Retroviral gene therapy can restore immunity to infants with X-linked severe combined immunodeficiency (XSCID) caused by mutations in the IL2RG gene encoding the common gamma chain (γc) of receptors for interleukins 2 (IL-2), −4, −7, −9, −15, and −21. We investigated the safety and efficacy of gene therapy as salvage treatment for older XSCID children with inadequate immune reconstitution despite prior bone marrow transplant from a parent. Subjects received retrovirus-transduced autologous peripherally mobilized CD34+ hematopoietic cells. T-cell function significantly improved in the youngest subject (age 10 years), and multilineage retroviral marking occurred in all 3 children.
Introduction
X-linked severe combined immunodeficiency (XSCID) is a primary immune deficiency that results from mutations in the IL2RG gene, which encodes the common gamma chain (γc) that is a component of and provides important signaling function for the receptors of interleukins 2 (IL-2), −4, −7, −9, −15, and −21.1,,–4 Infants with XSCID, who lack T and natural killer (NK) cells and have nonfunctional B cells, die of infections unless treated to establish functional immunity.1 Infants receiving a bone marrow transplant (BMT) from an HLA-matched sibling have almost 95% 5-year survival and achieve significant immune reconstitution.5,–7 However, most SCID patients lack an HLA-matched sibling. Although adequate reconstitution can follow HLA-haploidentical parental BM transplantation when performed within 3.5 months of birth, before severe infections occur,8 those receiving parental or unrelated donor BMT later in infancy have 5-year survival rates of only 55% to 75%.4,,–7,9,10 Furthermore, more than 50% of patients with XSCID or the JAK3-deficient type of SCID treated with BM transplantation fail to develop specific antibody responses, presumably because the presence of nonfunctional autologous B cells in these types of SCID may impede engraftment of functional donor B cells.9,11 Thus, after BM transplantation many XSCID patients remain dependent upon immunoglobulin infusions. Some BMT centers perform BM transplantation for SCID without conditioning, whereas others employ cytoreductive chemotherapy in an attempt to establish donor B-cell engraftment. Chemotherapy may adversely affect patient survival yet may not result in enhanced B-cell engraftment.9,–11 Furthermore, late-onset toxicities of chemotherapy include hypodontia, growth delay, and hormonal disturbances.12,–14 A subset of patients suffer progressive partial or total loss of their haploidentical T-cell graft over variable periods of time and present with susceptibility to infection, chronic diarrhea and pulmonary diseases, eczema, warts, and growth delay. These problems prompted our exploration of new treatments for these older children with XSCID who have waning immunity after BM transplantation. Gene therapy restored immune function to 12 of 14 infants with XSCID in 2 European trials.1,–3 Unfortunately, T-cell leukemias secondary to retroviral vector insertional mutagenesis occurred in 3 patients in 1 of these studies. At least 2 leukemias were in part caused by vector insertional activation of the LMO2 transcription factor gene.2,15,16
While retrovirally-induced leukemia is a serious risk, it must be balanced with the lack of satisfactory alternative treatments for the subset of XSCID children with profound impairment of T-cell immunity because of loss of donor T-cell number and function following haploidentical BM transplantation. These boys suffer progressive, chronic medical conditions despite intensive medical management. We enrolled 3 such older preadolescent XSCID patients in a clinical trial to assess safety and efficacy of treatment with ex vivo retroviral-transduced autologous GCSF-mobilized peripheral-blood CD34+ cells.
Patients, materials, and methods
Patients
Subject P1 presented at 6 months of age with untyped Pseudomonas sp and P. jiroveci pneumonia and widespread, chronic dermatitis infected with methicillin-resistant Staphylococcus aureus. His unusual IL2RG mutation at the poly-A addition site allowed expression of trace amounts of normal γc protein. Over a period of 4 years he received 4 T-cell–depleted, haploidentical parent–derived BMTs with no conditioning; however, he never achieved sustained engraftment. Although receiving intravenous immune gamma globulin (IVIG) and antibiotic, antiviral, and antifungal prophylaxis, he developed growth delay, chronic watery diarrhea, numerous upper- and lower-respiratory infections, eczema, and an esophageal stricture. He also had chronic scarring of his right middle lobe from previous recurrent pneumonias, significant loss of major upper-chest venous structures, and development of collateral venous drainage from long-term indwelling central venous catheters. He attended school but had frequent absences, primarily because of the pneumonias and recurrent diarrhea. At the time of gene therapy, no donor engraftment was detected in any lineage including purified mobilized CD34+ cells, using the polymerase chain reaction (PCR) quantitative analysis of informative microsatellite DNA sequences (“Quantification of retroviral vector copy number and chimerism analyses”).
