CD13/aminopeptidase N is a transmembrane peptidase that is induced in the vasculature of solid tumors and is a potent angiogenic regulator. Here, we demonstrate that CD13 controls endothelial cell invasion in response to the serum peptide bradykinin by facilitating signal transduction at the level of the plasma membrane. Inhibition of CD13 abrogates bradykinin B2 receptor internalization, leading to the attenuation of downstream events such as bradykinin-induced activation of Cdc42 and filopodia formation, and thus affects endothelial cell motility. Investigation into mechanisms underlying this block led us to focus on B2R internalization via membrane-dependent mechanisms. Membrane disruption by depletion of cholesterol or trypsinization halts B2R internalization, invasion, and filopodia formation, which can be recovered with addition of cholesterol. However, this functional recovery is severely impaired in the presence of CD13 antagonists, and the distribution of membrane proteins is disordered in treated cells, suggesting a role for CD13 in plasma membrane protein organization. Finally, exogenous expression of wild-type but not mutant CD13 further alters protein distribution, suggesting peptidase activity is required for CD13's regulatory activity. Therefore, CD13 functions as a novel modulator of signal transduction and cell motility via its influence on specific plasma membrane organization, thus regulating angiogenesis.
Introduction
Bradykinin has long been recognized as a component of an array of potent serum factors that maintain and regulate tissue perfusion by controlling the integrity of endothelial cells. This nonapeptide is the principal effector of the kallikrein-kinin system which functions in many normal and disease-related processes including pain perception, vascular homeostasis, smooth muscle contraction, coagulation, and fibrinolysis (reviewed in Blaukat1 and Prado et al2 ). Recently, bradykinin and its kininogen precursor have been implicated in angiogenesis, where the ischemic environment stimulates bradykinin production. In this setting, bradykinin acts immediately as a vasodilator to increase tissue perfusion and later as a long-term angiogenic stimulator.3,,,,–8
CD13 is a cell-surface peptidase that was originally defined as a myeloid-specific hematopoietic marker.9 More recently, however, we have shown that while normal endothelial cells are CD13−, neovessels in developing tumors express high levels of CD13.10 This induction is mediated at the transcriptional level in response to angiogenic stimuli in the tumor microenvironment11 through signals transduced via the Ras/MAPK pathway.12 Additional data support the notion that CD13 peptidase activity is required for endothelial invasion and morphogenic phases of tumor neovessel formation.11 CD13's role as a regulator of angiogenesis is clear: CD13 rescues angiogenesis in the presence of inhibitors of the Ras/MAPK pathway,12 and CD13 inhibition prevents tumor growth.10 However, the precise mechanisms mediating CD13's effects on tumor angiogenesis are largely unknown.
To determine the function of CD13 in angiogenesis we investigated its role in endothelial cell invasion. We find that bradykinin-induced invasion is highly dependent on CD13 functional activity, and that bradykinin-induced activation of the Rho-family GTPase Cdc42 and subsequent filopodia formation is inhibited by CD13 antagonists. Further investigation indicated that CD13 acts at the plasma membrane level, at a step subsequent to bradykinin binding but prior to receptor internalization. Experimental manipulation of membrane integrity via cholesterol depletion or trypsinization showed that CD13 is necessary for cells to recover from membrane perturbation, perhaps by participating in the assembly, trafficking, or maintenance of membrane proteins. Finally, overexpression of wild-type but not enzymatically inactive CD13 in endothelial cells alters the distribution of membrane proteins, suggesting that CD13 peptidase activity is required for this membrane regulatory function.
Materials and methods
Cell culture and plasmids
Human umbilical vein endothelial cells (HUVECs) were obtained from Clonetics (Lonza Group, Ltd, Basel, Switzerland) and maintained in endothelial growth medium-2 (EGM-2) medium plus serum (EGM complete [Clonetics], containing growth factors: insulin-like growth factor-1, epidermal growth factor, vascular endothelial growth factor, and basic fibroblast growth factor). For serum-free experiments, EGM-2 medium (containing growth factors) was supplemented with 0.2% BSA (EGM-2 SF). EOMA cells (CRL-2586; ATCC, Manassas, VA) were propagated in Dulbecco modified Eagle medium (DMEM; Gibco, Carlsbad, CA) with 10% fetal bovine serum, l-glutamine, penicillin, and streptomycin. MY7 and WM4.7 antibodies were obtained from Beckman Coulter (Fullerton, CA); high-molecular-weight kininogen (HK) was obtained from Calbiochem (San Diego, CA), and the Superdex 200 was obtained from Amersham Biosciences (Arlington Heights, IL). All other chemicals were obtained from Sigma (St Louis, MO). The antifascin antibody was obtained from Chemicon International (Temecula, CA); anti–Cav-1 was obtained from BD Biosciences Pharmingen (San Diego, CA); the anti-CD13 antibody used for Western blot analysis (clone H-300), B2 receptor, and flotillin-1 antibodies were obtained from Santa Cruz Biotechnologies (Santa Cruz, CA). Plasmids expressing wild-type and mutant CD13 in pcDNA3.112 were produced using the QuikChange site-directed mutagenesis kit (Stratagene, La Jolla, CA), and stably transfected single-cell clones in EOMA cells were produced according to published procedures.12
Invasion assays
Matrigel (Becton Dickinson, San Jose, CA) was diluted 1:5 with serum-free DMEM, and invasion chambers in 24-well plates were coated with 20 μL of diluted matrigel (Transwell; Corning 8-μm pore size; Corning, NY). Endothelial cells were trypsinized, washed once with serum-free DMEM, and added to the upper invasion chamber (5 × 104/100 μL), while the bottom chamber contained EGM-2 SF. Activators or inhibitors were added to appropriate concentrations. After incubation at 37°C for 16 hours, the upper side of the filter was cleaned with a cotton swab, and the cells on the bottom were stained with 2 μg/mL vital fluorescent dye calcein-AM (Molecular Probes, Eugene, OR) in Hanks balanced salt solution for 1.5 hours at 37°C. The number of invading cells was determined by fluorescence microscopy.
Immunofluorescence analysis and cell staining
Fascin staining.
HUVEC cells plated on cover slips in serum-containing medium were fixed with 100% methanol for 30 minutes and permeabilized with 0.5% Triton X-100 in PBS for 5 minutes at room temperature (RT). Cells were washed twice with 0.05% Tween-PBS and blocked by 1% BSA-PBS. A 1:100 dilution of antifascin antibody (clone 55K2; Chemicon) was added for 1.5 hours, followed by 1:100 dilution of mouse anti-IgG–FITC.
Filipin and phalloidin staining.
