Important predictors of adverse outcomes of thrombosis in children, including postthrombotic syndrome (PTS), have recently been identified. Given this knowledge and the encouraging preliminary pediatric experience with systemic thrombolysis, we sought to retrospectively analyze our institutional experience with a thrombolytic regimen versus standard anticoagulation for acute, occlusive deep venous thrombosis (DVT) of the proximal lower extremities in children in whom plasma factor VIII activity and/or D-dimer concentration were elevated at diagnosis, from within a longitudinal pediatric cohort. Nine children who underwent the thrombolytic regimen and 13 who received standard anticoagulation alone were followed from time of diagnosis with serial clinical evaluation and standardized PTS outcome assessments conducted in uniform fashion. The thrombolytic regimen was associated with a markedly decreased odds of PTS at 18 to 24 months compared with standard anticoagulation alone, which persisted after adjustment for significant covariates of age and lag time to therapy (odds ratio [OR] = 0.018, 95% confidence interval [CI] = < 0.001-0.483; P = .02). Major bleeding developed in 1 child, clinically judged as not directly related to thrombolysis for DVT. These findings suggest that the use of a thrombolysis regimen may safely and substantially reduce the risk of PTS in children with occlusive lower-extremity acute DVT, providing the basis for a future clinical trial.
Introduction
Over the past few decades, deep venous thrombosis (DVT) has emerged as a critical issue in pediatrics.1,–3 While the incidence of DVT is substantially lower among children than adults, with a recent rate of 5 cases per 10 000 children per year from the National Hospital Discharge Survey, it is clear that many cases of DVT and pulmonary embolism (PE) go unrecognized in part due to a low index of suspicion for young patients; consequently, these conditions are probably underdiagnosed in children.4,5 At the same time, the consequences of DVT and PE in children are significant. Sixteen percent to 20% of children with venous thromboembolism (VTE) have objectively confirmed PE,2,5,6 and the mortality rate for major vessel thrombosis in children is estimated at 1% to 4%.2,3,6,7 Thrombus recurrence has been reported in 6.5% to 21% of children with VTE.2,3,6,–8 Finally, postthrombotic syndrome (PTS), a syndrome of chronic venous insufficiency often associated with limitation in physical activities,9,10 appears to develop following DVT of the limbs at least as frequently in children as in adults, with reports of up to 70% of children affected.2,3,6,7,11,,,–15 Comprehensive pediatric thrombosis centers have just recently been formed to address these and other key concerns of thrombosis in children.6,–8,16
Given prior evidence of (1) a positive relationship between thrombus persistence and the development of PTS following DVT in adults,17,18 (2) the identification of elevations in D-dimer and factor VIII (FVIII) activity as prognostic indicators for thrombus persistence and recurrence in adults19,20 and as predictors of composite adverse thrombotic outcomes in children (persistent thrombosis, recurrent VTE, and PTS),13 (3) preliminary findings of safety and efficacy of thrombolysis with or without mechanical thrombectomy (particularly with regard to the reduction in PTS risk) in the acute treatment of symptomatic lower-extremity DVT in adults,21,–23 and (4) pilot data on systemic thrombolytic dosing, bleeding toxicity, clot lysis, and outcome in children with thrombosis,24,25 we sought to retrospectively analyze our single-institution experience with thrombolysis followed by standard anticoagulation vs standard anticoagulation alone for high-risk, occlusive, first-episode, acute DVT of the lower extremities from within the context of a longitudinal pediatric cohort. We hypothesized that (1) therapy consisting of thrombolysis followed by standard anticoagulation is associated with decreased odds for the development of PTS in children with high-risk occlusive DVT of the lower extremities compared with anticoagulation alone and that (2) age at DVT diagnosis and lag time from symptom onset to institution of antithrombotic therapy are important additional clinical factors modulating PTS risk in such children.
