Abstract
X-linked sideroblastic anemia with ataxia (XLSA/A) is a rare syndromic form of inherited sideroblastic anemia associated with spinocerebellar ataxia, and is due to mutations in the mitochondrial ATP-binding cassette transporter Abcb7. Here, we show that Abcb7 is essential for hematopoiesis and formally demonstrate that XLSA/A is due to partial loss of function mutations in Abcb7 that directly or indirectly inhibit heme biosynthesis.
Introduction
X-linked sideroblastic anemia with ataxia (XLSA/A) is a rare syndromic form of inherited sideroblastic anemia associated an early onset, non- or slowly progressive, predominantly truncal, spinocerebellar ataxia, accompanied by severe, selective cerebellar hypoplasia.1-5 The anemia of XLSA/A is typically mild and may be overlooked until after the neurologic presentation. The peripheral blood and bone marrow findings resemble mild typical X-linked sideroblastic anemia (XLSA) due to mutations in ALAS2.6 Affected males have a mildly decreased or low normal hemoglobin with a mild microcytosis and an increased red blood cell distribution width (RDW), often with erythrocyte dimorphism and Pappenheimer bodies. Bone marrow examination shows ringed sideroblasts
Mutation analysis in 3 families with XLSA/A has demonstrated ABCB7 missense mutations in each.1,2,4 ABCB7 is the human orthologue of the yeast mitochondrial ATP-binding cassette (ABC) transporter Atm1p, which has been implicated in the transport of a component required for the maturation of cytosolic iron-sulfur (Fe-S) cluster proteins out of mitochondria; yeast lacking ATM1 (Δatm1) are deficient in the holo-forms of cytosolic Fe-S proteins, are respiratory deficient, and accumulate pathologic iron deposits in mitochondria akin to mitochondrial iron seen in ringed sideroblasts.7-9 Complementation assays in yeast suggest that each of the human mutations is a mild partial loss of function allele.1,2
By using conditional gene targeting in mice, we previously demonstrated the essential nature of Abcb7 in the development of many tissues, and confirmed its role in cytosolic Fe-S protein maturation in mammals.10,11 Here, we show that Abcb7 is essential for hematopoiesis and formally demonstrate that the XLSA/A anemia is due to partial loss of function alleles.
Materials and methods
The generation of a conditionally targeted (“floxed”) allele of Abcb7 (Abcb7fl) has been described previously.10 A targeted point mutant allele was made by site-directed mutagenesis (QuickChange; Stratagene, La Jolla, CA) of the original gene targeting construct to create a glutamic acid-to-lysine mutation at position 433 (E433K) of the mouse protein corresponding to the hematologically most severe, E433K, human allele.2 All exons and intron/exon boundaries were resequenced in the mutagenized targeting construct. Newly targeted, homologously recombined embryonic stem cell colonies from the 129S4/SvJae background were analyzed for the mutation by sequencing genomic polymerase chain reaction (PCR) products. Similar phenotypic results were obtained from C57BL/6 chimeric animals produced from multiple independent embryonic stem (ES) cell lines with or without the NeoR cassette.
C57BL/6J-Mx1-Cre animals12 were obtained from Jackson Laboratories (Bar Harbor, ME) and bred to the previously described Abcb7fl allele10 on a mixed 129S4/SvJae × 129S6/SvEvTac background. F3 animals were used for most experiments. Induction of Cre expression was achieved in 4-day-old or 4-week-old animals by subcutaneous injection of 200 μg or intraperitoneal injection of 400 μg, respectively, polyinosine-polycytosine (pI-pC) on alternate days, for a total of 3 doses. Clinically ill animals were humanely killed in accordance with guidelines accepted by the Animal Care and Use Committee at Children's Hospital Boston. As a case control, a sex-matched, Mx1-Cre–negative animal injected with pI-pC was phenotyped in parallel with each moribund experimental animal. Complete blood counts, zinc protoporphyrin quantification, and Gpi1 isozyme chimerism analyses were performed as previously described.13
For electron microscopy studies, whole blood was processed as previously described.14 Electron microscopic thin sections were examined with and without lead and uranyl acetate staining on an FEI/Phillips EM 208S (FEI Electron Optics BV, Eindhoven, Netherlands) equipped with an AMT (Advanced Microscopy Techniques, Danvers, MA) digital camera. Light microscopic images were acquired on a Nikon Eclipse E600 microscope with a 100×/0.30 NA oil immersion lens and an RT Slider SPOT 2.3.1 camera (Diagnostic Instruments, Sterling Heights, MI) using SPOT Advanced software (version 3.5.9).
