) are not included in this analysis. Bars indicate means.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/109/8/10.1182_blood-2006-09-046672/4/m_zh80080711320003.jpeg?Expires=1769558746&Signature=PpYh5ao8Bocf5hQ8AkeXp8Ay2nmKgYjYZaJvHBIBVhVaPangMCxjlS2SzFGJjphLJD7XSG6HX0xHKiEVrzeUe0uG7iddaMmx5WKp~qdqMpMss3ByYxWLR8VCJ1D0IU8x5tfe-ty0abqeTrNGHgMGDtuGKN1brhdf~s0fRXiC-tIMkMY7zUyaug4~6-Yx~fogMNz6Bl5o5UegaZEubINcUGRDB5OEqHcs3ZU0PVLYzLZ~F91lBklv1cdpUppZ79yVQOrs3t4TO1lfFrZ1S0r8LY0we3DsmU5RLGexw5sGVKChhq~fBczkgnoS4fVzw~udRwlBwhGTcCwbyHVwHv4EAQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)