Patients, materials, and methods
Study design
In 2001, a comprehensive pediatric program for thrombosis and/or thrombophilia was established at the Mountain States Regional Hemophilia and Thrombosis Center and The Children's Hospital, Denver, with funding from the Centers for Disease Control and Prevention (CDC). Children in the present analysis were referred to the thrombosis program at the time of presentation with an acute DVT of the proximal lower extremity. All children were followed forward in time from initial diagnosis until at least 18 months after diagnosis, with thrombophilia testing, anticoagulation and thrombolysis regimens, therapeutic monitoring, serial clinical evaluation and imaging studies, and standardized PTS outcome assessments conducted in uniform fashion according to clinical protocols consistent with American College of Chest Physicians (ACCP) recommendations26 and best clinical care in pediatric thrombosis. A single-institution pediatric thrombosis cohort has been established and is retrospectively analyzed in the present study with approval by the Colorado Multiple Institutional Review Board (COMIRB no. 06–0194). Informed consent was provided, as required, in accordance with the Declaration of Helsinki. A study for the validation of the standardized PTS outcome instrument was also approved by the Colorado Multiple Institutional Review Board (COMIRB no. 02–904).
Study group eligibility and baseline laboratory testing
Inclusion criteria for the present study consisted of the following: (1) radiologically confirmed, completely venoocclusive, first-episode, acute DVT of the proximal lower extremity with or without extension into the inferior vena cava; (2) measured plasma FVIII activity 150 U/dL or greater and/or D-dimer level 500 ng/mL or greater; (3) age at DVT diagnosis ranging from 6 months to 21 years, inclusive; (4) complete data available for antithrombotic treatment and anticoagulant monitoring during the antithrombotic therapy period, serial follow-up imaging, and standardized PTS outcome assessment at 18 to 24 months after diagnosis of DVT; and (5) antithrombotic therapy consisting of either a standard anticoagulation regimen alone or an initial thrombolytic regimen followed by standard anticoagulation, as determined clinically by the treating physician.
All children underwent baseline coagulation screening prior to antithrombotic therapy, with prothrombin time (PT), activated partial thromboplastin time (aPTT), fibrinogen concentration, D-dimer level, and complete blood count with platelet count. In addition, comprehensive thrombophilia testing was performed at diagnosis of DVT (see “Laboratory methods”), as recommended by the Scientific and Standardization Committee, Subcommittee on Perinatal and Pediatric Hemostasis, of the International Society on Thrombosis and Hemostasis.27
Treatment
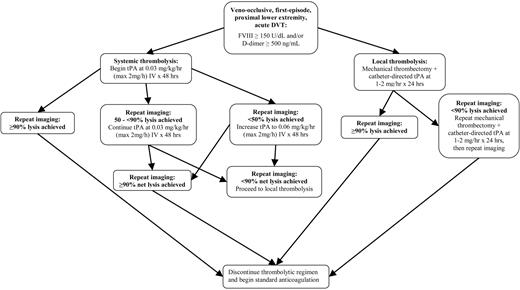
Thrombolytic therapy consisted of 1 of 2 regimens (Figure 1): (1) initial systemic intravenous administration of low-dose tissue-type plasminogen activator (tPA) with salvage mechanical thrombolysis and local (catheter-directed) tPA via an interventional radiologic approach for residual thrombus; or (2) primary local (catheter-directed) administration of tPA with mechanical thrombectomy via an interventional radiologic approach. These thrombolytic regimens have recently been described16 and are summarized in Figure 1, with the goal of rapid restoration of venous patency. In the case of systemic tPA, infusion was begun at 0.03 mg/kg/h with concomitant intravenous administration of unfractionated heparin at 10 U/kg/h, with the option to increase the tPA dose to 0.06 mg/kg/h at 24 to 48 hours for radiologic evidence of persistent thrombus, up to a maximum tPA rate of 2 mg/h. Systemic tPA infusion was discontinued upon radiologic documentation of complete thrombus resolution and was continued for a minimum of 48 hours in the absence of complete thrombus resolution prior to this time. Maximum duration of systemic tPA infusion was 96 hours.