Results and discussion
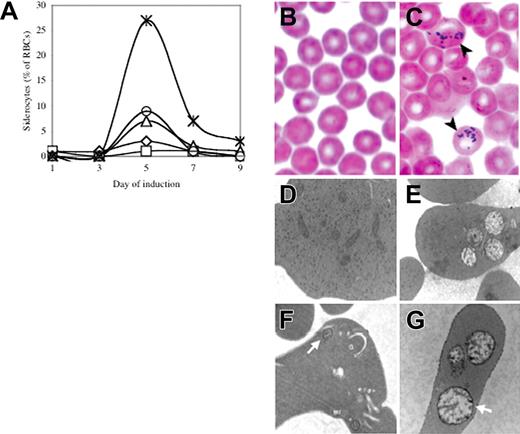
In order to evaluate the effect of Abcb7 deletion on hematopoiesis, we bred the Abcb7fl allele to the interferon-α–inducible Mx1-Cre line. As previously reported,12 following induction we found highly variable systemic deletion, including near-complete rearrangement in the bone marrow and liver, no effect in testes or brain, and intermediate levels of rearrangement in all other tissues examined (Figure S1, available on the Blood website; see the “Supplemental Figures” link at the top of the online article). Serial peripheral blood smears demonstrated the presence of a transient population of siderocytes that peaked at day 5 in Abcb7fl+Cre animals (Figure 1A,C and Figure S2); siderocytes were not present in animals without Cre.
Transient siderocytosis following Mx1-Cre–mediated Abcb7 gene deletion. (A) Serial peripheral blood siderocyte counts in 5 representative Abcb7fl+Mx1-Cre animals induced at 4 days of age. Iron-stained peripheral blood smears of Abcb7fl−Mx1-Cre (B) and Abcb7fl+Mx1-Cre (C) animals. Black arrows indicate cells with siderotic granules. Transmission electron micrographs of reticulocytes in Abcb7fl animals with or without Mx1-Cre (D) −Cre, conventional electron microscopy (EM) stain; (E) +Cre, conventional EM stain; (F) −Cre, EM Fe stain; (G) +Cre EM Fe stain. White arrows indicate a representative mitochondrion in each field. Note the increased electron density of the mitochondrial membranes in panel G. Electron micrographs: original magnification, ×24 000.
Transient siderocytosis following Mx1-Cre–mediated Abcb7 gene deletion. (A) Serial peripheral blood siderocyte counts in 5 representative Abcb7fl+Mx1-Cre animals induced at 4 days of age. Iron-stained peripheral blood smears of Abcb7fl−Mx1-Cre (B) and Abcb7fl+Mx1-Cre (C) animals. Black arrows indicate cells with siderotic granules. Transmission electron micrographs of reticulocytes in Abcb7fl animals with or without Mx1-Cre (D) −Cre, conventional electron microscopy (EM) stain; (E) +Cre, conventional EM stain; (F) −Cre, EM Fe stain; (G) +Cre EM Fe stain. White arrows indicate a representative mitochondrion in each field. Note the increased electron density of the mitochondrial membranes in panel G. Electron micrographs: original magnification, ×24 000.
Transmission electron micrographs of day-5 peripheral blood showed a subset of reticulocytes from experimental animals with clusters of swollen, pale, damaged mitochondria; electron-dense intramitochondrial deposits, typical of siderocytes, were not seen (Figure 1E). However, treatment of whole blood with acid ferrocyanide iron stain prior to processing the cells for electron microscopy highlighted the inner and outer membranes of abnormal mitochondria in mutant cells (Figure 1G). Consequently, there is iron deposited in mitochondria insufficient to produce electron-dense deposits, but adequate enough to produce a detectable histochemical reaction. Marrow ringed sideroblasts were not present in either group, as assessed by light microscopy iron staining, during the entire time course (data not shown).