Subject P2 developed multiple respiratory infections, oral candidiasis, chronic diarrhea, and failure to thrive by 6 months of age. At 11 months, when XSCID was diagnosed, he had graft-versus-host disease (GVHD) from maternal T cells presumably acquired perinatally that caused a rash and permanent hair loss. He received a maternal T-cell–depleted haploidentical BMT without bone marrow conditioning. After 2 years without a major infection, he developed severe diarrhea and failure to thrive. Because of low T-cell counts and poor proliferative responses, a second maternal T-cell–depleted BM transplantation without conditioning was performed. He experienced no improvement and remained dependent on gastrostomy feedings, IVIG, and antibiotic prophylaxis. Despite this prophylaxis, he suffered from recurrent viral and bacterial pneumonias as well as recurrent sinusitis. He has never attended school. At the time of gene therapy, 51% of circulating T cells were of donor (maternal) origin, but there was no detectable engraftment in any other lineage or in mobilized CD34+ cells.
Subject P3 had repeated respiratory infections and oral thrush in early infancy and, at 3 months of age, P jiroveci pneumonia. Laboratory studies revealed decreased T-cell counts and no mitogen responses. He received a T-cell–depleted paternal haploidentical BMT with no bone marrow conditioning at age 6 months. He experienced GVHD with rash, loss of fingernails, and diarrhea for 6 months. He continued to receive IVIG and antibiotic prophylaxis but had sinusitis and 1 bacterial pneumonia requiring hospitalization. At 8 years of age, respiratory infections became frequent and bronchiectasis and growth delay were diagnosed. Immunologic studies showed lymphopenia and poor lymphoproliferative responses to mitogens. However, he appeared to retain adequate lymphocyte proliferation to Candida antigen. He continued to suffer from frequent infections of the bronchiectatic areas of his lungs. At the time of gene therapy, 100% of T cells were of donor origin, but no donor engraftment was found in any other lineage or in mobilized CD34+ cells.
Informed consent process
The protocol, consent, and minor assent documents were approved by the National Institute of Allergy and Infectious Diseases (NIAID) institutional review board and designated as National Institutes of Health (NIH) protocol 02-I-0057. The protocol was also approved by the NIH Institutional Biosafety Committee and the NIH Office of Biotechnology Activities' Recombinant DNA Advisory Committee; and the US Food and Drug Administration (FDA) approved treatment under its Investigational New Drug (IND) authority.
Informed consent was obtained in accordance with the Declaration of Helsinki. The informed consent process involved 3 stages. First, the principal investigators and staff discussed the protocol with parents and with the potential child subject, following which, the parents and child were given the consent and protocol to read and discuss with the home physician. Several days to weeks later, all components of the protocol and elements of the informed consent and minor assent documents were discussed in detail with the parents and child patient, and the consent and minor assent documents were signed and witnessed. The third stage involved a detailed interview by a member of the NIH Clinical Center patient care Ethics Office to ascertain the level of understanding. A written confirmation regarding adequate understanding was added to the medical chart by the NIH ethics consultant.
Retroviral vector GALV-MFGS-γc
The open reading frame of human IL2RG cDNA was inserted into the NcoI-BamHI cloning site of the murine leukemia virus–based retroviral vector MFGS.17 To obtain gibbon ape leukemia virus (GALV)–pseudotyped MFGS-γc vector, we transduced PG13-packaging cells with MFGS-γc.17 A high-titer producer cell clone was selected and expanded. Supernatant containing vector produced under good manufacturing practices and subject to all of the FDA regulation–required safety testing was stored in 100-mL bags at −70°C. The biologic titer was sufficient in preclinical studies to reproducibly transduce more than 40% of human CD34+ cells in a culture containing 90% supernatant and 10% medium. The estimated multiplicity of infection (MOI) for the clinical transductions was 1 to 2.