Summary schema of thrombolytic regimen. DVT indicates deep venous thrombosis; FVIII, factor VIII activity; and tPA, tissue-type plasminogen activator.
Summary schema of thrombolytic regimen. DVT indicates deep venous thrombosis; FVIII, factor VIII activity; and tPA, tissue-type plasminogen activator.
If upon cessation of systemic tPA at 48 to 96 hours there was radiologic evidence of persistent thrombus, salvage therapy was considered with pharmacomechanical thrombolysis via an interventional radiologic approach using the Clot Buster Amplatz Thrombectomy Device (Microvena, White Bear Lake, MN), AngioJet (Possis Medical, Minneapolis, MN), or equivalent device, with catheter-directed local administration of tPA at 1 to 2 mg/h for 24 to 48 hours. In the case of primary local (catheter-directed) tPA regimen, local tPA and mechanical thrombectomy were performed identically to their use in the aforementioned salvage protocol for systemic tPA.
In all children, contraindications to the use of anticoagulation or thrombolysis were carefully assessed and consistently enforced, as previously described.16 PT, aPTT, fibrinogen concentration, D-dimer level, hematocrit, platelet count, and plasminogen concentration were measured daily during systemic tPA infusion. Fresh frozen plasma, cryoprecipitate, and/or platelet concentrates were administered as needed to maintain PT 3 seconds or less above the upper limit of normal, fibrinogen concentration 1 g/L (100 mg/dL) or greater, platelet count 50 × 109/L or greater, and plasminogen activity 50% or greater.
Initial anticoagulation for children on standard therapy or following thrombolysis consisted of unfractionated heparin adjusted to achieve a plasma anti-Xa activity of 0.3 to 0.7 U/mL or enoxaparin adjusted to 0.5 to 1.2 anti-Xa activity U/mL for at least 7 days. Long-term anticoagulation was maintained with enoxaparin or warfarin, with goal anti-Xa activity of 0.5 to 1.2 U/mL or international normalized ratio (INR) of 2.0 to 3.0 (2.5-3.5 if antiphospholipid antibody positive), respectively, for a minimum total anticoagulation therapy duration of 3 months, according to ACCP guidelines.26 Indications for further extension of anticoagulant therapy included antiphospholipid antibody syndrome, severe congenital thrombophilia, or multiple thrombophilia traits. Extension of anticoagulation was also considered as an option in cases of persistent occlusive thrombosis at 3 months.
Laboratory methods
Plasma fibrinogen concentration was measured by the clotting method of Clauss, D-dimer by latex agglutination assay, FVIII activity by 1-stage aPTT-based clotting assay, antithrombin and protein C activities by chromogenic assay, free protein S antigen and lipoprotein(a) concentrations by enzyme-linked immunosorbent assay (ELISA), homocysteine concentration by gas chromatography and mass spectroscopy, and lupus anticoagulant by dilute Russell viper venom time. IgG and IgM antibodies to cardiolipin and beta-2-glycoprotein-1 were determined in serum by ELISA. Factor V Leiden and prothrombin 20210 mutations were identified by polymerase chain reaction (PCR) technique in DNA isolated from buffy-coat leukocytes. All positive antiphospholipid antibody results at diagnosis of DVT were repeated in follow-up at 1 and 3 to 6 months.
Radiologic imaging
Both at diagnosis and serial follow-up, compression ultrasound with Doppler was the primary modality used to image the extent and occlusiveness of lower-extremity DVT, with additional computerized tomography used to define the extent and occlusiveness of iliac venous and/or inferior vena caval involvement not visualized by ultrasound. Serial follow-up imaging to determine thrombus response to therapy was performed at 1, 3, 6, and 12 months.