Cells plated on glass coverslips in the presence of bradykinin or inhibitors were paraformaldehyde fixed (4% in PBS, 30 minutes at RT), stained with 50 μg/mL filipin (Cayman Chemical, Ann Arbor, MI) in PBS for 15 hours at RT, or permeabilized with 0.1% Triton X-100 and stained with TRITC-phalloidin (Molecular Probes) and analyzed by fluorescence microscopy using a Zeiss Axioplan 2 (63 ×) attached to a Zeiss AxioCam HRC digital camera (Zeiss, Thornwood, NY). Alternatively, filopodia formation was induced by plating HUVECs on Alcian Blue–coated coverslips13 in the presence or absence of bestatin and was microscopically evaluated for the presence of phenotypically characteristic filopodia-like structures consisting of thin cytoplasmic projections extending from the cell.
GTPase activation
Adherent cells were washed and preincubated in EGM-2 SF for 1 hour followed by the addition of 200 nM bradykinin for 15 minutes at 37°C. Activation of Cdc42 and Rac was determined using EZ-Detect Small GTPase Activation Kits (Pierce, Rockford, IL), which detect active small GTPases by differential binding to their effector PAK.
Bradykinin binding/internalization
Adherent cells were washed with EGM-2 SF medium and incubated with 3H-labeled bradykinin (Perkin-Elmer, Shelton, CT) in the same medium for 1 hour. Cells were washed with SF DMEM, and the amount of receptor-bound bradykinin was calculated by measuring the radioactivity released from the cells in the presence of an excess of nonradioactive bradykinin (100 μM in SF DMEM for 5 minutes at RT). Bradykinin internalization was determined by measuring the radioactivity remaining in the adherent cells. Nonspecific binding as determined by measuring internalization at 0°C was negligible, as was nonspecific internalization measured at 0°C.
CD13 activity
Stably transfected EOMA cells were scraped in PBS and lysed with a glass-Teflon homogenizer (12-15 strokes) followed by 3 cycles of freeze/thaw. The lysate was centrifuged at 10 000g for 10 minutes at 4°C to collect the insoluble fraction. Protein content was determined by the Bradford assay (BioRad, Hercules, CA). CD13 activity was expressed as micromoles of the alanine p-nitroanalide substrate14 converted into cleavage products in 1 hour at 37°C by 1 mg of insoluble cell lysate proteins (units per milligram of protein).
Gel filtration chromatography
Fetal bovine serum (0.5 mL) was separated using Superdex 200 (Amersham Biosciences) gel filtration chromatography using a modified procedure by De Pauw et al.15 The serum sample (0.5 mL) was loaded onto a 50-mL Superdex 200 column (separation range of 10-600 kDa). Fractions of 1 mL (total of 60 mL) were eluted with PBS, collected, and tested in the invasion assay. The molecular weights of eluted fractions were determined using gel filtration standards (BioRad).
Membrane fractionation
Cells were washed with PBS and scraped in 900 μL scraping buffer (PBS containing 1 mM EDTA, 1 mM PMSF, and protease inhibitors). After homogenization on ice by multiple passages through a 26-gauge needle, the cell suspension was centrifuged at 1000g for 5 minutes to remove debris. The remaining supernatant was supplemented with Triton X-100 to 1% and further homogenized. As an alternative to the detergent lysis, homogenates were sonicated in the absence of detergent (3 × 10 seconds) after the 5-minute centrifugation step. Sucrose was added to samples adjusted to contain equivalent protein levels to obtain a 40% solution. A total of 1.5 mL of the mixture was overlaid with 4.5 mL of cold 30% sucrose solution and 4.5 mL of 5% sucrose solution in scraping buffer (plus 1% Triton X-100). After centrifugation for 16 hours at 180 000g, the tube was punctured with a 19-gauge needle, and 10 fractions of 0.5 mL were collected. Fractions were assayed by Western blot analysis.16
Statistical analysis
In some experiments, the maximum activation of endothelial invasion or 3H-bradykinin binding was extrapolated from experimentally generated dose-response curves using nonlinear regression analysis. Curve-fitting and all other statistical analysis was performed using GraphPad Prism Software version 3.00 for Windows (San Diego, CA). Statistical significance was set at P value greater than .05.
Results
CD13-dependent invasion of endothelial cells is mediated by high-molecular-weight kininogen
We have previously established that CD13 is required for angiogenesis,10 where it plays a critical role in endothelial cell invasion through the extracellular matrix in response to serum.11,12 To elucidate the molecular mechanisms regulating CD13-dependent endothelial invasion, we initially sought to define the critical serum components participating in this process. As previously shown, invasion of human endothelial cells through a layer of basement membrane proteins (Matrigel) is highly serum inducible (< 110-fold; Figure 1A). While the addition of saturating concentrations of critical growth factors that modulate endothelial metabolism (insulin-like growth factor-1, epidermal growth factor, vascular endothelial growth factor, and basic fibroblast growth factor) induced a significant 4-fold increase in invasion compared with serum-free conditions (Figure 1A), this induction was considerably lower than that observed in the presence of serum. This result suggests that invasion is only partially mediated by these growth factors present in the serum. Importantly, the strong serum-induced invasion is CD13 dependent because it is suppressed by either the CD13 inhibitor bestatin or CD13-blocking antibodies in a dose-dependent manner (Figure 1B). In contrast, a CD13-binding monoclonal antibody that does not inhibit CD13 activity (WM4.7) or an irrelevant protease inhibitor failed to have significant effects on endothelial invasion. This activity is completely abrogated by heat treatment of serum (65°C, 10 minutes), indicating that the factor(s) responsible for induction is most likely a heat labile protein (data not shown).
Serum proteins modulate CD13 dependent endothelial invasion in vitro. (A) Primary endothelial cells (HUVECs) were plated in the top chamber of Matrigel-coated transwell plates in the presence (▩) or absence (□) of added growth factors or 10% FBS (■) and the number of cells invading through the Matrigel barrier assessed after 24 hours. The CD13 functional antagonists bestatin (100 μg/mL) and MY7 antibody (1:100 dilution; CD13 mAb) were added to medium containing 10% FBS. (B) Invasion was assessed in the presence of increasing doses of the CD13 antagonists or irrelevant inhibitors or noninhibitory CD13 antibodies. The highest concentration of each agent used is set at 100%. For bestatin (○) and soybean trypsin inhibitor (□), 100% equals 100 μg/mL, while for antibodies MY7 (●; neutralizing mAb) and WM4.7 (■; noninhibitory mAb) 100% equals 1:40 dilution. (C) FBS was fractionated by gel filtration, and fractions were assayed for their ability to induce endothelial invasion. (D) Invasion was assessed in the presence of increasing concentrations (“Materials and methods”) of the plasma components fibronectin (♦), vitronectin (▾), plasminogen (▴), fibrinogen (□), high-molecular-weight kininogen (HK; ●), and low-molecular-weight kininogen (○), LDL (*), and HDL (▵). (E) HK (100 μg/mL; ▩)–induced invasion was assessed in the presence of the CD13 inhibitors bestatin or MY7 antibody (CD13 mAb, 1:100 dilution) or the bradykinin B2 receptor antagonist HOE140 (10 μM). (F) Effect of increasing concentrations of B2 receptor antagonist HOE140 on invasion induced by 10% FBS. Relative invasion in treated cells is expressed as percentage of controls. Data are shown as means plus or minus a standard deviation (SD), n = 3.
