Abstract
Human hematopoietic stem cells (HSCs) exposed to cytokines in vitro rapidly divide and lose their characteristic functional properties presumably due to the alteration of a genetic program that determines the properties of an HSC. We have attempted to reverse the silencing of this HSC genetic program by the sequential treatment of human cord blood CD34+ cells with the chromatin-modifying agents, 5-aza-2′-deoxycytidine (5azaD) and trichostatin A (TSA). We determined that all CD34+CD90+ cells treated with 5azaD/TSA and cytokines after 9 days of incubation divide, but to a lesser degree than cells exposed to only cytokines. When CD34+CD90+ cells that have undergone extensive number of cell divisions (5-10) in the presence of cytokines alone were transplanted into immunodeficient mice, donor cell chimerism was not detectable. By contrast, 5azaD/TSA-treated cells that have undergone similar numbers of cell divisions retained their marrow repopulating potential. The expression of several genes and their products previously implicated in HSC self-renewal were up-regulated in the cells treated with 5azaD/TSA as compared to cells exposed to cytokines alone. These data indicate that HSC treated with chromatin-modifying agents are capable of undergoing repeated cell divisions in vitro while retaining their marrow-repopulating potential.
Introduction
Primitive hematopoietic stem cells (HSCs) are relatively quiescent cells that reside in the G0/G1 phase of the cell cycle.1,2 Recently, HSCs have been shown to slowly cycle in vivo during steady-state hematopoiesis.3-10 Self-renewal of HSCs has been tracked in mice by marking HSCs with retroviral vectors.11 Quantitative studies have also documented HSC expansion following stem cell transplantation.12 The maintenance of the size of the HSC pool during adult life is a consequence of HSCs undergoing asymmetrical cell divisions, whereas the expansion of the HSC compartment that occurs during fetal life or following HSC transplantation likely requires symmetrical HSC divisions.8,13-15
Numerous investigators have attempted to create ex vivo conditions that favor HSC self-renewal.16-23 The clinical use of such ex vivo expanded grafts could theoretically shorten the time required for successful hematopoietic engraftment to occur following HSC transplantation. Widespread use of such expanded HSC grafts has been limited due to the lack of the detailed understanding of factors that regulate symmetrical HSC division as well as access to culture conditions that maintain HSCs in an uncommitted state.16 Previous attempts to create an in vitro microenvironment that would allow HSC symmetrical cell division to occur have, however, resulted in the progressive loss of the numbers of marrow-repopulating cells and generation of large numbers of committed hematopoietic progenitor cells.20,21
The ability of human cells to engraft immunodeficient mice has been used as a surrogate assay for human HSCs. In our earlier studies we have demonstrated that a 10-fold expansion of severe combined immunodeficiency (SCID) mouse repopulating cells (SRCs) can be achieved when cord blood (CB) CD34+ cells are treated with 5-aza-2′-deoxycytidine (5azaD)/trichostatin A (TSA) in the presence of an optimal cytokine combination.24 Extensive (> 10-fold) expansion of human HSCs, which retain their in vivo marrow-repopulating potential has not been previously possible.20,21 The loss of HSC function observed following previous attempts to expand human HSCs in vitro has been related to the transit of HSCs through specific phases of the cell cycle.25 Gothot et al have shown that the transition of human HSCs from G0 into G1 is associated with the rapid loss of HSC transplantability.6 We have shown that the expansion of CB SRCs in the presence of 5azaD/TSA treatment is not due to selection of a nondividing primitive HSC population retained in the culture but is rather associated with the active cellular division of SRCs present within a CD34+CD90+ cell subpopulation.24,26 In our current studies we have used the same culture strategy to explore the functional potential of reisolated CD34+CD90+ cells that have undergone progressively greater numbers of cell divisions.
We have previously shown that 5azaD/TSA treatment affects methylation patterns of CpG sites of γ-globin promoter26 and induces changes in the acetylation status at the histone H4 region.24 Both of these events are likely to affect the gene expression patterns of SRCs present within the CD34+CD90+ cell population, which possibly favors expansion of in vivo repopulating HSCs. Recent studies have identified candidate intracellular factors that might regulate self-renewal of HSCs.27-41 In our current studies we have examined whether chromatin-modifying agents permit expansion of SRCs in vitro by promoting their division and the expression of the genes required for HSC self-renewal.
Materials and methods
Isolation of CB CD34+ cells
Fresh CB collections were obtained from the Placental Blood Program of the New York Blood Center (New York, NY) according to guidelines established by the University of Illinois at Chicago Institutional Review Board. CB cells were isolated by density-gradient centrifugation on Ficoll-Paque (< 1.077 g/mL) (Amersham Biosciences, Uppsala, Sweden). CD34+ cells were immunomagnetically enriched using the magnetic-activated cell sorting (MACS) CD34 progenitor kit (Miltenyi Biotech, Auburn, CA) as previously described.22,24 The purity of CB CD34+ cells ranged between 90% and 99%.
Ex vivo cultures
The human CB CD34+ cells (5 × 104/well) were cultured in Iscove modified Dulbecco medium (IMDM; BioWhittaker, Walkersville, MD) containing 30% fetal bovine serum (FBS; HyClone Laboratories, Logan, UT) supplemented with 100 ng/mL stem cell factor (SCF), 100 ng/mL FLT-3 ligand (FL), 100 ng/mL megakaryocyte growth and development factor (MGDF), and 50 ng/mL interleukin 3 (IL-3). The cytokines were a gift of Amgen (Thousand Oak, CA) and incubated as described previously.24 After an initial 16 hours of incubation, the cells were exposed to 5azaD (Pharmachemie, Haarlem, The Netherlands). After 48 hours the cells were washed and then distributed to new culture plates containing SCF, FL and MGDF, and TSA (Sigma, St Louis, MO). The cultures were then continued for an additional 7 days. The cultures lacking 5azaD/TSA treatment were incubated in the identical culture condition supplemented with cytokines (IL-3, SCF, FL, MGDF for the initial 48 hours and SCF, FL, MGDF for the terminal 7 days) only.