Standardized PTS outcome assessment
Children were evaluated for PTS at 1, 3, 6, and 12 months and then yearly using a standardized PTS outcome instrument.28 This scoring system encompasses assessment of both physical-examination findings and functional limitations (ie, chronic lower-extremity pain that limits age-appropriate activity) and was derived from the validated international adult PTS scale of the American Venous Forum's Ad Hoc Committee on Venous Outcomes,10 with minor adaptation for pediatric application. Specifically, the modification consisted of the use of the Wong-Baker Faces Pain Scale previously validated for pain assessment in children.29 Data for the validation of this instrument for lower-extremity DVT in children are given in “Results.”
Physical outcome was scored as zero for no physical findings of chronic venous insufficiency. Individual findings of edema (> 1-cm increase in midcalf or midthigh circumference in the affected extremity compared with the contralateral extremity), dilated superficial collateral veins, venous stasis dermatitis, and venous stasis ulcers were each assessed 1 point, for a maximum physical-exam score of 4.
Functional limitation due to chronic lower-extremity pain was scored for each of 3 levels of activity: none (ie, rest); activities of daily living (eg, walking at school or home, climbing steps); and aerobic activity (eg, running on the playground, participation in organized sports), with 1 point given for pain limitation to each activity or at rest. PTS was defined as a composite (physical exam plus functional assessment) score of 1 or more, and abnormalities in the 2 components of the instrument were also separately reported and analyzed.
Assessment of bleeding complications
Bleeding was monitored clinically in an inpatient setting during the acute antithrombotic period and both by history and clinical exam upon serial follow-up as an outpatient throughout the extended anticoagulation therapy period (at 1 and 3 months in all patients, and additionally at 6 and 12 months and annually thereafter in patients who met clinical criteria for long-term anticoagulation). Medical records were requested and reviewed for any bleeding events for which treatment was sought at outside facilities. Bleeding episodes were defined as “major” if any of the following 4 circumstances existed, as previously established30 : (1) central nervous system (CNS) hemorrhage; (2) retroperitoneal hemorrhage; (3) hemorrhage associated with a decline in hemoglobin level of 20 g/L (2 g/dL) or greater in a 24-hour period; or (4) hemorrhage prompting surgical intervention to achieve hemostasis. Minor bleeding episodes, including bruising or oozing at venipuncture sites, were not systematically recorded and analyzed.
Statistical methods
In the validation of the PTS instrument for lower-extremity DVT, descriptive statistics were used to establish distributions and corresponding parameters of normal contralateral differences in lower-extremity circumference measurements in healthy children. The upper limit of normal values for these differences was calculated using the following expression: median + (1.5 × interquartile range), given nonparametric distribution of these data. Interrater reliability was measured by percent agreement, calculated as the number of agreeing observations divided by the total number of observations, and multiplied by 100.
All statistical analyses in the study cohort used SAS 9.1 statistical software (SAS Institute, Cary, NC). Descriptive statistics were used for explanatory variables (consisting of known and hypothesized risk factors for and prognostic indicators in DVT and PTS) to define distributions and corresponding parameters of continuous variables and frequencies and proportions of categoric variables by treatment regimen. These distributions and proportions were compared between treatment regimens (thrombolytic therapy followed by standard anticoagulation versus standard anticoagulation alone) via Mann-Whitney and chi-square testing, respectively. Fisher exact test was used in the instance of any 2 × 2 table with frequency values of 5 or less in any one cell.
Univariate and multivariate analyses to determine potential associations between explanatory variables and PTS outcome used logistic regression, with treatment regimen, age, and lag time from symptom onset to institution of antithrombotic therapy serving as a priori primary and secondary hypothesized explanatory variables and using an a priori–defined significance threshold of P less than .2 for inclusion of other explanatory variables subsequently into multiple logistic regression. Otherwise, for all hypothesis testing, a P value of less than .05 was considered statistically significant. The PTS odds estimate for the primary variable of interest (therapy regimen) was not adjusted in multivariate analysis by variables believed to be in the casual pathway of the relationship between treatment regimen and PTS outcome (specifically, degree of clot resolution at 1 year). All hypothesis tests were 2-sided. Estimation of risk of developing PTS at 18 to 24 months after DVT used odds ratios (ORs), with 95% confidence intervals (CIs) calculated by the Wald method.