Collection and transduction of GCSF-mobilized CD34+ peripheral-blood stem cells (PBSCs)
Patients received 10 μg/kg of granulocyte colony-stimulating factor (G-CSF; Filgrastim; Amgen, Thousand Oaks, CA) daily for 5 days. Increases in peripheral CD34+ cell counts were comparable to those of healthy controls and subjects with chronic granulomatous disease.18,19 Peripheral-blood mononuclear cells (PBMCs) were collected by apheresis on days 4 and 5 of the G-CSF schedule, and CD34+ cells were isolated as reported previously.18
CD34+ cells were thawed and cultured in flexible and air-permeable bags in serum-free medium (X-VIVO 10; BioWhittaker X-VIVO Media Systems, Cambrex BioScience, Walkersville, MD) supplemented with 1% human serum albumin (GIBCO culture products; Invitrogen, Carlsbad, CA) and 50 ng/mL FLT3 ligand, 50 ng/mL stem cell factor, 25 ng/mL IL-6, 50 ng/mL thrombopoietin, and 5 ng/mL IL-3 (all cytokines purchased from R&D Systems, Minneapolis, MD). Bags had been previously coated with recombinant fibronectin fragment CH296 (RetroNectin, kindly provided by Takara Bio, Shiga, Otsu, Japan). Beginning 16 hours after culture initiation, CD34+ cells were transduced 6 hours/day on 4 consecutive days using 100 mL of vector supernatant and 10 mL of medium per 100 × 106 cells, supplemented with 6 μg/mL protamine and cytokines at the same concentration as the culture medium. Transduced cells were washed with phosphate-buffer saline (PBS) and resuspended in 20 mL of PBS with 1% HSA.
Quantification of retroviral vector copy number and chimerism analysis
The presence of the retroviral vector in leukocyte lineages was quantified using real-time PCR. Cells were sorted using a Ficoll gradient and immunomagnetic beads (Dynalbeads; Invitrogen, Brown Deer, WI), with specificity for CD3 (T cells), CD19 (B cells), and CD14 (monocytes). CD15-specific beads were used to isolate granulocytes from the red blood cell fraction of the gradient. Genomic DNA was obtained from the sorted cells with QiaAmp DNA blood mini kit (Qiagen, Valencia, CA). Real-time quantitative PCR was performed using primers and labeled probes listed below to quantify the amplification of a specific sequence of the MFGS vector: forward primer, 5′-CGCAACCCTGGGAGACGTCC-3′; reverse primer, 5′-CGTCTCCTACCAGAACCACATATCC-3′; probe, 5′-6-FAM-CCGTTTTTGTGGCCCGACCTGA-TAMRA.
The retroviral copy number was established using a standard curve of increasing numbers of a single clone of transduced K562 cells containing one proviral copy of the MFGS vector per cell. Donor and recipient cells were detected by quantitative analysis of informative microsatellite DNA sequences, as previously described.19
Immunologic evaluation
Lymphocyte proliferation to mitogens and antigens was measured using a carboxy-fluorescein diacetate succinimidyl ester (CFSE) assay.20 T-cell receptor excision circles (TRECs) from total PBMCs were quantified by real-time PCR according to Douek et al.21 We also analyzed the diversity of the T-cell receptor (TCR) Vβ repertoire (spectratyping).22
Safety tests: replication-competent retrovirus and analysis of retroviral insertion sites
PBMC samples were obtained at baseline, every 3 months for the first year, and every 6 months thereafter. Replication-competent retrovirus safety testing was performed on blood cell samples at the Indiana University Vector Production Facility (Indiana University School of Medicine, Indianapolis, IN) where PCR was used to detect retrovirus envelope sequence. In our laboratory, blood cell samples were analyzed to assess the clonality and identity of retroviral insertion sites using 2 methods: linear amplification-mediated PCR (LAM-PCR)23,24 and the linker-mediated PCR (LM-PCR)25 using MseI digestion. Insertion site sequences were aligned to the human genome sequence using the UCSC genome browser (http://genome.ucsc.edu/).