Flow cytometric analysis
Briefly, cells were stained with anti–human CD34 monoclonal antibody (mAb) conjugated to fluorescein isothiocyanate (FITC) and anti–human CD90 conjugated to phycoerythrin (PE). Cells were analyzed on a FACSCalibur flow cytometer (Becton Dickinson, San Jose, CA) and at least 10 000 live cells were acquired. All mAbs were purchased from Becton Dickinson PharMingen (San Diego, CA).
CFSE labeling to assess cell division
Primary CB CD34+ cells were labeled for 10 minutes at 37°C with 0.5 μM carboxyfluorescein diacetate succinimidyl ester (CFSE; Molecular Probes, Eugene, OR) in DPBS as described previously.24,26,42 After 9 days of culture the cells were labeled with anti–CD34-allophycocyanin and anti–CD90-PE and analyzed for a progressive decline of fluorescence intensity of CFSE, using flow cytometry as described earlier.24,26,42 The cells were sorted based on their cell division history using FACSVantage (Becton Dickinson).
Cell cycle analysis
Cell cycle kinetics of the CB CD34+ cells in the expansion culture with or without 5azaD/TSA treatment was determined by using a thymidine analog, BrdU (Sigma) in conjunction with propidium iodide (PI; Sigma) as described previously with minor modifications.5,43 After 5 days of initial culture with or without 5azaD/TSA, reisolated CD34+ cells were pulsed with 5 μg/mL BrdU for 2 hours, and then cultured in the presence of cytokines (SCF, FL, MGDF) without BrdU for an additional 48 hours. After the identified intervals, cells were harvested and fixed in 70% ethanol. BrdU quantitation and DNA contents of the cells were analyzed by flow cytometry as described previously.5,43 The fraction of CD34+ cells in S phase marked with BrdU was traced during the subsequent 48 hours in the culture without any additional BrdU or 5azaD/TSA.
Colony-forming cell assays
Colony-forming cells (CFCs) were assayed in semisolid media as previously described.24,26 Briefly, 5 × 102 cells were plated per dish in duplicate cultures containing 1 mL IMDM with 1.1% methylcellulose supplemented with 30% FBS, 5 × 10−5 M 2-ME (StemCell Technologies, Vancouver, BC, Canada), to which 100 ng/mL SCF, 100 ng/mL FL, 50 ng/mL IL-3, 50 ng/mL IL-6, 50 ng/mL granulocyte-macrophage colony-stimulating factor, and 5 U/mL erythropoietin (EPO; Amgen) was added as described previously.24 The colonies were enumerated after 14 days using standard criteria.24,26
Cobblestone area-forming cell assays
NOD/SCID assay for in vivo marrow repopulating potential of ex vivo expanded CB HSCs
NOD/SCID mice were purchased from the Jackson Laboratories (Bar Harbor, ME). The NOD/SCID assay was performed as previously described.24,26 CD34+ cells cultured in the presence of cytokines with or without 5azaD/TSA treatment were harvested after 9 days, CD34+CD90+ cells that have undergone 4 or fewer divisions or 5 or more divisions were reisolated by flow cytometry and then injected (5 × 104 CD34+CD90+ cells/mouse) through the tail vein intravenously to nonobese diabetic/SCID (NOD/SCID) mice 12 to 16 hours after a sublethal dose of total body irradiation (300 cGy). Bone marrow (BM) cells from NOD/SCID mice were analyzed flow cytometrically to detect human cell engraftment after 8 weeks of transplantation as previously described.24,26,44,45
Secondary transplantation
NOD/SCID mice were treated with a single intraperitoneal injection (200 μg/mouse) of TM-β1 (BD Biosciences PharMingen) within 4 hours after total body irradiation as a conditioning for the stem cell transplantation assay. TM-β1 is a mAb directed against the murine IL-2Rβ to eliminate residual natural killer cell activity. BM from chimeric primary recipient mice engrafted with 5azaD/TSA-expanded cells were harvested and injected into secondary NOD/SCID recipients without further reisolation of human cells. BM from a single chimeric mouse was transplanted into a single secondary mouse. Mouse BM cells were stained for human cell chimerism after 7 weeks of transplantation using a panel of mAbs as described earlier to assess multilineage human hematopoietic engraftment.24,26
Differentiation potential of 5azaD/TSA-treated expanded CB cells in subsequent in vitro culture
After 9 days of initial 5azaD/TSA treatment in culture, cells were placed in a secondary culture supplemented with GM-CSF, G-CSF, SCF, IL-3, IL-6, and EPO in the absence of 5azaD/TSA treatment. At the end of 7 days of secondary culture (total 16 days), cytospin preparations (Shandon, Pittsburgh, PA) of cultured cells were stained with Wright-Giemsa stain and viewed under a light microscope (objective used 40×/0.75 NA). The phenotype of cells following secondary culture (day 16) was determined by staining with mAbs directed toward CD34, CD90, CD14, CD15, CD36, and lineage markers (CD2, CD14, CD15, CD19, glycophorin A).