Results
Validation of the PTS instrument for lower-extremity DVT in children
All parameters of the PTS instrument were formally assessed in a separate derivation cohort (n = 78) consisting of healthy children aged 12 months to 21 years who were without known underlying medical conditions, were taking no medications, and had no personal or first-degree family history of venous thromboembolism, myocardial infarction, or ischemic stroke prior to 55 years of age. The age-grouped distributions of these findings in the derivation cohort are shown in Table 1. Based upon these observations, the upper limit of normal for the contralateral difference in lower-extremity circumference was determined to be 1.0 cm for midthigh and midcalf, irrespective of age group. This value was then uniformly used in the evaluation of presence versus absence of lower-extremity edema in patients with DVT in the present study. Furthermore, dilated superficial collateral veins, venous stasis dermatitis, venous stasis ulcers, and chronic lower-extremity pain limiting aerobic activities, limiting activities of daily living, or at rest were all absent in the derivation cohort, such that their presence was defined as abnormal on the physical exam and pain components of the PTS instrument when uniformly used in patients with DVT in the present study.
Interrater reliability in each parameter of the PTS instrument was evaluated in a cohort (n = 50) composed of both healthy children as described at the beginning of this section and children with a history of lower-extremity DVT aged 12 months to 21 years. PTS assessment using the standardized pediatric outcome instrument was independently performed by 2 trained clinicians who were blinded to each other's findings. Values for interrater reliability, measured as percent agreement, are given in Table 2.
Study groups
Twenty-two children with lower-extremity DVT met criteria for inclusion in the study. Demographic characteristics, underlying medical conditions, and other clinical prothrombotic risk factors, treatment regimens, and outcomes for each patient are shown in Tables 3 and 4. Based upon clinical judgment, 9 of these children were treated by thrombolysis and 13 by standard anticoagulation alone. Among the former group, 7 received systemic tPA (2 of whom underwent salvage mechanical thrombectomy with additional local, catheter-directed tPA) and 2 underwent local thrombolysis alone, via mechanical thrombectomy and catheter-directed tPA. One child (subject no. 9) in whom systemic tPA infusion was prematurely discontinued due to need for an invasive procedure was maintained in the analysis, as was 1 child (subject no. 7) in whom systemic tPA was prematurely discontinued and a subsequent course of standard anticoagulation was withheld due to a minor hemorrhagic complication (see “Bleeding complications”). Two children treated with systemic thrombolysis alone had been included in earlier reports.24,25
Table 5 summarizes patient characteristics, including hypothesized and known risk factors for and prognostic indicators in pediatric DVT and PTS, by treatment group (thrombolytic therapy followed by standard anticoagulation versus standard anticoagulation alone). As shown in Table 5, the 2 treatment groups were well-matched for all characteristics, with the exception that the proportion of patients having concomitant PE at DVT diagnosis was significantly greater in the thrombolytic regimen group than in the standard anticoagulation group (44.% vs 7.7%, respectively; P = .04). Specifically, as shown in Table 5, children in the 2 groups did not differ significantly in age; extent of DVT at diagnosis (as measured by number of venous segments involved by DVT, counting the inferior vena cava [IVC] and each unilateral iliac, femoral, and popliteal vein separately); FVIII activity or D-dimer levels at DVT diagnosis; lag time from symptom onset to initiation of antithrombotic therapy; relationship of the thrombus to an indwelling central venous catheter (CVC); or presence of the lupus anticoagulant (LA), antiphospholipid antibody syndrome, non-LA thrombophilia, or genetic thrombophilia in particular.