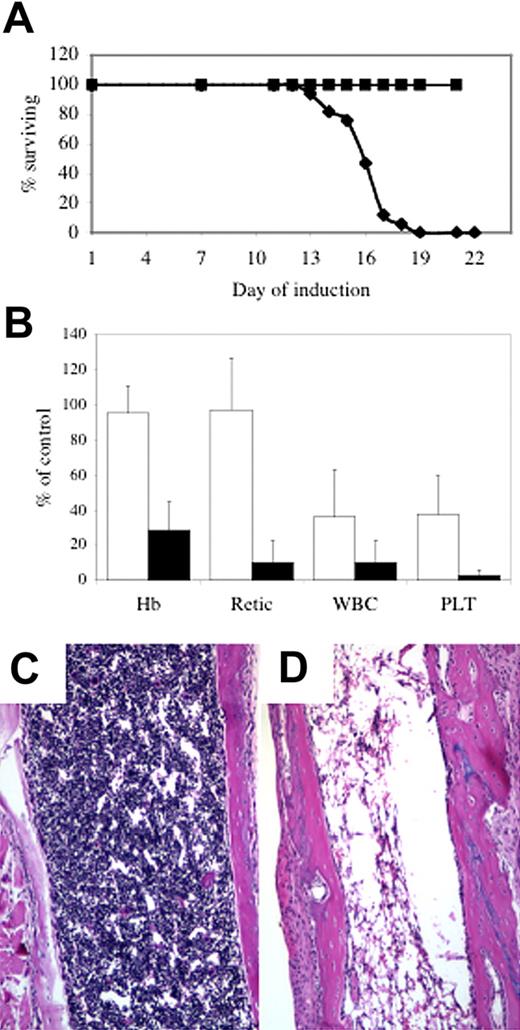
At 1 to 2 weeks after the disappearance of siderocytes, Abcb7fl+Cre animals became clinically ill, with signs including lethargy, rubor, epistaxis, and rectal bleeding, necessitating that the animals be humanely killed. The median survival of +Cre newborns was 16 days (Figure 2A and Figure S3). Postmortem gross and microscopic examination typically showed histologic evidence of gastrointestinal, urologic, and/or intracranial hemorrhage with or without microscopic evidence of systemic bacterial infection. Consistent with these findings, complete blood counts (CBCs) revealed severe pancytopenia (Figure 2B), and the bone marrow was markedly hypocellular (Figure 2D). CBCs taken from animals at day 11 of the induction protocol, prior to the onset of clinical signs, showed that the platelet and white blood counts were less than 25% of control values (Figure 2B). There was partial or near-complete gene deletion in tissues outside the hematopoietic system, but apart from characteristic hepatocellular iron deposits fully described elsewhere,10,11 we did not observe gross or microscopic phenotype in other tissues. It is possible that with time we would see other abnormalities, but the uniform and rapidly fatal bone marrow failure phenotype precluded their ascertainment.
Abcb7 is essential for hematopoiesis. (A) Survival curve of newborn Abc7fl animals with or without MX1-Cre induced with pI-pC. (▪) −Cre, (♦) +Cre. (B) Peripheral blood parameters of animals shown in panel A at day 11 (□) and immediately before death (▪). Values represent the mutant value expressed as a percentage of the control value. Hb indicates hemoglobin; Retic; absolute reticulocyte count; WBC, white blood cells; PLT, platelets. Femoral bone marrow in 4-week-old Abcb7fl+/−Mx1-Cre case controlled animals induced with pI-pC. (C) −Cre, (D) +Cre. Note extreme hypocellularity in panel D.
Abcb7 is essential for hematopoiesis. (A) Survival curve of newborn Abc7fl animals with or without MX1-Cre induced with pI-pC. (▪) −Cre, (♦) +Cre. (B) Peripheral blood parameters of animals shown in panel A at day 11 (□) and immediately before death (▪). Values represent the mutant value expressed as a percentage of the control value. Hb indicates hemoglobin; Retic; absolute reticulocyte count; WBC, white blood cells; PLT, platelets. Femoral bone marrow in 4-week-old Abcb7fl+/−Mx1-Cre case controlled animals induced with pI-pC. (C) −Cre, (D) +Cre. Note extreme hypocellularity in panel D.
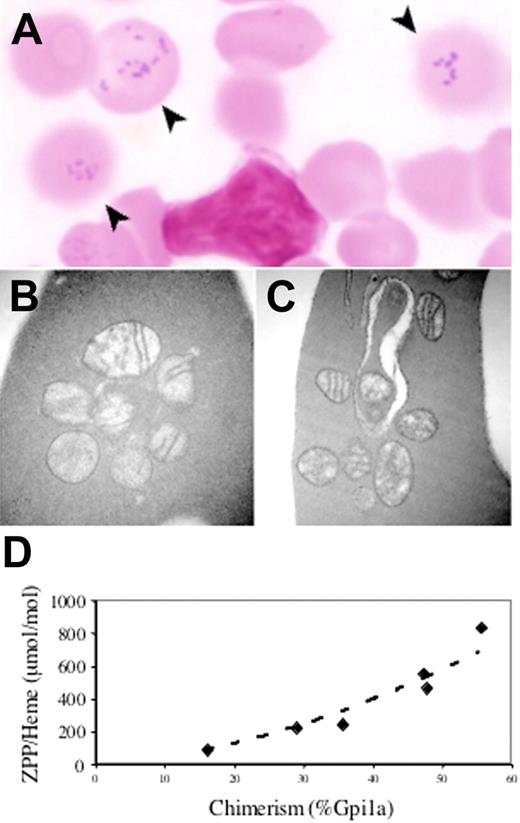
In order to circumvent the bone marrow–dependent lethality, we created a targeted point mutant allele corresponding to the hematologically most severe human E433K mutation.2 Male chimeras were, however, infertile due to testicular atrophy and incomplete spermatogenesis associated with interstitial iron deposition (not shown), which is a phenotype not reported in human subjects with XLSA/A. In other nonhematopoietic tissues, despite substantial chimerism we observed no gross or histologic abnormalities. In particular, animals did not appear ataxic or otherwise neurologically compromised. In the blood, however, a population of siderocytes was present in proportion to the degree of chimerism, as assessed by Gpi1 isozyme analysis (Figure 3A). Similar to the Abcb7fl+Mx1-Cre animals, abnormal mitochondria were readily seen in reticulocytes; however, iron deposition could be demonstrated only by acid ferrocyanide enhancement (Figure 3B–C), and marrow ringed sideroblasts were not visualized by either method (data not shown).