RNA extraction and real-time PCR
Total RNA was extracted from CB cells cultured in the presence of cytokines with or without 5azaD/TSA treatment at the indicated time points (day 5 and day 9) using RNeasy Mini kit (Qiagen, Valencia, CA) or TRIzol (Invitrogen, Carlsbad, CA). Total RNA was subjected to DNase digestion to remove gDNA contaminant. Approximately 1 μg total RNA was reverse-transcribed by use of a GeneAmp cDNA synthesis kit with random hexamer primers (Roche Molecular Systems, Applied Biosystems, Branchburg, NJ). To quantitate the level of mRNAs expression, we carried out polymerase chain reaction (PCR) amplification using the 7700 Sequence detector (PE Applied Biosystems, Foster City, CA), and the PCR products were detected by use of SYBR green technology (ABI, Foster City, CA). PCR cycling conditions were standard except for annealing/elongation temperature, which ranged between 57°C and 62°C and was chosen based on preliminary primer optimization experiments. GAPDH mRNA quantification was used as internal calibrator and the standard curve method was used for relative mRNA quantitation. Measurements were done in triplicate and a negative control (lacking cDNA template) was included in each assay.46-48
The primer sequences used in real-time PCR assays are as follows: GATA 2: forward primer: 5′-GATACCCACCTATCCCTCCTATGTG-3′, reverse primer: 5′-GTGGCACCACAGTTGACACACTC-3′; NOTCH 1: forward primer: 5′-GAGGCGTGGCAGACTATGC-3′, reverse primer 5′-CTTGTACTCCGTCAGCGTGA-3′; BMI 1: forward primer: 5′-TGGCTCTAATGAAGATAGAGG-3′, reverse primer: 5′-TTCCGATCCAATCTGTTCTG-3′; HOXB4: forward primer: 5′- TCCCACTCCGCGTGCAAAGA-3′, reverse primer: 5′-GCCGGCGTAATTGGGGTTTA- 3′; P21: forward primer: 5′-GTCTTGTACCCTTGTGCCTC-3′, reverse primer: 5′-GGTAGAAATCTGTCATGCTGG-3′; P27: forward primer: 5′-TTTAATTGGGTCTCAGGCAAACTCT-3′, reverse primer: 5′-CCGTCTGAAACATTTTCTTCTGTTC-3′; C-MYC: forward primer: 5′-TCCTCGGATTCTCTGCTCTC-3′, reverse primer: 5′-CTTGTTCCTCCTCAGAGTCG-3′; MPO: forward primer: 5′-ACCCTCATCCAACCCTTC-3′, reverse primer: 5′-GTCAATGCCACCTTCCAG-3′; GATA 1: forward primer: 5′-ACAAGATGAATGGGCAGAAC-3′, reverse primer: 5′-TACTGACAATCAGCGCTTC-3′.
Western blotting analysis
Whole-cell lysates were prepared from CB cells cultured for 9 days with or without 5azaD/TSA in the presence of cytokines using the Mammalian Cell Extraction Kit (BioVision, Mountain View, CA) as described previously.24 Equal amounts of protein were subjected to Western blotting analysis. Samples were separated by 12% sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and transferred to polyvinylidene diflouride membranes. The membranes were probed with HOXB4 (Santa Cruz Biotechnology, Santa Cruz, CA), Bmi-1 (Upstate Biotechnology, Lake Placid, NY), P21 (Santa Cruz Biotechnology), and β-actin (Sigma) and developed using the enhanced chemiluminescence system with horseradish peroxidase-conjugated secondary antibody (Amersham Biosciences, Piscataway, NJ) as described previously.24
Statistical analysis
Results are expressed as mean ± SE when appropriate. Statistical differences were evaluated using the student t test with significance at P of .05 or less.
Results
5azaD/TSA affects the division of CD34+CD90+ cells
The optimal combination of cytokines to be used for in vitro HSC expansion remains the topic of continued investigation. Most investigators have concluded that a cytokine cocktail including the early-acting cytokines, SCF, FL, and MGDF, is effective or at least serves as a core cytokine combination to which additional cytokines may be added for these purposes.49-51 After 9 days of culture of CB CD34+ cells, the addition of 5azaD/TSA to the combination of SCF, FL, and MGDF promoted far greater expansion of the number of CD34+CD90+ cells than observed with the addition of the same cytokines alone lacking 5azaD/TSA treatment.24
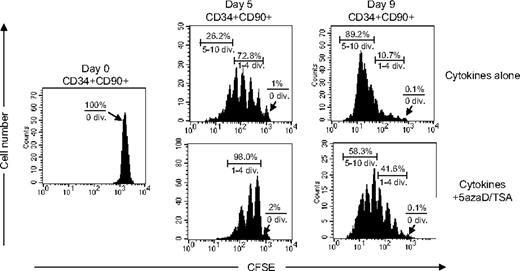
We have previously demonstrated that 5azaD/TSA-treated CD34+CD90+ cells, but not CD34+CD90− cells are responsible for in vivo SRC potential of such expanded cell products.24 The cell division history of CB CD34+CD90+ cells after in vitro culture was assessed using CFSE staining, a fluorescent cytoplasmic dye, which is equally distributed between daughter cells after each cell division. Irrespective of the treatment with 5azaD/TSA, virtually no CD34+CD90+ cells (0.1%) remained quiescent after 9 days of culture. In the absence of 5azaD/TSA treatment, about 73% of the CD34+CD90+ cells had divided up to 4 times and 26% had divided 5 to 10 times after 5 days of culture, whereas 98% of 5azaD/TSA-treated culture of CD34+CD90+ cells had divided only 1 to 4 times (Figure 1). After 9 days of culture in the absence of 5azaD/TSA treatment, 90% of CD34+CD90+ cells already has divided 5 to 10 times, whereas in the presence of 5azaD/TSA treatment 42% of CD34+CD90+ had divided 1 to 4 times and 58% of CD34+CD90+ cells had undergone 5 to 10 cell divisions (Figure 1). These findings suggest that treatment with 5azaD/TSA is associated with CD34+CD90+ cell division occurring at a rate much slower than that observed in the presence of cytokines alone.
The cell division history of CD34+CD90+ cells during ex vivo culture. CFSE-labeled CD34+ cells were cultured in the presence of cytokines with or without 5azaD/TSA treatment for 9 days. The panel shows a representative (1 of 3 experiments) flow cytometric profile of CFSE fluorescence intensity after 5 days and 9 days of culture. The arrow indicates the fraction of cells that have not undergone cell division.
The cell division history of CD34+CD90+ cells during ex vivo culture. CFSE-labeled CD34+ cells were cultured in the presence of cytokines with or without 5azaD/TSA treatment for 9 days. The panel shows a representative (1 of 3 experiments) flow cytometric profile of CFSE fluorescence intensity after 5 days and 9 days of culture. The arrow indicates the fraction of cells that have not undergone cell division.
5azaD/TSA-treated CD34+ cells cycle relatively slowly
For precise examination of the rate of cycling of 5azaD/TSA-expanded CD34+ cells, we have reisolated CD34+ cells from cultures with or without 5azaD/TSA treatment and pulsed with BrdU. The BrdU positively marked cells from both cultures were chased for the subsequent 48 hours at different time intervals in identical cultures (SCF, FL, TPO). The populations of cells that divide faster are expected to re-enter into S phase faster than cells cycling relatively slower possessing a longer doubling time.43 The passage of the cells from 5azaD/TSA-treated culture during the G2/M though the G1 phase paralleled that of the cells obtained from culture lacking 5azaD/TSA treatment (Figure 2). However, re-entry into the S phase of BrdU+ cells cultured in the presence of 5azaD/TSA was significantly delayed by approximately 6 hours in comparison to cells in the absence of 5azaD/TSA treatment. These results indicate that cells expanded in the presence of chromatin-modifying agents cycle relatively slowly in comparison to untreated cells.