Outcomes
As shown in Table 5, the proportion of children who had evidence of PTS at 18 to 24 months (either physical-exam abnormalities, functional limitations, or both, as determined by standardized pediatric PTS outcome assessment), was significantly lower for the thrombolytic regimen than for standard anticoagulation (22.2% vs 76.9%, respectively; P = .01). As is consistent with the therapeutic goal of the thrombolytic regimen, a significantly greater proportion of patients exhibited 90% or greater thrombus resolution (characterized by no residual thrombus or only a small amount of mural thrombus) at 1 year in the thrombolytic regimen group compared with the standard anticoagulation group (88.9% vs 41.7%, respectively; P = .03). While the difference in proportion of children who developed abnormalities on both the physical exam and functional outcome components of the PTS instrument was not statistically significant when comparing the thrombolytic regimen and standard anticoagulation groups (11.1% vs 30.8%), this difference appears to be clinically meaningful.
In univariate logistic regression (Table 6), treatment regimen was significantly and most strongly associated with PTS (P = .02). The thrombolytic regimen was associated with a markedly decreased odds of PTS (OR = 0.086, 95% CI = 0.011-0.655) compared with standard anticoagulation alone, representing a 91% decrease in the odds of PTS. In addition, thrombus resolution (≥ 90% versus < 90% clot resolution at 1 year) was significantly associated with the development of PTS, such that the odds of PTS were 91% decreased in children who exhibited complete or near-complete thrombus resolution at 1 year compared with those in whom the thrombus persisted (OR = 0.089, 95% CI = 0.008-0.960; P = .046).
Multivariate analysis evaluated the odds of PTS for the primary explanatory variable (therapy regimen) after adjustment for those explanatory variables that were hypothesized a priori to be important explanatory variables (specifically, age at DVT diagnosis and lag time from symptom onset to initiation of antithrombotic therapy) as well as those explanatory variables found to be potentially statistically significant (using a threshold of P ≤ .2) by univariate logistic regression. Interestingly, the former 2 variables (age and lag time to therapy) were also the only additional explanatory variables that met the latter criterion for inclusion in multiple logistic regression (P = .10 and P = .20, respectively; Table 5). Explanatory variables that were in the a priori–hypothesized causal pathway of the relationship between therapy regimen and PTS development (specifically, thrombus resolution) were accordingly not included in the multivariate model. Importantly, after adjustment for age and lag time to therapy, therapy regimen was significantly and independently associated with the development of PTS (P = .02). The thrombolytic regimen remained associated with a markedly decreased odds of PTS (OR = 0.018, 95% CI = < 0.001-0.483) compared with standard anticoagulation alone, representing a 98% decrease in the odds of PTS.
Bleeding complications
Major bleeding complications occurred in one child with near-complete occlusion of both proximal pulmonary arteries due to PE at the time of acute DVT. This patient suffered a pulmonary hemorrhage in association with local pulmonary arterial mechanical thrombolysis performed for rapidly progressive right-heart failure. He was successfully supported with packed red blood cell transfusion and continuous positive airway pressure via mechanical ventilation to tamponade the pulmonary hemorrhage, and he is alive and well 2 years following the event. Notably, this bleeding event was clinically judged to be related to the urgent pulmonary arterial mechanical thrombolytic procedure (immediately prior to which systemic tPA was discontinued) rather than directly to the thrombolytic regimen for DVT per se. An additional child was noted to develop a pelvic hematoma near the site of recent femoral CVC placement, a minor procedure for which administration of systemic tPA had been clinically judged to present an acceptable risk of bleeding; this bleeding episode did not meet criteria for a major bleeding episode (see “Assessment of bleeding complications”).