Results
Patient characteristics before gene therapy
The patients in our study (P1, P2, and P3) had proven IL2RG mutations26 ; had received from 1 to 4 parental T-cell–depleted BMTs early in life after initial presentation; and had significant impairment of T-cell function. They were treated with gene therapy at 11, 10, and 14 years of age, respectively. Pretreatment histories were notable for growth delay, frequent infections, and chronic diarrhea (Table 1). All patients had very low numbers of CD4+ T cells and few or no NK cells; P1 and P2 also had low numbers of CD8+ T cells. P1 had no detectable donor engraftment in any blood cell lineage, whereas P2 and P3 had detectable donor engraftment of CD3+ T cells only (Table 1). T-cell receptor excision circles (TRECs) that are characteristically found in newly produced T cells21 were undetectable in all 3 patients, and proliferative responses to mitogens were depressed. All 3 patients demonstrated adequate mobilization of CD34+ cells from the bone marrow in response to daily administration of granulocyte colony-stimulating factor, allowing hematopoietic stem cells (HSCs) to be harvested from peripheral blood by apheresis. Importantly, the HSC-enriched CD34+ cells in all cases were exclusively of patient origin, explaining the absence of durable multilineage engraftment from their previous BMTs (not shown).
Ex vivo transduction of patient CD34+ PBSCs and infusion of transduced cells
From 17.9 to 27.7 million cryopreserved autologous CD34+ cells/kg (Table 1) were thawed as described (“Patients, materials, and methods, Collection and transduction of GCSF-mobilized CD34+ peripheral-blood stem cells”).27,–29 After 4 daily transductions with GALV-enveloped MFGS-γc vector (MOI of 1-2),21 the cells expanded 3.4- to 4.3-fold while remaining 80% to 90% CD34+; 39% to 45% of total cells in the culture were MFGS-γc–transduced as assessed by flow cytometry expression of the γc transgene. Based on these measurements, each patient received approximately 30 × 106 transduced CD34+ cells/kg intravenously. Vector copy number per transduced cell was 1.1 to 3.7.
Gene marking and changes in number and function of immune cell lineages
All 3 patients have retroviral marking in multiple leukocyte lineages (Table 2). Consistent with the selective growth and survival advantage conferred upon T cells by functional γc, there has been consistently higher marking in T cells than in CD15+ myeloid cells. At latest measurement, mean provirus copy number per CD3+ T cell for P1, P2, and P3, respectively, was 0.06, 1.0, and 0.22. Corrected B cells also exhibit a selective enrichment, although less marked than in T cells (Table 2).