Serum proteins modulate CD13 dependent endothelial invasion in vitro. (A) Primary endothelial cells (HUVECs) were plated in the top chamber of Matrigel-coated transwell plates in the presence (▩) or absence (□) of added growth factors or 10% FBS (■) and the number of cells invading through the Matrigel barrier assessed after 24 hours. The CD13 functional antagonists bestatin (100 μg/mL) and MY7 antibody (1:100 dilution; CD13 mAb) were added to medium containing 10% FBS. (B) Invasion was assessed in the presence of increasing doses of the CD13 antagonists or irrelevant inhibitors or noninhibitory CD13 antibodies. The highest concentration of each agent used is set at 100%. For bestatin (○) and soybean trypsin inhibitor (□), 100% equals 100 μg/mL, while for antibodies MY7 (●; neutralizing mAb) and WM4.7 (■; noninhibitory mAb) 100% equals 1:40 dilution. (C) FBS was fractionated by gel filtration, and fractions were assayed for their ability to induce endothelial invasion. (D) Invasion was assessed in the presence of increasing concentrations (“Materials and methods”) of the plasma components fibronectin (♦), vitronectin (▾), plasminogen (▴), fibrinogen (□), high-molecular-weight kininogen (HK; ●), and low-molecular-weight kininogen (○), LDL (*), and HDL (▵). (E) HK (100 μg/mL; ▩)–induced invasion was assessed in the presence of the CD13 inhibitors bestatin or MY7 antibody (CD13 mAb, 1:100 dilution) or the bradykinin B2 receptor antagonist HOE140 (10 μM). (F) Effect of increasing concentrations of B2 receptor antagonist HOE140 on invasion induced by 10% FBS. Relative invasion in treated cells is expressed as percentage of controls. Data are shown as means plus or minus a standard deviation (SD), n = 3.
To identify the protein(s) responsible for the serum induction of invasion, fetal bovine serum proteins were separated by gel filtration, and the resulting fractions were analyzed for their ability to induce invasion in serum-free medium supplemented with growth factors (Figure 1C). In these experiments, a single major peak of activity was identified within the relatively high-molecular-weight range of 44 to 158 kDa (Figure 1C). We then tested purified preparations of several serum proteins of the correct molecular weight that are essential for maintaining endothelial cell integrity (fibronectin, vitronectin, plasminogen, fibrinogen, and high- and low-molecular-weight kininogen) and 2 protein-lipid complexes (LDL and HDL) for their ability to induce CD13-dependent invasion (Figure 1D). In these experiments, the maximum induction of invasion for each sample was extrapolated from the dose-response curves generated for each of the serum factors examined. The highest maximal induction of invasion (18-fold) was obtained with HK, a serum protein with a molecular weight ranging between 88 and 120 kDa depending on its extent of glycosylation.17 A significant, but lower, induction was also detected with HDL (3.5-fold), while the other proteins examined showed only very modest effects (0.42- to 1.0-fold increase over controls). Importantly, invasion in response to HK is completely abrogated by bestatin or anti-CD13 monoclonal antibodies, indicating that CD13 is critical for endothelial invasion induced under these experimental conditions (Figure 1E).
Physiologically, the proteolytic processing of HK releases the biologically active, 9-amino-acid peptide bradykinin that mediates numerous biological processes via binding to its G-protein–coupled receptor B2 (B2R; reviewed in Blaukat1 ). To determine if HK induces endothelial invasion via bradykinin, we tested the effect of the B2R antagonist HOE140 on HK-mediated invasion. The complete inhibition of HK-stimulated invasion in the presence of this antagonist indicates that bradykinin is responsible for the HK-stimulated invasion via B2R-dependent mechanisms (Figure 1E). Furthermore, treatment with the B2R receptor antagonist HOE140 also suppressed serum-induced invasion (EC50 = 3.3± 1.1 μM; Figure 1F), while treatment with the B1R-specific antagonist (Des-Arg10)-HOE140 had no effect (data not shown) indicating that in our system, bradykinin is an important contributor to serum-stimulated endothelial invasion.
Bradykinin's effect on endothelial invasion is regulated at the membrane level by CD13
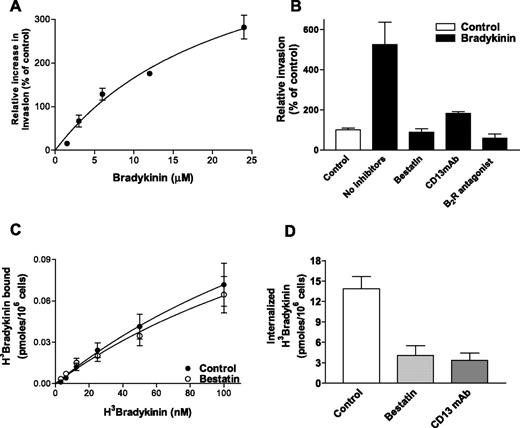
To further investigate B2R signaling in endothelial cell invasion and to confirm the role of CD13 in this process, we verified that purified bradykinin induces endothelial invasion in a dose-dependent manner when added to serum-free medium supplemented with growth factors (Figure 2A). The short half-life of bradykinin necessitates the addition of relatively high bradykinin concentrations in this long-term assay, whereas much lower concentrations are effective in short-term assays. Similar to the results obtained using serum and HK, bradykinin-induced invasion was inhibited by both bestatin and CD13-blocking antibodies (Figure 2B). To begin to determine at which step CD13 may regulate bradykinin activity, we performed a short-term assay designed to measure only receptor-ligand interaction at the membrane. Radiolabeled bradykinin binding to endothelial cells was unaffected in the presence of bestatin (Figure 2C). The extrapolated maximum binding (control = 0.25 ± 0.12, bestatin = 0.21 ± 0.09 pmoles/106 cells) and EC50 values (control = 247.8 ± 154.5nM, bestatin = 230.0 ± 137.1 nM) were also not significantly different (P = .725 vs P = .672).