Effect of 5azaD/TSA treatment on cell cycle progression. After 5 days of initial culture, CD34+ cells were reisolated from culture containing 5azaD/TSA treatment or cytokines alone. Reisolated CD34+ cells were pulse-labeled with BrdU and then cultured without BrdU or additional 5azaD/TSA treatment for an additional 48 hours. The harvested cells were stained with FITC-anti-BrdU mAb and PI, and BrdU+ S phase cells quantitated every 6 to 12 hours by flow cytometry. The results show the percentage of BrdU+ cells in the S phase at indicated time intervals.
Effect of 5azaD/TSA treatment on cell cycle progression. After 5 days of initial culture, CD34+ cells were reisolated from culture containing 5azaD/TSA treatment or cytokines alone. Reisolated CD34+ cells were pulse-labeled with BrdU and then cultured without BrdU or additional 5azaD/TSA treatment for an additional 48 hours. The harvested cells were stained with FITC-anti-BrdU mAb and PI, and BrdU+ S phase cells quantitated every 6 to 12 hours by flow cytometry. The results show the percentage of BrdU+ cells in the S phase at indicated time intervals.
In vitro behavior of CD34+CD90+ cells undergoing multiple cell divisions
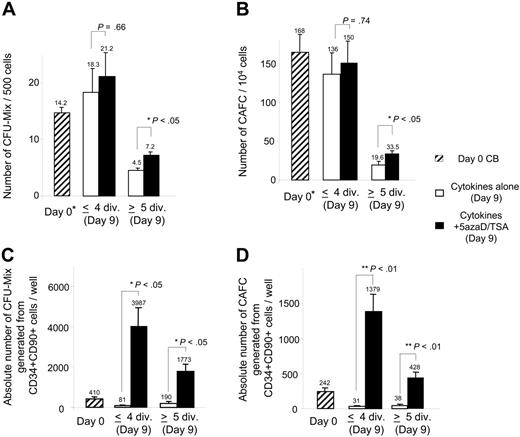
Reisolated CD34+CD90+ cells that had undergone various numbers of cell divisions (1-4 or 5-10 cell divisions) were assayed for mixed colony-forming units (CFUs-Mix) as well as CAFCs. Cells undergoing 1 to 4 divisions contained greater numbers of these primitive hematopoietic progenitors than cells undergoing 5 to 10 cell divisions irrespective of the culture conditions used (Figure 3A–B). Cells undergoing 5 to 10 cell divisions in the presence of chromatin-modifying agents contained statistically greater numbers of assayable CFUs-Mix and CAFCs than CD34+CD90+ cells that had undergone similar numbers of cell divisions in the presence of cytokines alone after 9 days of culture (P ≤ .05; Figure 3A–B). The absolute numbers of CFUs-Mix and CAFCs generated under each culture condition after 5 and 9 days were also determined. The absolute numbers of CFUs-Mix and CAFCs assayed from CD34+CD90+ cells that had undergone 1 to 4 or 5 to 10 cell divisions were far greater in the 5azaD/TSA-treated cultures than similar cell populations exposed to cytokines alone (Figure 3C–D). Absolute numbers of CD34+CD90+ cells from 5azaD/TSA-treated cultures that have undergone 1 to 4 divisions was 38-fold greater than the CD34+CD90+ cells undergoing equal numbers of divisions from cultures receiving cytokines alone. Furthermore, absolute numbers of CD34+CD90+ cells from cultures receiving the 5azaD/TSA that have undergone 5 to 10 divisions was 6.4-fold higher than the CD34+CD90+ cells from cultures receiving cytokines alone (Table 1). These findings indicate that 5azaD/TSA treatment promotes the retention of functional potential of CD34+CD90+ cells even after they have undergone extensive rounds of cell division.
The influence of cell division history of CD34+CD90+ cells on frequency and absolute numbers of CFUs-Mix and CAFCs following ex vivo culture. CD34+ cells cultured in the presence of cytokines with or without 5azaD/TSA treatment after 9 days were harvested and CD34+CD90+ cells that have undergone 4 or fewer divisions or 5 or more divisions were reisolated and assayed for CFCs and CAFCs. (A) Frequency of CFUs-Mix generated from populations of day 0 CD34+CD90+ cells and CD34+CD90+ cells undergoing 4 or fewer divisions or 5 or more divisions (day 9) was determined. The bar graph indicates the numbers of CFUs-Mix assayed in 500 CD34+CD90+ cells plated. (B) Numbers of CAFCs/104 cells assayed from day 0 CD34+CD90+ cells and CD34+CD90+ cells having undergone 4 or fewer divisions or 5 or more cell divisions (day 9). (C) The absolute numbers of CFUs-Mix/well generated from populations of day 0 CD34+CD90+ cells and CD34+CD90+ cells undergoing 4 or fewer divisions or 5 or more divisions (day 9) present in an individual well was determined by the following calculation: (numbers of CFUs-Mix assayed in 500 CD34+CD90+ cells plated that have undergone ≤ 4 or ≥ 5 cell divisions) × (total number of CD34+CD90+ cells having undergone ≤ 4 divisions or ≥ 5 cell divisions present in an individual well). (D) The absolute number of CAFCs/well was determined by the following calculation: (number of CAFCs/104 cells assayed from reisolated CD34+CD90+ cells having undergone ≤ 4 divisions or ≥ 5 cell divisions in an individual well after 9 days of culture) × (total number of CD34+CD90+ cells having undergone ≤ 4 divisions or ≥ 5 cell divisions present in an individual well). The bar graphs represent mean ± SE of 3 independent experiments. *Previously published data, reprinted with permission.24