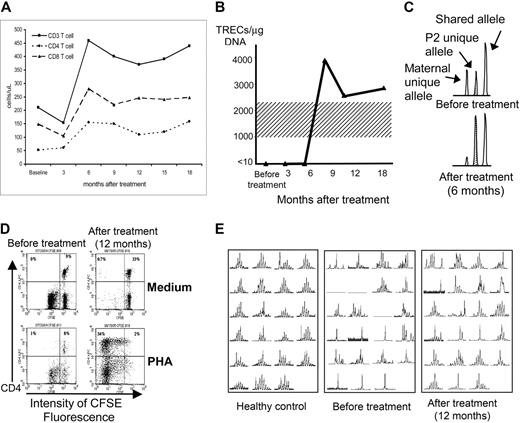
Although T-cell counts have not normalized in any of the patients, P2 has shown improvement in several immunologic parameters (Table 3; Figure 1). His CD4+ and CD8+ T-cell numbers increased (Figure 1A); and CD4+ CD45RA+ naive cells (Table 3) and TRECs from total PBMCs have appeared and persisted (Figure 1B), signifying new thymic output. The ratio of host versus donor origin of his T cells also shifted (Figure 1B). Before gene therapy, 49% of his very low number of T cells was autologous, whereas 51% derived from his maternal BM transplant. However, at 6 months and thereafter, he had only 13% donor cells. Taking into account his higher total T-cell counts, the maternal donor fraction stayed constant while autologous T cells increased in both absolute and relative number. Normal in vitro proliferation to mitogens PHA, ConA, and PWM and to Candida antigen has also been restored (Figure 1D and not shown).20 Finally, following gene therapy, T cells from P2 showed increased T-cell antigen receptor diversity as assessed by Vβ TCR spectratype analysis (Figure 1E).22
Improvements in immune function of patient P2 following gene therapy. (A) Increases in peripheral-blood CD4+, CD8+, and total CD3+ T-cell counts at 6 months after gene therapy. (B) TRECs, initially absent from peripheral-blood T cells, appearing at 9 months after gene therapy (normal adult range 1000-2200 TRECs/μg DNA, shading). (C) Shift in T-cell chimerism after gene therapy (area under PCR-amplified polymorphic allele peaks; Cofiler; Applied Biosystems, Foster City, CA), from before gene therapy (top set of peaks) where there is 51% maternal bone marrow donor (left peak) and 49% P2 host (stippled middle peak) to 6 months after gene therapy (bottom set of peaks) where the ratio is 13% donor versus 87% host. (D) CD4+ T-cell proliferation measured by decrease in fluorescence in response to PHA mitogen stimulation, absent before gene therapy (empty top left quadrant of the bottom left panel) but normal at 12 months after gene therapy (top left quadrant of the bottom right panel). P2 blood mononuclear leukocytes were labeled with the permanent cell-membrane–binding dye CFSE and cultured for 5 days in medium alone (control; top panels) or PHA (stimulus; bottom panels), then labeled with phycoerythrin-labeled anti-CD4 antibody. CD4+ T cells appear in the top half of each panel, and proliferation of the CD4+ T cells (dilution of CSFE fluorescence) is seen to occur only after gene therapy and only in response to PHA stimulation (bottom right panel). Not shown is that CD8+ T-cell response to PHA and both CD4+ and CD8+ responses to ConA, PWM, and Candida antigen increased similarly as measured by the CSFE assay. (E) Spectratyping of the Vβ TCR repertoire of CD3+ T cells23 from P2, demonstrating very restricted diversity before gene therapy (middle panel; total absence of representation of 3 Vβ families and almost monoclonal single-peak representation within 6 or 7 families) but increased diversity at 12 months after gene therapy (right panel; some representation in all the Vβ families, almost monoclonal single-peak representation only within 3 or 4 families, and clear improvement in multipeak polyclonality within 12 of the 23 families represented). A typical healthy control is shown in the left panel.
Improvements in immune function of patient P2 following gene therapy. (A) Increases in peripheral-blood CD4+, CD8+, and total CD3+ T-cell counts at 6 months after gene therapy. (B) TRECs, initially absent from peripheral-blood T cells, appearing at 9 months after gene therapy (normal adult range 1000-2200 TRECs/μg DNA, shading). (C) Shift in T-cell chimerism after gene therapy (area under PCR-amplified polymorphic allele peaks; Cofiler; Applied Biosystems, Foster City, CA), from before gene therapy (top set of peaks) where there is 51% maternal bone marrow donor (left peak) and 49% P2 host (stippled middle peak) to 6 months after gene therapy (bottom set of peaks) where the ratio is 13% donor versus 87% host. (D) CD4+ T-cell proliferation measured by decrease in fluorescence in response to PHA mitogen stimulation, absent before gene therapy (empty top left quadrant of the bottom left panel) but normal at 12 months after gene therapy (top left quadrant of the bottom right panel). P2 blood mononuclear leukocytes were labeled with the permanent cell-membrane–binding dye CFSE and cultured for 5 days in medium alone (control; top panels) or PHA (stimulus; bottom panels), then labeled with phycoerythrin-labeled anti-CD4 antibody. CD4+ T cells appear in the top half of each panel, and proliferation of the CD4+ T cells (dilution of CSFE fluorescence) is seen to occur only after gene therapy and only in response to PHA stimulation (bottom right panel). Not shown is that CD8+ T-cell response to PHA and both CD4+ and CD8+ responses to ConA, PWM, and Candida antigen increased similarly as measured by the CSFE assay. (E) Spectratyping of the Vβ TCR repertoire of CD3+ T cells23 from P2, demonstrating very restricted diversity before gene therapy (middle panel; total absence of representation of 3 Vβ families and almost monoclonal single-peak representation within 6 or 7 families) but increased diversity at 12 months after gene therapy (right panel; some representation in all the Vβ families, almost monoclonal single-peak representation only within 3 or 4 families, and clear improvement in multipeak polyclonality within 12 of the 23 families represented). A typical healthy control is shown in the left panel.