CD13 regulates bradykinin-induced invasion and internalization, but not its binding to endothelial cells. (A) Effects of increasing concentrations of bradykinin (in serum-free medium containing growth factor supplement) on endothelial invasion. (B) Sensitivity of bradykinin-induced invasion (12.5 μM; ■) to CD13 inhibition by bestatin (100 μg/mL) or MY7 antibody (CD13 mAb, 1:100 dilution) or inhibition of bradykinin signaling by the B2R antagonist HOE140 (10 μM). (C) Binding of 3H-bradykinin to HUVECs at 0°C in the presence or absence of bestatin. (D) Effects of CD13 inhibition by bestatin (100 μg/mL) or MY7 antibody (CD13 mAb, 1:100 dilution) on 3H-bradykinin (50 nM) internalization at 37°C. Data are shown as mean (± SD, n = 3).
CD13 regulates bradykinin-induced invasion and internalization, but not its binding to endothelial cells. (A) Effects of increasing concentrations of bradykinin (in serum-free medium containing growth factor supplement) on endothelial invasion. (B) Sensitivity of bradykinin-induced invasion (12.5 μM; ■) to CD13 inhibition by bestatin (100 μg/mL) or MY7 antibody (CD13 mAb, 1:100 dilution) or inhibition of bradykinin signaling by the B2R antagonist HOE140 (10 μM). (C) Binding of 3H-bradykinin to HUVECs at 0°C in the presence or absence of bestatin. (D) Effects of CD13 inhibition by bestatin (100 μg/mL) or MY7 antibody (CD13 mAb, 1:100 dilution) on 3H-bradykinin (50 nM) internalization at 37°C. Data are shown as mean (± SD, n = 3).
Following bradykinin binding to its receptor, the bradykinin-B2R complex is internalized, a step linked to signaling/desensitization of the B2 receptor.1,2 To determine if CD13 participates in B2R internalization, we cultured endothelial cells in the presence of bestatin or CD13-blocking antibodies and measured uptake of radiolabeled bradykinin-B2R complexes (Figure 2D). Internalization was significantly decreased under CD13-inhibitory conditions, indicating that CD13 participates in events subsequent to bradykinin binding to its receptor but prior to or coincident with receptor complex internalization. These results were reinforced by experiments using stable CD13-overexpressing cell lines that showed a significant increase in relative bradykinin internalization while binding was not significantly altered (Figure S1, available on the Blood website; see the Supplemental Figure link at the top of the online article), consistent with our findings using CD13 inhibitors.
CD13 is required for bradykinin-induced, Cdc42-dependent filopodia formation
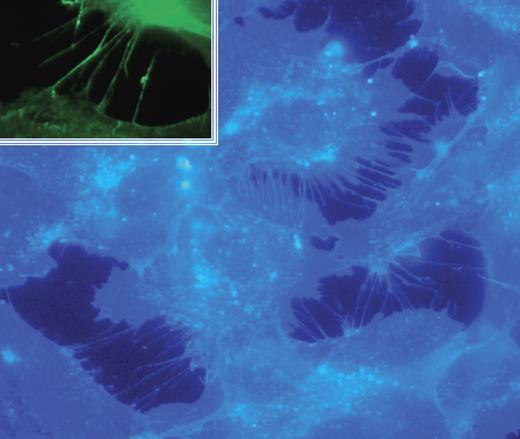
Bradykinin has been shown to contribute to fibroblast morphology and motility by inducing the rapid formation of filopodia that function as environmental sensors to guide cell movement.18,19 To investigate if filopodia formation is responsible for bradykinin's effects on endothelial cell invasion, HUVECs were incubated with bradykinin and stained with the fluorescent cholesterol-binding dye filipin (Figure 3). Although filopodia are often detected by staining actin filaments with phalloidin (Figure S2), we find that filipin staining significantly improves our ability to visualize these structures at high resolution. Our results confirm that similar to fibroblasts, in endothelial cells bradykinin induces the striking formation of actin-containing (Figure S3) structures that resemble filopodia and express the filopodia marker protein fascin20 (Figure 3; inset).
Bradykinin induces filopodia formation in endothelial cells. HUVECs were plated in serum-free medium containing growth factor supplement followed by the addition of bradykinin (15 minutes; 200 nM). Cells were fixed and stained with filipin; images were captured with a Zeiss Axioplan 2 (Thornwood, NJ) fluorescence microscope with 63×/1.4 NA oil objective, a Zeiss Axiocam HRC digital camera with plug-in v. 2.0 acquisition software, and processed with Photoshop 1 (Adobe, San Jose, CA). Inset shows immunofluorescence analysis of the filopodia marker fascin.
Bradykinin induces filopodia formation in endothelial cells. HUVECs were plated in serum-free medium containing growth factor supplement followed by the addition of bradykinin (15 minutes; 200 nM). Cells were fixed and stained with filipin; images were captured with a Zeiss Axioplan 2 (Thornwood, NJ) fluorescence microscope with 63×/1.4 NA oil objective, a Zeiss Axiocam HRC digital camera with plug-in v. 2.0 acquisition software, and processed with Photoshop 1 (Adobe, San Jose, CA). Inset shows immunofluorescence analysis of the filopodia marker fascin.
To confirm the role of CD13 in filopodia formation, we pretreated cells with bestatin and assessed filopodia formation in the presence of bradykinin (Figure 4A; Figure S4). The number, length, and width of filopodial structures in response to both stimuli are consistently suppressed by CD13 inhibition with no effect on total cholesterol levels (Figure S5), thus supporting a role for CD13 in this process. Activation of the small GTPase Cdc42 plays a crucial role in filopodia formation in many mammalian cell systems,21 and specifically in bradykinin-induced filopodia formation in fibroblasts.18 Consistent with these results, activity assays of bradykinin-treated HUVECs indicate that Cdc42 is specifically activated in response to bradykinin, and this activation is inhibited by bestatin treatment (Figure 4B). However, this inhibition is specific to the bradykinin-Cdc42-filopodia pathway since the activity of Rac1, a second member of the small GTPase family that regulates cellular motility via lamellipodia formation, is unaffected under identical conditions (Figure 4B). Similarly, filopodia formation induced by a distinct stimulus13 is also unaffected, again suggesting specificity for the Cdc42 pathway (Figure S6). Therefore, our data are consistent with CD13 participating in bradykinin-induced endothelial motility downstream of bradykinin-B2R interaction and upstream of Cdc42 activation.
Suppression of CD13 activity inhibits bradykinin-induced filopodia formation. (A) HUVECs were trypsinized and replated with bradykinin in the presence or absence of bestatin (100 μg/mL) or MY7 antibody (CD13 mAb, 1:100 dilution) and stained with filipin as in Figure 3. (B) Bestatin (100 μg/mL) inhibits bradykinin signal transduction, resulting in inhibition of Cdc42 but not Rac activation in HUVECs.