The influence of cell division history of CD34+CD90+ cells on frequency and absolute numbers of CFUs-Mix and CAFCs following ex vivo culture. CD34+ cells cultured in the presence of cytokines with or without 5azaD/TSA treatment after 9 days were harvested and CD34+CD90+ cells that have undergone 4 or fewer divisions or 5 or more divisions were reisolated and assayed for CFCs and CAFCs. (A) Frequency of CFUs-Mix generated from populations of day 0 CD34+CD90+ cells and CD34+CD90+ cells undergoing 4 or fewer divisions or 5 or more divisions (day 9) was determined. The bar graph indicates the numbers of CFUs-Mix assayed in 500 CD34+CD90+ cells plated. (B) Numbers of CAFCs/104 cells assayed from day 0 CD34+CD90+ cells and CD34+CD90+ cells having undergone 4 or fewer divisions or 5 or more cell divisions (day 9). (C) The absolute numbers of CFUs-Mix/well generated from populations of day 0 CD34+CD90+ cells and CD34+CD90+ cells undergoing 4 or fewer divisions or 5 or more divisions (day 9) present in an individual well was determined by the following calculation: (numbers of CFUs-Mix assayed in 500 CD34+CD90+ cells plated that have undergone ≤ 4 or ≥ 5 cell divisions) × (total number of CD34+CD90+ cells having undergone ≤ 4 divisions or ≥ 5 cell divisions present in an individual well). (D) The absolute number of CAFCs/well was determined by the following calculation: (number of CAFCs/104 cells assayed from reisolated CD34+CD90+ cells having undergone ≤ 4 divisions or ≥ 5 cell divisions in an individual well after 9 days of culture) × (total number of CD34+CD90+ cells having undergone ≤ 4 divisions or ≥ 5 cell divisions present in an individual well). The bar graphs represent mean ± SE of 3 independent experiments. *Previously published data, reprinted with permission.24
Effects of 5azaD/TSA treatment on the marrow-repopulating potential of reisolated CD34+CD90+ cells based on their cell division history
To further examine the functional potential of CD34+CD90+ cells cultured in presence or absence of 5azaD/TSA, CD34+CD90+ cells were reisolated based on their cell division history after 9 days of culture and were transplanted into NOD/SCID mice following total body irradiation. When 5 × 104 CD34+CD90+ cells that had undergone 1 to 4 cell divisions after 5azaD/TSA treatment were injected into NOD/SCID mice, all mice displayed human multilineage hematopoietic cell engraftment after 8 weeks of transplantation (Figure 4A). In addition, 50% of mice receiving CD34+CD90+ cells treated with 5azaD/TSA in the culture that had undergone 5 to 10 cellular divisions still possessed evidence of human multilineage hematopoietic cell engraftment (Figure 4A–B). By contrast, when an equivalent number of CD34+CD90+ cells that had undergone 5 to 10 cellular divisions isolated from cultures receiving cytokines alone were transplanted, none of the recipient mice had any evidence of detectable human hematopoietic cell engraftment (Figure 4A). Since 90% of CD34+CD90+ cells in the cultures exposed to cytokines alone lacking 5azaD/TSA treatment had undergone 5 or more cell divisions by day 9 (Figure 1), only a small fraction of CD34+CD90+ cells (0.15% of total cells) remained within the range of 1 to 4 divisions (Table 1), making it technically challenging to directly assess their marrow-repopulating potential. The transplantation of CD34+CD90+ cells cultured in the presence of 5azaD/TSA treatment that had undergone 1 to 4 cell divisions led to a greater degree of human chimerism than that observed following the transplantation of CD34+CD90+ cells that had undergone 5 to 10 cell divisions (Figure 4A), although the difference was not statistically significant (P = .13).
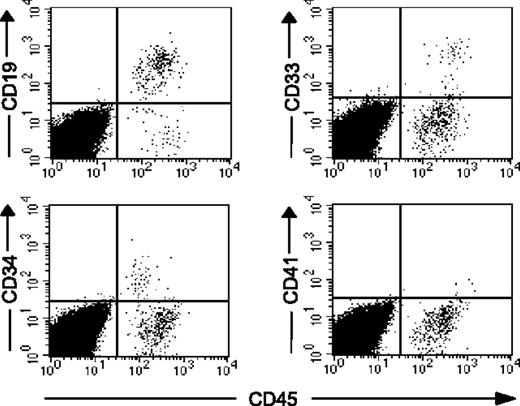
The percentage of human cell chimerism following transplantation of reisolated CD34+CD90+ cells that have undergone 5 or more cell divisions following ex vivo culture. CD34+ cells cultured in the presence of cytokines with or without 5azaD/TSA treatment were harvested after 9 days; the CD34+CD90+ cells that have undergone 4 or fewer divisions and 5 or more divisions were reisolated, then injected into NOD/SCID mice. (A) NOD/SCID (n = 21, data pooled from 2 independent experiments) engraftment achieved with CD34+CD90+ cells (≤ 4 divisions and ≥ 5 divisions). The percent of human hematopoietic cell chimerism is plotted as dots and their mean values indicated as horizontal bars. (B) Representative flow cytometric analysis of multilineage hematopoietic differentiation potential of engrafted human hematopoietic cells in NOD/SCID mice given transplants with reisolated CD34+CD90+ cells (≥ 5 divisions) is shown. *Previously published data, reprinted with permission.24
The percentage of human cell chimerism following transplantation of reisolated CD34+CD90+ cells that have undergone 5 or more cell divisions following ex vivo culture. CD34+ cells cultured in the presence of cytokines with or without 5azaD/TSA treatment were harvested after 9 days; the CD34+CD90+ cells that have undergone 4 or fewer divisions and 5 or more divisions were reisolated, then injected into NOD/SCID mice. (A) NOD/SCID (n = 21, data pooled from 2 independent experiments) engraftment achieved with CD34+CD90+ cells (≤ 4 divisions and ≥ 5 divisions). The percent of human hematopoietic cell chimerism is plotted as dots and their mean values indicated as horizontal bars. (B) Representative flow cytometric analysis of multilineage hematopoietic differentiation potential of engrafted human hematopoietic cells in NOD/SCID mice given transplants with reisolated CD34+CD90+ cells (≥ 5 divisions) is shown. *Previously published data, reprinted with permission.24
Self-renewal and long-term reconstitution function of HSCs is generally demonstrated by reconstitution of secondary recipients after serial transplanrtation.52 To evaluate whether 5azaD/TSA-treated expanded CB cells still retain their self-renewal capacity after primary transplantation, BM cells from the primary NOD/SCID recipients were transplanted into secondary NOD/SCID recipients. Five of 6 secondary NOD/SCID mice receiving BM from primary mice engrafted with the cells treated with 5azaD/TSA resulted in human cell engraftment (Table 2). Furthermore, these cells were capable of differentiating into cells belonging to multiple hematopoietic lineages in secondary recipients (Figure 5).