An Abcb7E433K mutation results in siderocytosis with increased zinc protoporphyrin. (A) Siderocytes (arrows) in the peripheral blood of an Abcb7E433K chimera (Fe stain). Transmission electron micrographs of Abcb7E433K chimera reticulocytes unstained (B) or with Fe stain (C). Original magnification ×44 000. (D) RBC zinc protoporphyrin-to-heme ratio as a function of Abcb7E433K chimerism measured by Gpi1 isozyme. Normal is typically less than 100 ng/μg for mice.
An Abcb7E433K mutation results in siderocytosis with increased zinc protoporphyrin. (A) Siderocytes (arrows) in the peripheral blood of an Abcb7E433K chimera (Fe stain). Transmission electron micrographs of Abcb7E433K chimera reticulocytes unstained (B) or with Fe stain (C). Original magnification ×44 000. (D) RBC zinc protoporphyrin-to-heme ratio as a function of Abcb7E433K chimerism measured by Gpi1 isozyme. Normal is typically less than 100 ng/μg for mice.
Our ability to characterize the biochemical phenotype of the Abcb7E433K allele was severely restricted by the unexpected male infertility, which precluded germ line propagation. Nonetheless, analysis of chimeric blood demonstrated that the proportion of protoporphyrin IX chelated with zinc (zinc protoporphyrin IX [ZPP]) compared with iron (heme) was increased in animals with a greater proportion of mutant RBCs (Figure 3D).
In toto, our data demonstrate that Abcb7 is essential not only for erythropoiesis, but hematopoiesis in general, and that the XLSA/A anemia phenotype is due to partial loss of function alleles in ABCB7. As in several other murine models of sideroblastic anemia, including vitamin B6 (pyridoxine) nutritional deficiency,15 isoniazid (INH) toxicity, flexed-tail (f),16 and Sod2 deficiency,14 we did not observe pathologic mitochondrial iron deposits in nucleated erythroid precursors characteristic of sideroblastic anemias in humans in either the Abcb7fl+Mx1-Cre or the Abcb7E433K model; true sideroblasts have been seen only in murine erythroid precursors lacking Alas2, and even then, they have been associated with peculiar, diffuse cytosolic iron staining.17,18 This would further suggest that there is something biologically distinctive between human and murine precursors with respect to mitochondrial iron handling and/or toxicity. For example, it is possible that in mice, mitochondrial iron accumulation, the cessation of heme biosynthesis, and mitochondrial senescence occur later in comparison to morphologic maturation than in humans. Furthermore, as ferrochelatase is required for the formation of zinc protoporphyrin IX,19 the observation that the RBC ZPP/heme ratio increases in proportion to the contribution of Abcb7E433K mutant cells would suggest that Abcb7 has an effect on heme biosynthesis independent of a negative effect on ferrochelatase.20 Rather, it would appear that Abcb7 loss of function directly or indirectly alters the availability of reduced iron required to synthesize heme from protoporphyrin IX.
Authorship
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Mark D. Fleming, Department of Pathology, Enders 1116.1, Children's Hospital Boston, 320 Longwood Ave, Boston, MA 02115.
Presented in part at the 46th Annual Meeting of the American Society of Hematology, San Diego, CA, December 3-7, 2004.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
This work was supported by National Institutes of Health grant DK62 474, the Wilkes Fund of the Children's Hospital Department of Pathology, and the Pew Biomedical Scholars Program (M.D.F.). C.P. was funded in part by L'Association pour la Recherche sur le Cancer (ARC).