Suppression of CD13 activity inhibits bradykinin-induced filopodia formation. (A) HUVECs were trypsinized and replated with bradykinin in the presence or absence of bestatin (100 μg/mL) or MY7 antibody (CD13 mAb, 1:100 dilution) and stained with filipin as in Figure 3. (B) Bestatin (100 μg/mL) inhibits bradykinin signal transduction, resulting in inhibition of Cdc42 but not Rac activation in HUVECs.
CD13 is necessary for restoration of filopodia formation after depletion of membrane cholesterol
Bradykinin internalization occurs via a lipid raft/caveolae-dependent mechanism.22,,–25 Lipid rafts and caveolae are specialized membrane domains enriched in cholesterol, glycosphingolipids, and proteins that have been implicated in numerous signaling processes. Because CD13 inhibition prevents B2R internalization, and CD13 has been associated with lipid-rich microdomains/rafts in multiple cell types,26,,–29 CD13 may participate in membrane microdomain function. To investigate this possibility, we perturbed the plasma membrane and disrupted raft/caveolar integrity by sequestering cell membrane cholesterol with methyl-β-cyclodextrin (M-β-CD). M-β-CD treatment of endothelial cells abrogated endothelial invasion in a dose-dependent manner (Figure 5A). In agreement with published results,22,,–25 M-β-CD treatment also inhibited the internalization of the bradykinin-B2R receptor complex (Figure 5B), which was clearly dependent on membrane cholesterol since we were able to restore internalization of the complexes by cholesterol reloading subsequent to the M-β-CD treatment (Figure 5B). Importantly, treatment of cells with bestatin or the CD13-blocking antibody during cholesterol reloading prevented the restoration of both B2 receptor internalization (Figure 5B) and filopodia formation (Figure 5C). Similarly, we have found that bestatin treatment following membrane perturbation by trypsinization of HUVECs also attenuates invasion and filopodia formation (data not shown). Since depriving cells of their extracellular matrix attachments (as a result of trypsinization) causes the rapid internalization of lipid rafts/caveolae in fibroblasts,30,31 our findings are consistent with a specific role for CD13 in the proper assembly or reorganization of cholesterol-dependent membrane structures. Taken together, our results support a functional role for both CD13- and cholesterol-rich microdomains in bradykinin induction of filopodia formation and endothelial invasion.
Cholesterol depletion suppresses bradykinin-induced filopodia formation. (A) HUVEC invasion in the presence of increasing concentrations of the cholesterol depleting agent M-β-CD (0.15%-2%, 0.5 hours). (B) Effects on internalization of 3H-bradykinin in HUVECs depleted of cholesterol with M-β-CD (MβCD; 2%, 0.5 hours) or depleted with M-β-CD then replenished with cholesterol (CH; 20 μg/mL, 3 hours) in the presence of the CD13 antagonists bestatin (100 μg/mL) or MY7 antibody (CD13 mAb; 1:100 dilution). (C) Effects on bradykinin-induced filopodia formation in HUVECs depleted of cholesterol with M-β-CD or depleted with M-β-CD and replenished with cholesterol in the presence of bestatin (100 μg/mL) or MY7 antibody (CD13 mAb; 1:100 dilution) Data are shown as means (± SD, n = 3).
Cholesterol depletion suppresses bradykinin-induced filopodia formation. (A) HUVEC invasion in the presence of increasing concentrations of the cholesterol depleting agent M-β-CD (0.15%-2%, 0.5 hours). (B) Effects on internalization of 3H-bradykinin in HUVECs depleted of cholesterol with M-β-CD (MβCD; 2%, 0.5 hours) or depleted with M-β-CD then replenished with cholesterol (CH; 20 μg/mL, 3 hours) in the presence of the CD13 antagonists bestatin (100 μg/mL) or MY7 antibody (CD13 mAb; 1:100 dilution). (C) Effects on bradykinin-induced filopodia formation in HUVECs depleted of cholesterol with M-β-CD or depleted with M-β-CD and replenished with cholesterol in the presence of bestatin (100 μg/mL) or MY7 antibody (CD13 mAb; 1:100 dilution) Data are shown as means (± SD, n = 3).
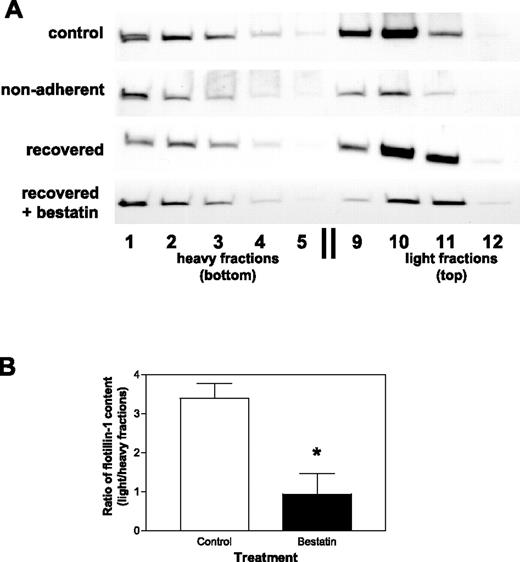
CD13 is necessary for restoration of membrane organization after membrane cholesterol depletion
To gain further insight into the possible role of CD13 in membrane assembly, we physically separated membrane proteins and tracked the distribution of the light fraction-resident protein flotillin-132 as a measure of membrane integrity under conditions shown to disrupt filopodia formation. We hypothesized that if CD13 regulates membrane organization, CD13 antagonists may affect the targeting or distribution of proteins in various membrane fractions. Others have recently shown that disengaging fibroblasts from their extracellular matrix contacts leads to lipid raft internalization, while allowing them to re-adhere after trypsinization (“recover”) restores intact membrane organization.30,31 In agreement with these studies, in controls, cultured HUVECs flotillin-1 is predominantly localized in the light buoyant membrane fractions (Figure 6A; fractions 9-11; “control”). In contrast, loading of equivalent amounts of protein from sonicated, detergent-free lysates prepared from trypsinized endothelial cells plated under conditions that hamper the re-establishment of extracellular contacts shows that flotillin-1 redistributes from the light to the heavy membrane fractions (Figure 6A; fractions 1-5; “nonadherent”), consistent with disrupted membrane organization. When cells are allowed to re-adhere and “recover,” there is a significant shift in flotillin-1 to light fractions (Figure 6A; fractions 9-11, “recovered”) with a distribution similar to control cells, indicating that proper organization has been re-established. However, flotillin-1 remains primarily in the heavy fractions in trypsinized cells that “recover” in the presence of the CD13 antagonist (Figure 6A; “recovered + bestatin”), suggesting that CD13 is necessary for proper membrane protein organization. Relative quantification of flotillin-1 protein showed that flotillin-1 is predominantly found in the light fractions in control preparations (relative flotillin-1 levels in light/heavy fractions = 3.39 ± 0.67; Figure 6B), while a significant and reproducible decrease is seen in light fraction-localized flotillin-1 in cells recovered in the presence of bestatin (0.94 ± 0.92; P = .02). Redistribution of additional relevant light fraction resident proteins is observed in the presence of bestatin following membrane disruption by either trypsinization (B2R; Figure S7A) or cholesterol depletion (caveolin-1; Figure S7B). Therefore, since intact membrane organization is required for optimal bradykinin B2 receptor signal transduction, the participation of CD13 in membrane protein organization may influence bradykinin-induced endothelial invasion and angiogenesis.