The cells treated with 5azaD/TSA retain long-term repopulating ability after serial transplantation into secondary NOD/SCID recipients. Representative flow cytometric analysis of multilineage hematopoietic differentiation potential of engrafted human hematopoietic cells in secondary NOD/SCID mice (no. 2 secondary mouse BM as shown in Table 2).
The cells treated with 5azaD/TSA retain long-term repopulating ability after serial transplantation into secondary NOD/SCID recipients. Representative flow cytometric analysis of multilineage hematopoietic differentiation potential of engrafted human hematopoietic cells in secondary NOD/SCID mice (no. 2 secondary mouse BM as shown in Table 2).
5azaD/TSA-treated cells possess the multiple lineage differentiation potential in subsequent in vitro culture
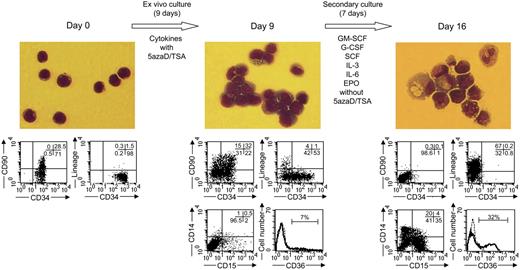
Previously we have demonstrated that human BM CD34+ cells treated with 5azaD/TSA results in an expansion of HSCs capable of differentiating into multiple hematopoietic lineages, both in vitro and in vivo like primary BM CD34+ cells.26 To evaluate the differentiation potential of the CB cells expanded following treatment with 5azaD/TSA, the cells were placed in a secondary culture containing GM-CSF, G-CSF, SCF, IL-3, IL-6, and EPO in the absence of 5azaD/TSA for an additional 7 days to assess their ability to terminally differentiate. After an additional 7 days of culture (total 16 days), the cells were found to be capable of differentiating into multiple blood cell lineages as evident by examining both morphology as well as their immunophenotype (Figure 6). Furthermore, we show that 5azaD/TSA-treated cells are capable of differentiating into both myeloid and lymphoid lineages including CD41+ megakaryocytes following transplantation in vivo (Figure 4B). These findings thus fur suggest that the 5azaD/TSA-treated CB cells are likely to function normally for possible therapeutic use.
Differentiation potential of the cells expanded following 5azaD/TSA treatment in the ex vivo culture. After 9 days of initial culture (as described in “Material and methods”), 5azaD/TSA-treated cells were placed in a secondary culture supplemented with GM-CSF, G-CSF, SCF, IL-3, IL-6, and EPO in the absence of additional 5azaD/TSA treatment. Cytospin preparations were stained with Giemsa and Wright stains and viewed with a light microscope (objective used 40×/0.75 NA) equipped with an Axiocam camera (Zeiss, Thornwood, NY). Images were processed using Zeiss Axiovision software version 4.1. The phenotype of cells was determined by staining with mAb directed toward CD34, CD90, CD14, CD15, CD36, and lineage markers (CD2, CD14, CD15, CD19, glycophorin A).
Differentiation potential of the cells expanded following 5azaD/TSA treatment in the ex vivo culture. After 9 days of initial culture (as described in “Material and methods”), 5azaD/TSA-treated cells were placed in a secondary culture supplemented with GM-CSF, G-CSF, SCF, IL-3, IL-6, and EPO in the absence of additional 5azaD/TSA treatment. Cytospin preparations were stained with Giemsa and Wright stains and viewed with a light microscope (objective used 40×/0.75 NA) equipped with an Axiocam camera (Zeiss, Thornwood, NY). Images were processed using Zeiss Axiovision software version 4.1. The phenotype of cells was determined by staining with mAb directed toward CD34, CD90, CD14, CD15, CD36, and lineage markers (CD2, CD14, CD15, CD19, glycophorin A).
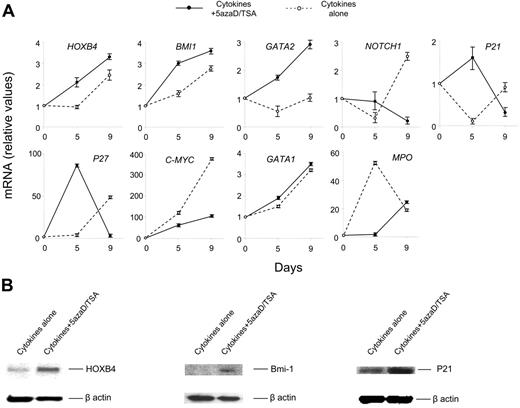
Treatment of CB cells with 5azaD/TSA modulates expression of genes implicated in HSC self-renewal
To understand the molecular mechanism responsible for the expansion of functional HSCs observed following 5azaD/TSA treatment, we examined transcription levels of several genes implicated in self-renewal of HSCs using real-time quantitative PCR. Primers specific for HOXB4, BMI 1, GATA 2, NOTCH 1, P21, P27, C-MYC, GATA 1, and myeloperoxidase (Mpo) were used. Total RNA from cells after 5 and 9 days of culture was extracted. We observed relatively higher levels of transcripts for HOXB4, Bmi-1, and GATA-2 genes in cells treated with 5azaD/TSA in the culture. In addition, the transcript levels of the genes regulating cell cycle, such as P21 and P27 were increased to a greater degree in 5azaD/TSA-treated cells after 5 days but not after 9 days of culture (Figure 7A). The higher transcript level of P21 and P27 in 5azaD/TSA-treated cells is consistent with the proposed role of these gene products in regulating the cycling behavior of HSCs.35,36 Furthermore, the expression of another transcription factor, C-MYC, which is involved in the regulation of cellular proliferation was also examined. A significantly lower level of C-MYC transcript was observed in the 5azaD/TSA-treated cells in comparison to the cells cultured with cytokines alone. The expression of NOTCH 1, which has been implicated in promoting stem cell expansion, was similarly examined.53 Surprisingly Notch expression was increased in cells exposed to cytokines alone but not the chromatin-modifying agents.