Discussion
Thrombolysis has been applied to the treatment of venous thrombosis in children for 20 years,24,25,31,–33 albeit in highly limited and selected fashion. Until recently, the use of thrombolysis in adults with venous thrombosis (in whom local thrombolysis is primarily used) has been reserved for thrombi that are life- or limb-threatening or that have progressed on standard anticoagulant therapies. Broad application of thrombolysis to DVT has not yet occurred, primarily due to concerns regarding major hemorrhagic complications of thrombolysis and the need to appropriately risk-stratify patients who are most likely to benefit from this intensive management approach. Recently, it has been recognized that risk of major hemorrhage related to thrombolytic therapy is relatively low in young patients when appropriate contraindications are strictly observed.21,,,–25 Although the risk for bleeding (including both major and minor hemorrhage) is increased with systemic tPA compared with standard anticoagulation, careful selection of patents, diligent surveillance for signs of possible bleeding complications, and proactive management of hemorrhage can all render this risk quite acceptable, given the observation that the rate and severity of PTS after DVT appears to be decreased when venous flow is rapidly restored via thrombolysis.23 Finally, biomarkers such as FVIII and D-dimer have emerged as prognostic indicators in venous thrombosis, in turn making risk stratification more feasible.13,19,20
To date, published pediatric data regarding the safety of thrombolysis (whether systemic or local, and with or without mechanical thrombectomy) are limited, and the efficacy of thrombolytic approaches in the prevention of PTS in children has not been evaluated. We previously reported decreased signs of PTS in children treated with urokinase thrombolysis in whom greater than 75% restoration in venous blood flow was achieved within 48 hours of initiation of therapy.25 Based on our encouraging preliminary experience with systemic thrombolysis in a small number of children with DVT of the limbs, we developed a thrombolytic management paradigm aimed at achieving rapid restoration of venous blood flow in children with occlusive lower-extremity DVT. We used a step-wise approach to venous flow restoration typically beginning with systemic low-dose continuous intravenous infusion of tPA and using local mechanical thrombectomy with catheter-directed tPA administration as salvage therapy for persistent thrombosis. In some instances, the same local thrombolytic approach was used without prior systemic tPA administration. The current report is among the first to describe thrombus resolution and PTS outcomes of thrombolysis for a subset of children in whom mechanical thrombolysis was specifically used.
The present data support our prior findings that, in the context of a rigorously applied clinical pathway, thrombolysis appears to be appropriately safe in children with completely venoocclusive lower-extremity DVT. Furthermore, the present findings suggest that the use of a thrombolysis regimen may substantially reduce the risk of PTS in children with occlusive proximal lower-extremity acute DVT whose plasma levels of FVIII activity are 150 U/dL or greater and/or whose D-dimer concentrations measure 500 ng/mL or greater. In this cohort-study analysis, in which treatment groups were well matched for VTE risk factors and prognostic indicators at DVT diagnosis, we observed a 98% relative reduction in the odds of PTS for children treated with the thrombolytic regimen followed by standard anticoagulation compared with standard anticoagulation alone, after adjustment for both age at DVT diagnosis and lag time from symptom onset to institution of antithrombotic therapy. Seventy-seven percent of children in the standard anticoagulation group developed PTS compared with only 22% in the thrombolytic group. While a statistically significant difference in the proportion of patients who developed both physical and functional findings of PTS was not detected between the 2 treatment groups, this difference (31% for standard anticoagulation vs 11% for the thrombolytic regimen) may indeed be clinically significant, and the detection of statistical significance is probably impaired by the relatively small size of the study population. Furthermore, it is noteworthy that the only child in the thrombolytic group who developed moderate-severe PTS (subject no. 9, who was also the only child in this group in whom thrombus persisted at 1 year) had experienced a lag time of greater than 1 month from onset of symptoms to initiation of antithrombotic therapy and had received only an abbreviated course of systemic tPA due to the need for an invasive procedure.