CD13 is necessary for the efficient restoration of membrane organization. (A) HUVECs were analyzed for distribution of the marker protein flotillin-1 under conditions that inhibit filopodia formation. Control HUVECs (control) indicate normal flotillin-1 distribution. Sonicated cell lysates containing equivalent total protein levels from trypsinized HUVECs were plated in noncoated plastic dishes and rocked in serum-containing medium (nonadherent), plated in serum-containing medium in tissue culture–coated dishes in the absence (recovered), or the presence of bestatin (recovered + bestatin; 100 μg/mL) were analyzed by sucrose gradient separation and fractions assayed for flotillin-1 by Western blot analysis. Heavy fractions (fractions 1-5) represent higher-density protein complexes, whereas light fractions (fractions 9-12) contain proteins complexed with lipids, such as lipid rafts/caveolae. Results are representative of 3 separate experiments. (B) The relative distribution of flotillin-1 in light versus heavy sucrose gradient fractions in cells “recovered” in the presence of bestatin versus control vehicle was calculated using densitometric quantification of Western blots. The overall distribution of flotillin-1 in the gradient fractions was significantly different between the 2 conditions (P < .02). Data are shown as means (± SD, n = 3).
CD13 is necessary for the efficient restoration of membrane organization. (A) HUVECs were analyzed for distribution of the marker protein flotillin-1 under conditions that inhibit filopodia formation. Control HUVECs (control) indicate normal flotillin-1 distribution. Sonicated cell lysates containing equivalent total protein levels from trypsinized HUVECs were plated in noncoated plastic dishes and rocked in serum-containing medium (nonadherent), plated in serum-containing medium in tissue culture–coated dishes in the absence (recovered), or the presence of bestatin (recovered + bestatin; 100 μg/mL) were analyzed by sucrose gradient separation and fractions assayed for flotillin-1 by Western blot analysis. Heavy fractions (fractions 1-5) represent higher-density protein complexes, whereas light fractions (fractions 9-12) contain proteins complexed with lipids, such as lipid rafts/caveolae. Results are representative of 3 separate experiments. (B) The relative distribution of flotillin-1 in light versus heavy sucrose gradient fractions in cells “recovered” in the presence of bestatin versus control vehicle was calculated using densitometric quantification of Western blots. The overall distribution of flotillin-1 in the gradient fractions was significantly different between the 2 conditions (P < .02). Data are shown as means (± SD, n = 3).
CD13 enzymatic activity is required for proper membrane protein distribution
Our data indicate that CD13 participates in the recovery of membrane organization after disruption. If this were the case, manipulation of either CD13 levels or enzymatic activity might be expected to alter the protein distribution on the membrane. To answer this question, we constructed 2 mutant expression clones containing alterations in the invariant histidine residues of the critical CD13 zinc coordination site that are predicted to affect enzyme activity (…. 387AHELAHQ393…;9 clones H388A and H392A). Wild-type and mutant CD13 expression plasmids were stably transfected into a murine hemangioendothelioma-derived endothelial cell line (EOMA). We have previously shown that EOMA cells express very low levels of endogenous CD13.11 Stably transfected cells were serially sorted for high CD13 expression levels to obtain final pools expressing comparable levels of transfected CD13 (approximately 10-fold increase over vector control; data not shown). Determination of the effects of the mutations on CD13 enzymatic activity showed that the H388A substitution completely abrogated CD13 activity, while clone H392A retained approximately 50% of wild-type activity (Figure 7B). Using flotillin-1 as a marker of membrane protein distribution, we found that although total flotillin-1 protein levels were identical in control cells and the 3 CD13-overexpressing pools (Figure 7E), the distribution of flotillin-1 appears to be altered in the presence of high levels of functional CD13 (Figure 7C). When results are expressed as a relative ratio of levels of flotillin-1 protein found in the light versus heavy fractions, a 3-fold increase is seen in light fraction-localized flotillin-1 in the presence of wild-type CD13 (Figure 7D), indicating a role for CD13 in the assembly, trafficking, or control of membrane organization. Interestingly, the changes seen in the distribution of flotillin-1 in the CD13-overexpressing clones parallels their CD13 activity, suggesting that enzymatically active CD13 is required for these effects.
Enzymatic activity of CD13 is needed for optimal organization of membrane proteins. (A) CD13 enzyme activity was determined in vector-transfected EOMA cells (control) and in cells transfected with expression plasmids containing wild-type human CD13 (CD13 [WT]), CD13 point mutants His388Ala (H388A) or His392Ala (H392A). (B) Total levels of flotillin-1 protein are equivalent in cell lysates from all 4 EOMA lines by Western blot analysis. (C) Triton-solubilized cell lysates from control, wild-type, or mutant-transfected cells were analyzed by sucrose gradient separation and fractions assayed for flotillin-1 by Western blot analysis. High-density fractions (fractions 1-5) represent soluble proteins and light fractions (fractions 9-12) contain proteins complexed with lipids. (D) Ratios of flotillin-1 detected in light and heavy sucrose gradient fractions were calculated using densitometric quantification of Western blots of the 4 EOMA lines. Data are shown as means (± SD, n = 3).
Enzymatic activity of CD13 is needed for optimal organization of membrane proteins. (A) CD13 enzyme activity was determined in vector-transfected EOMA cells (control) and in cells transfected with expression plasmids containing wild-type human CD13 (CD13 [WT]), CD13 point mutants His388Ala (H388A) or His392Ala (H392A). (B) Total levels of flotillin-1 protein are equivalent in cell lysates from all 4 EOMA lines by Western blot analysis. (C) Triton-solubilized cell lysates from control, wild-type, or mutant-transfected cells were analyzed by sucrose gradient separation and fractions assayed for flotillin-1 by Western blot analysis. High-density fractions (fractions 1-5) represent soluble proteins and light fractions (fractions 9-12) contain proteins complexed with lipids. (D) Ratios of flotillin-1 detected in light and heavy sucrose gradient fractions were calculated using densitometric quantification of Western blots of the 4 EOMA lines. Data are shown as means (± SD, n = 3).