Treatment of CB cells with 5azaD/TSA modifies the expression of transcription levels of genes and their products implicated in HSC self-renewal. (A) Effects of 5azaD/TSA treatment on the relative transcript levels of genes (HOXB4, BMI 1, GATA 2, NOTCH 1, P21, P27, C-MYC, GATA 1, and MPO) were measured by real-time quantitative PCR. Total RNA was extracted from CB CD34+ cells (day 0) or cells obtained after 5 and 9 days of culture in the presence of cytokines with or without 5azaD/TSA treatment. Relative mRNA levels in cultured cells (day 5 and day 9) to primary CB cells (day 0) was determined by real-time quantitative PCR. GAPDH was used as internal calibrator (control gene), the standard curve method was used for relative mRNA quantitation. Measurements were obtained in triplicate and a negative control (lacking the cDNA template) was included for each assay. (B) Detection of HOXB4, Bmi-1, and P21 proteins in the cells cultured with or without 5azaD/TSA treatment in the presence of cytokines after 9 days using Western blotting analyses as described in “Materials and methods.” Equal loading of protein was verified with anti–β-actin antibody on the same membrane.
Treatment of CB cells with 5azaD/TSA modifies the expression of transcription levels of genes and their products implicated in HSC self-renewal. (A) Effects of 5azaD/TSA treatment on the relative transcript levels of genes (HOXB4, BMI 1, GATA 2, NOTCH 1, P21, P27, C-MYC, GATA 1, and MPO) were measured by real-time quantitative PCR. Total RNA was extracted from CB CD34+ cells (day 0) or cells obtained after 5 and 9 days of culture in the presence of cytokines with or without 5azaD/TSA treatment. Relative mRNA levels in cultured cells (day 5 and day 9) to primary CB cells (day 0) was determined by real-time quantitative PCR. GAPDH was used as internal calibrator (control gene), the standard curve method was used for relative mRNA quantitation. Measurements were obtained in triplicate and a negative control (lacking the cDNA template) was included for each assay. (B) Detection of HOXB4, Bmi-1, and P21 proteins in the cells cultured with or without 5azaD/TSA treatment in the presence of cytokines after 9 days using Western blotting analyses as described in “Materials and methods.” Equal loading of protein was verified with anti–β-actin antibody on the same membrane.
The expression of lineage-specific genes such as MPO and GATA-1 were also examined by real-time quantitative PCR. GATA1 is a transcription factor, important for commitment of HSCs to adult erythroid and megakaryocytic lineages,54,55 whereas the expression of MPO is associated with granulocytic differentiation.56 The GATA1 transcript level progressively increased to a similar degree in cultures receiving cytokines alone as well as culture receiving the chromatin-modifying agents. Higher transcript levels of MPO were observed in cultures exposed to cytokines alone as compared to cells cultured in the presence of 5azaD/TSA.
To confirm the role of higher transcript levels of genes observed, protein levels of HOXB4, BMI 1, and P21 were examined in the CB cell cultures treated with and without 5azaD/TSA. A significantly higher degree of HOXB4, Bmi-1, and P21 protein expression was detected in the cells treated with 5azaD/TSA in comparison to the cells treated with cytokines alone (Figure 7B).
Discussion
Previous attempts to promote the expansion of human HSCs in vitro have led either to HSC differentiation resulting in HSC exhaustion or, at best, asymmetrical HSC divisions and maintenance of the numbers of HSCs.20,21 We hypothesize that previously used ex vivo culture conditions for HSC expansion result in the silencing of pivotal genes that are crucial for HSCs to undergo symmetrical cell division. In this study chromatin-modifying agents were used to alter the methylation and acetylation status of promoters of critical genes that are pivotal for the retention of the characteristic biological properties of dividing HSCs. Our data suggest that chromatin-modifying agents not only prevent the loss of HSCs in the culture but promote symmetrical division of SRCs resulting in the expansion of the numbers of marrow-repopulating cells.24,26,57 Although a more careful safety assessment of 5azaD/TSA-treated expanded CB cells is required before proceeding with clinical development of this technology, the expanded CB cells show preliminary therapeutic potential as an alternative graft for adult recipients. The 5azaD/TSA-treated cells are not only capable of differentiating into myeloid and lymphoid lineages like unmanipulated primary CB CD34+ cells in vivo but they can also terminally differentiate in vitro when further cultured in an appropriate environment.
HSCs slowly cycle in vivo; however, in the presence of a variety of cytokine combinations in vitro CD34+ cells rapidly cycle and undergo repeated cell divisions resulting frequently in the generation of large numbers of differentiated progenitor cells but a decline in the number of marrow-repopulating cells.5,6,9,43 The residual marrow-repopulating potential of such ex vivo generated grafts has been reported to reside either within a population of HSCs that had either remained quiescent or had undergone a limited numbers of divisions.58-60 In the present studies, we confirm CB CD34+CD90+ cells that have undergone 5 to 10 cell divisions in vitro in response to a cytokine combination lack in vivo marrow-repopulating potential. By contrast, CD34+CD90+ cells exposed to 5azaD/TSA, which also had undergone 5 to 10 cell divisions, still contained assayable SRCs. We conclude that exposure of the SRCs within the CD34+CD90+ cell population to cytokines alone results in their rapid cell division and their terminal differentiation, whereas exposure of SRCs to 5azaD/TSA in the culture results in these same cells dividing at a slower rate and retention of their marrow-repopulating potential. In an additional experiment we have demonstrated that 5azaD/TSA-treated cells re-enter into S phase significantly slower in contrast to the cells cultured in cytokines alone. These findings support the concept that the kinetics of HSC progression through the cell cycle is associated with or determined by the decision of an HSC to undergo self-renewal or differentiation. The chromatin-modifying agents might influence the doubling time by regulating cell cycle machinery, but it is more likely that chromatin-modifying agents in our culture system result in the expansion of more primitive HSCs, which cycle slowly. It has been suggested by several investigators that primitive HSCs divide more slowly than the relatively committed progenitor cells.3,4,43,57
The expressions of a number of genes have been implicated in determining the function and fate of HSCs. These genes include HOXB4, Bmi-1, GATA-2, Notch-1, P21, P27, GATA 2, and possibly other as of now unidentified genes. GATA 2 is a transcription factor expressed by HSCs, which has been reported to be necessary for maintenance of HSCs and primitive hematopoietic progenitor cells.33 In this report significantly higher transcript levels of GATA 2 were observed exclusively in 5azaD/TSA-treated cells after 5 and 9 days of culture. The increased transcript level of GATA 2 observed in cells expanded with chromatin-modifying agents indicates that GATA-2 is likely one of the contributing factors that determines the characteristic properties of an HSC.