Several limitations of the study must be recognized. Given the rather small number of children treated by the thrombolytic regimen, accurate measurement of safety with respect to bleeding complications is limited, and firm conclusions as to safety should await formal analysis of safety data derived from a clinical trial. Secondly, the treatment allocation in this study was determined clinically, and was nonrandomized, such that selection biases are possible. However, statistical analyses revealed that the 2 treatment groups were well matched with respect to possible predictor variables of age, lag time to therapy, and frequencies of CVC association, genetic thrombophilia, lupus-anticoagulant positivity, antiphospholipid antibody syndrome, and non–lupus-anticoagulant–acquired thrombophilia. Moreover, it is reasonable to postulate that children with greatest severity of thrombosis and judged at highest risk for PTS were most likely to be chosen for thrombolytic therapy, such that children in the thrombolytic group might have been at increased a priori risk for PTS. The significantly higher prevalence of PE in the thrombolytic group supports this notion.
An additional limitation of the present analysis is that thrombolytic outcomes were summarized for children treated either by continuous systemic low-dose tPA with subsequent local pharmacomechanical thrombolysis as salvage therapy or by local pharmacomechanical thrombolysis without prior systemic tPA. Given the size of the study population, trends toward superiority of 1 of the 2 approaches cannot be detected. While the feasibility of mechanical thrombolysis is limited by both local expertise and size constraints of the child (the smallest child in this series treated with pharmacomechanical thrombolysis via an interventional radiologic approach was 29 kg), infants as small as 10 kg with acute venous thrombosis have been treated by pharmacomechanical thrombolysis in our thrombosis program, in the case of superior vena cava obstruction.
When evaluating the applicability of the present analysis, it is important to consider both the risk and potential benefits of thrombolytic therapy. Because children require venous sufficiency and capacity for aerobic exercise for normal growth and development, the negative impact of PTS will be especially pronounced in children. Furthermore, because children are anticipated to live 6 or more decades after an episode of acute DVT, the long-term costs and morbidity of PTS are likely to be substantial. On the other hand, the bleeding risks of aggressive antithrombotic therapies such as systemic and/or local thrombolysis are not so well understood in children as to permit their broad application without risk stratification.
The current finding that a thrombolytic regimen is associated with a statistically significant decrease in the odds of PTS in general, but not in the odds of PTS that involves both physical-exam abnormalities and functional limitation (perhaps due to the relatively small size of the study population), emphasizes the need to select the highest possible PTS risk group among children with lower-extremity DVT for investigation of the safety and efficacy of thrombolysis in a clinical trial setting. In addition, these observations underscore the need for evaluation of both physical exam and functional outcomes for PTS in future studies. Based in part upon the present results, a multicenter clinical trial of thrombolysis in children with high-risk, acute lower-extremity DVT is in development through the Hemophilia and Thrombosis Research Society network.
An Inside Blood analysis of this article appears at the front of this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank: Elizabeth Pounder, Jasper Hillhouse, and Christopher Bombardier for data collection; Rick Shearer for data management; Carissa Smith for study coordination; and Dr Taru Hays for collaborative patient care.
This work was supported by grants from the Centers for Disease Control and Prevention (UR6/CCU820552) and the General Clinical Research Centers Program, National Center for Research Resources, National Institutes of Health (MO1-RR00069).
N.A.G. is supported by an American Society of Hematology Scholar Award in Clinical/Translational Research and a National Hemophilia Foundation Clinical Fellowship Award.
National Institutes of Health
Authorship
Contribution: N.A.G. and M.J.M.-J. designed research, performed research, analyzed data, and wrote the paper. J.D.D. and R.K.-C. designed research and performed research.
N.A.G. and M.J.M.-J. contributed equally to this work.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Marilyn J. Manco-Johnson, Mountain States Regional Hemophilia and Thrombosis Center, PO Box 6507, Aurora, CO 80045; e-mail: marilyn.manco-johnson@uchsc.edu.