Discussion
We have previously reported that the CD13 cell-surface peptidase is required for endothelial cell invasion.11 To more precisely define the stimulus driving this CD13-dependent process, we fractionated serum proteins and found that HK potently induced invasion. Bradykinin is the primary cleavage product of HK and the major effector of the kallikrein-kinin system; we found that this nonomeric peptide is also responsible for HK-induced invasion. CD13 antagonists (either inhibitors or specific antibodies to CD13) prevented internalization of the bradykinin-B2R ligand receptor complex, but had no effect on bradykinin binding. These results indicate that CD13 functions at the plasma membrane level, which is consistent with its cell-surface localization. Bradykinin regulates cell motility by activating the small GTPase Cdc42, with the resultant formation of filopodial structures. CD13 antagonists eliminated the bradykinin-dependent activation of Cdc42 and filopodia formation, supporting a role for CD13 at the onset of bradykinin signaling that is required for subsequent bradykinin-dependent processes. Because productive bradykinin signaling requires intact cholesterol-rich membrane domains, we investigated the effects of CD13 antagonists on membrane cholesterol-containing microdomains. We found that inhibition of CD13 activity during the cell recovery phase following cholesterol depletion or trypsinization prevents filopodia formation and disrupts the distribution of membrane proteins. Overexpression of functional, but not enzymatically inactive CD13 alters the cellular distribution of cell surface proteins, consistent with a role in the formation or organization of membrane proteins crucial for transduction of signals from numerous receptor systems.33,,–36
Bradykinin is a well-characterized inflammatory mediator and initiator of vascular homeostatic processes as well as peripheral pain signals. It is produced by endothelial cells at the site of tissue injury, where it increases tissue permeability by signaling primarily through its B2-type receptor.1,2 Recently, bradykinin and its kininogen precursor have also been shown to be proangiogenic, since kininogen-deficient rats show impaired angiogenic responses,3 and antibodies that prevent the binding of HK to endothelial cells are potent inhibitors of neovascularization.4,5 A more direct role for bradykinin in angiogenesis has also been demonstrated in studies showing impaired tumor angiogenesis in B2R-deficient murine models.6 Consistent with these observations, the proangiogenic effects of ACE inhibitors have also been shown to be B2R mediated, presumably due to the attenuation of bradykinin breakdown by ACE, bradykinin accumulation, and augmentation of bradykinin-dependent angiogenesis.7
The molecular mechanisms implicated in mediating bradykinin regulation of angiogenesis often include the induction of various proangiogenic genes such as angiogenic growth factors,8,37 suggesting a more indirect role. In contrast, our finding that bradykinin is a potent mediator of invasion is supported by data linking bradykinin more directly to tumor cell invasion,38 as well as showing that bradykinin has a role in Cdc42-dependent filopodia formation.18 Filopodia are slender cell extensions consisting of membrane-covered bundles of actin filaments that have important roles in cell-cell communication during development.19 Similar cellular extensions are often found on moving cells where they participate in guided migration and cell movement (reviewed in Jacinto and Wolpert19 and Rorth39 ). We find that bradykinin plays a direct role in endothelial invasion by initiating a program of cytoskeletal rearrangement resulting in filopodia formation. We have shown that CD13 inhibition blocks bradykinin signal transduction by influencing membrane protein distribution with subsequent inhibition of Cdc42 activation and filopodia formation. These results provide a plausible molecular mechanism for the participation of CD13 in endothelial invasion.
Our present study indicates that CD13 functions in the initial steps of bradykinin signal transduction, after bradykinin binding but prior to ligand-receptor internalization. The molecular steps which follow binding of bradykinin to B2R involve well-characterized signaling mechanisms described for the 7 transmembrane G-protein–coupled receptor family.1,2 Bradykinin stimulation of the B2R has been frequently thought to involve the activation-dependent redistribution of the ligand-receptor complex on the cell surface, although its final location remains controversial and may include either translocation out of25 or into caveolae22,24 or into lipid-rich microdomains/lipid rafts.24 B2R activation in response to ligand binding also triggers its internalization via caveolae, an event that may ultimately affect B2R signaling and/or desensitization.1,2 CD13 could act at any of several steps affecting bradykinin signal transduction and internalization, such as membrane domain organization, receptor localization, or caveolar endocytosis. While the concept of membrane lipid microdomains remains controversial,40,–42 it is well accepted that the distribution of most plasma membrane proteins is heterogeneous and that their organization is regulated by other interacting proteins.16,42 Our data suggest that once the membrane is perturbed by cholesterol depletion or trypsinization, the recovery of a proper distribution of membrane proteins among fractions of different buoyant density requires CD13 activity. Thus, the requirement for CD13 in this experimental model of acute membrane disruption may reflect its role in the more dynamic process of membrane maintenance. Interestingly, CD13 has been shown to be physically associated with cholesterol in human bile and involved in the pathogenesis of cholesterol gallstone disease.43,–45 More recently, CD13 has been proposed to be a target of the cholesterol absorption inhibitor ezetimibe in the intestine,46 raising the possibility that CD13 participates in membrane assembly by regulating membrane cholesterol. Distinguishing among these possibilities and elucidating their precise regulatory mechanisms is the subject of active investigation in our laboratory.
Consistent with our current localization of CD13 in lipid-rich membrane fractions of endothelial cells, CD13 has been associated with similar lipid-rich microdomains/rafts of synoviocytes,26 monocytes,27 fibroblasts,28 and enterocytes.29 One well-characterized function of CD13 in these microdomains is as a receptor for certain subtypes of coronaviruses.47,48 Virus binding to CD13 triggers its internalization via a receptor-dependent mechanism that involves CD13 redistribution to caveolin-positive membrane clusters and endocytosis via caveolae.28,49 Depletion of cholesterol inhibits both receptor redistribution and internalization but not coronavirus binding, suggesting that this process requires the proper organization of lipid-rich domains in the membrane as well.28 Taken together, these observations support a role for CD13 in membrane integrity and internalization, where it may act to facilitate the partitioning of integral membrane components, a function that has been exploited by the coronavirus to gain entry into the cell.