Members of the Polycomb group of genes such as BMI 1 and members of the homeobox family of genes such as HOXB4 may bias HSCs to undergo self-renewal rather than differentiation.27 Many of these pathways interact with the basic cell cycle machinery.27 We have detected a high transcript level of both HOXB4 and BMI 1 in human CB CD34+ cells treated with chromatin-modifying agents. HOXB4 requires a functional BMI 1 to execute its function as an HSC activator.32 Overexpression of HOXB4 and Bmi-1 genes has been shown to induce self-renewal of long-term multilineage repopulating HSCs without causing leukemia.27-32 The greater expression of HOXB4 and BMI 1 transcripts present in CD34+ cells treated with chromatin-modifying agents is therefore consistent with the proposed roles of these genes in HSC self-renewal.
The activity of these cyclin-dependent kinases is regulated by a number of inhibitors, including P21 and P27, which have been shown to play a role in determining HSC behavior. Cheng and coworkers have indicated that the absence of P21 leads to expansion of the stem cell pool, more active HSC cycling, and greater sensitivity of the HSC pool to exhaustion in response to a variety of challenges.35 By contrast P27 has been shown not to affect stem cell kinetics but rather to determine progenitor cell proliferation and the size of HSC pool.36 The transcript level of both P21 and P27 were increased after 5 days in the cultures exposed to the chromatin-modifying agents. Although the transcript levels of P21 and P27 in 5azaD/TSA-treated cells declined after 9 days of culture, the expression of P21 protein level in the chromatin-modifying agents treated cells remained higher in comparison to the untreated cells. These findings could be partially responsible for the expansion of CD34+CD90+ cells after 9 days of culture but the decline of CD34+CD90+ cell numbers observed after 14 days of culture (H.A. and N.M., unpublished observation, 2006).
Studies of the effect of C-MYC on stem cell behavior has resulted in conflicting information.37,61 Our studies revealed progressively greater amounts of C-MYC transcripts in cells exposed to cytokines alone, which did not contain assayable SRCs as compared to cells exposed to the chromatin-modifying agents. These findings indicate that C-MYC expression alone is unlikely to define the functionality of HSCs after in vitro division but might affect in vivo HSC self-renewal by altering the interaction between an HSC and its niche within the marrow microenvironment as suggested by Wilson et al.37 In our current studies we detected greater transcript levels of NOTCH 1 in the cultures performed in the absence of 5azaD/TSA treatment than observed in cultures exposed to the chromatin-modifying agents. These findings are similar to those reported by DeFelice et al who demonstrated that Notch-1 pathway was not associated with ex vivo expansion of primitive hematopoietic progenitor cells treated with another HDAC inhibitor, valproic acid.62 GATA 1 expression is associated with HSC commitment to erythropoiesis and megakaryocytopoiesis,54,55 whereas MPO expression is associated with terminal myeloid maturation.56 GATA 1 transcript levels increased progressively in cells exposed to cytokines alone to a similar degree as cells receiving the chromatin-modifying agents. MPO transcript levels peaked after 5 days of incubation in cultures exposed to cytokines alone but maximal expression was delayed in cultures containing the chromatin-modifying agents until day 9 of incubation. The methylation of the MPO promoter in primitive hematopoietic progenitor cells may be sensitive to the action of chromatin modifying agents.56
Collectively, our data suggest that epigenetic mechanisms likely result in the loss of in vivo marrow-repopulating potential of CB HSCs following ex vivo culture in the presence of cytokines alone. This loss of HSC function is at least in part accounted for by a faster rate of HSC division occurring in the CD34+ cells exposed to cytokines alone. However, this loss of SRCs can be circumvented by the use of chromatin-modifying agents, which results in a slower rate of cell division associated with higher expressions of a group of HSC regulatory genes including HOXB4, BMI 1, GATA 2, P21, as well as P27. The exact functional roles of these genes for maintaining self-renewal of HSCs have yet to be fully elucidated. The possibility remains where the expression of additional or yet to be identified HSC regulatory genes may also contribute to the retention of the marrow-repopulating potential of 5azaD/TSA-treated CB CD34+ cells. The global gene expression pattern of CD34+ cells following treatment with chromatin-modifying agents is of immense interest and currently being sought in our laboratory.
Authorship
Contribution: H.A. designed and performed research, interpreted the data, and wrote the paper; K.Y. performed research; P.B. and Y.Z. performed and analyzed PCR data; R.H. was responsible for critical reading of the manuscript for important intellectual content; and N.M. was responsible for the study concept, design, execution of the research, interpretation of data, and writing and revising the draft paper.
Conflict-of-interest disclosure: The authors have no competing financial interests.
Correspondence: Nadim Mahmud, University of Illinois, 909 S Wolcott Ave, COMRB, Rm 3095, M/C 734 Chicago, IL 60612; e-mail: nadim@uic.edu.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This work was supported in part by grants from the State of Illinois through Illinois Regenerative Medicine Institute (N.M.) and grants from the Prudence Cole Foundation (N.M.) and start-up funding from the Department of Medicine and the University of Illinois Cancer Center (N.M.).
We would like to thank Edward Bruno, Rifat Rahman, and Sakina M. Petiwala for their excellent technical assistance. We gratefully acknowledge the helpful comments of Joseph DeSimone and Donald Lavelle. Dolores Mahmud is acknowledged for critical reading of the manuscript. We wish to thank Amgen Inc (Thousand Oaks, CA) for providing cytokines. We are indebted to Pablo Rubenstein and Luda Dobrila of New York Blood Center (New York, NY) for providing CB units.