CD13's cell-surface expression dictates that its function in certain tissues is dependent on the peptide substrates available in the extracellular space. Thus, in the central nervous system it processes neuroactive peptides, while in the intestine CD13 participates in the scavenging of amino acids from peptides during digestion (reviewed in Riemann et al50 ). In each of these roles, CD13 enzymatic activity is central to its function. However, CD13-dependent coronavirus infection proceeds normally in the presence of CD13 inhibitors or mutant receptors analogous to those used in our study, strongly suggesting that CD13 enzymatic activity is not required for viral infection.47,48,51,52 In contrast, we see a clear effect of both peptide inhibitors and monoclonal antibodies on filopodia formation and endothelial invasion, and the observed alterations in membrane protein distribution correlate with enzymatic activity. However, the binding of certain antibodies to CD13 has been shown to induce conformational changes that expose cryptic epitopes on the molecule.53 It is possible that inhibitor binding and amino acid substitution provokes similar structural changes that affect other CD13 functions in addition to its enzymatic activity. Therefore, while our data strongly suggest that CD13 regulates membrane integrity, further studies are necessary to definitively determine the role of CD13 enzymatic activity and the mechanisms of CD13 function in these processes.
Finally, the question of whether CD13 effects are specific for endothelial bradykinin signal transduction or has broader implications for other G-protein–coupled receptors, other cell types, or raft/caveola-mediated signal transduction in general, remains to be explored. In this regard, endothelial invasion in response to the chemokine SDF-1 and the bioactive lipid mediator sphingosine-1-phosphate (S1P) are also exquisitely sensitive to CD13 antagonists (N.P. and L.H.S., unpublished observations, May, 2005). Since both of these mediators transduce signals via cell surface G-protein–coupled receptors,54,–56 this observation would support a more pervasive role for CD13 in endothelial G-protein–coupled receptor (GPCR)–mediated signal transduction. In addition, a more general role for CD13 in membrane organization would be consistent with reports of the inhibitory affects of CD13 antagonists on signal transduction pathways downstream of T-cell activation,57 a process in which lipid raft involvement has also been implicated.35 Further exploration of the role of CD13 in endothelial cell signal transduction will allow us to determine precisely how CD13 functions to create or maintain the endogenous cell membrane microenvironment, and thus regulates cell functions critical to normal and pathologic processes including cell migration, invasion, and metastasis.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We would like to thank Kevin Claffey, Stephan Pfeiffer, Xiufang Liu, Kathleen Mahoney, and Melissa Bryant for technical assistance and for helpful discussions; Dr Carol Pilbeam for use of photomicrographic equipment; and Dr David Shapiro for critical reading of the manuscript.
This work was supported by National Institutes of Health grants R01 CA 85714 and 1 R01 CA 106345, and BCTR0402745 from the Susan G. Komen Foundation to L.H.S.
National Institutes of Health
Authorship
Contribution: N.P., W.S., and L.H.S. designed research; N.P., W.S., J.R.G., C.A.O., R.E.C., B.W., P.M.-O., and L.H.S. performed research; N.P., W.S., P.M.-O., R.E.C., C.A.O., B.W., and L.H.S. analyzed data; and N.P. and L.H.S. wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Linda H. Shapiro, Center for Vascular Biology MC3501, Department of Cell Biology, University of Connecticut Health Center for Vascular Biology, 263 Farmington Ave, Farmington, CT 06030-3501; e-mail: lshapiro@neuron.uchc.edu.






![Figure 7. Enzymatic activity of CD13 is needed for optimal organization of membrane proteins. (A) CD13 enzyme activity was determined in vector-transfected EOMA cells (control) and in cells transfected with expression plasmids containing wild-type human CD13 (CD13 [WT]), CD13 point mutants His388Ala (H388A) or His392Ala (H392A). (B) Total levels of flotillin-1 protein are equivalent in cell lysates from all 4 EOMA lines by Western blot analysis. (C) Triton-solubilized cell lysates from control, wild-type, or mutant-transfected cells were analyzed by sucrose gradient separation and fractions assayed for flotillin-1 by Western blot analysis. High-density fractions (fractions 1-5) represent soluble proteins and light fractions (fractions 9-12) contain proteins complexed with lipids. (D) Ratios of flotillin-1 detected in light and heavy sucrose gradient fractions were calculated using densitometric quantification of Western blots of the 4 EOMA lines. Data are shown as means (± SD, n = 3).](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/110/1/10.1182_blood-2006-02-002931/2/m_zh80130703420007.jpeg?Expires=1766072409&Signature=BGd3mGzpymFq999FwqxNx1R~tlXtrEhf47W59Nr4Si8xB6J7iWSMqMIl7VFTCIDdxwMJbHQ3mbR9VLzsEuZ0s4wVyb8xImrcYmJ6H6Y2b88SHZqztlPZuT~lIsjyRdDcwaYxlIWVYaduq528ohtWI9UhGrJlam~8etIbGEoUxthBsbQDg97GtMXCXmLcn8igp36EHHOAgw3CUUsMNUx6vY9H59TF-wZkVJiuXQcfjTPS7MloDLt3JBpocSFg3BMg394U1jHer9jS2wkOJtO9Gim9CdOXeFhzGGk95dduRTmdjC8QVLbGUwERwwyrRIRn0CAIFotPYyndJK8bV8m8Gg__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)





![Figure 7. Enzymatic activity of CD13 is needed for optimal organization of membrane proteins. (A) CD13 enzyme activity was determined in vector-transfected EOMA cells (control) and in cells transfected with expression plasmids containing wild-type human CD13 (CD13 [WT]), CD13 point mutants His388Ala (H388A) or His392Ala (H392A). (B) Total levels of flotillin-1 protein are equivalent in cell lysates from all 4 EOMA lines by Western blot analysis. (C) Triton-solubilized cell lysates from control, wild-type, or mutant-transfected cells were analyzed by sucrose gradient separation and fractions assayed for flotillin-1 by Western blot analysis. High-density fractions (fractions 1-5) represent soluble proteins and light fractions (fractions 9-12) contain proteins complexed with lipids. (D) Ratios of flotillin-1 detected in light and heavy sucrose gradient fractions were calculated using densitometric quantification of Western blots of the 4 EOMA lines. Data are shown as means (± SD, n = 3).](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/110/1/10.1182_blood-2006-02-002931/2/m_zh80130703420007.jpeg?Expires=1766352000&Signature=dl0TIb4kfZtDbH7eksH~zxMUWBBaMHFhgHzgXRkBPv~z0yU9xUDE2RJ7RSGqBYvjZFFXOYwVnaO1w1ww-NE-2LLV3ylP5txJ2Hzh~aKk8fXDSI4bNdWbqi5HXdOPOAFjfJ8ocZMD6FXevvwYwgMe6ddTPA5-wGiaL2AwdrUBvvoDz1P9FuU1ZFLXwZhkQaJ0fAqNEeIzU4Tbnh-r29F5QZ~iLs-WxukWRNfLydtfDJIvmSf9SiBw2ncUQdE8PB-H3hOfPn07nxu1Do1SuAMtcBL5D2WW-CpjSd5Ifuwyt04aQEMzlCHdixecGlRWIKeeHbYjGtHp3vrAU2Bczq9DLQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)