No similar improvements in lymphocyte functions were measured in P1 or P3. However, prior to gene therapy, T cells of P3 were 100% of donor origin, but by 9 and 12 months following gene therapy a small number of T cells of host origin became detectable. For all 3 patients, NK cells increased from 0 to 10 cells/μL at baseline to 10 to 20 cells/μL at latest follow-up (not shown). B-cell counts and serum IgA, IgM, and IgE levels did not change, and all 3 patients remain on their pre–gene therapy schedule of periodic intravenous pooled immune globulin treatments.
General clinical status after gene therapy
There have been no complications to date, with 30, 23, and 12 months of follow-up for P1, P2, and P3, respectively. All have reported improved well-being and stamina, reduced frequency of diarrhea and respiratory infections, and resolution of rashes. Furthermore, all have experienced linear growth, P1 gaining 4.9 cm (1.6 cm/year since treatment); P2 gaining 6.9 cm (3 cm/year); and P3 gaining 3.3 cm (3.3 cm/year). P1 and P3 have had fewer school absences than before gene therapy, and home-schooled P2 has had fewer doctor visits. At 6 months after gene therapy, P1 developed 0.5-cm bilateral submandibular lymph nodes and visible tonsillar tissue that have persisted without changing.
Safety monitoring
Safety monitoring has revealed, in addition to negative tests for replication-competent retrovirus, consistently polyclonal assessment of retroviral insertion sites using LAM-PCR; (Figure 2)23,24,30 and LM-PCR.25 From a sample of the preinfusion-transduced CD34+ cells from P1, 203 insertion sites were isolated, sequenced, and assigned to specific genomic locations. As previously noted for murine leukemia virus vectors, insertions near the 5′ end of genes were more frequent than predicted by random integration models,25,31 but no insertions were found near the LMO2 locus. Similarly, posttreatment peripheral-blood samples from all 3 patients have yielded 260 distinct insertion sites (24 from P1, 219 from P2, and 17 from P3) but none within 5 million base-pairs of LMO2 or ZNF217 and only one 58-kb 5′ to CCND2 (genes suggested to be common integration sites with oncogenic potential).
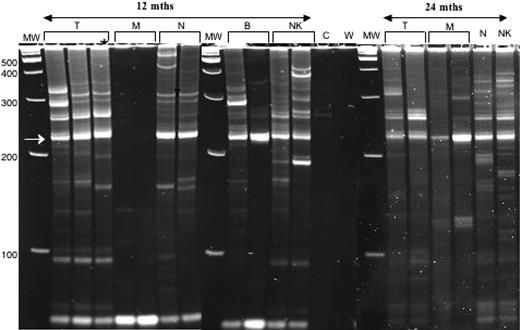
Polyclonality of vector inserts in blood leukocyte lineages of P2 at 12 and 24 months after gene therapy. Retroviral vector insertions are assessed by the LAM-PCR DNA amplification method22 ; amplified bands of multiple sizes are then separated by gel electrophoresis, where the various bands correspond to different retroviral-genomic DNA junctions. Shown in this figure are 3 electrophoresis gels cropped to show the relevant adjacent lanes. The different gels are separated by a vertical white line. Gels A and B were run at the same time and amplifications of DNA from the various blood-cell lineages separated from a single blood sample from subject P2 were obtained at 12 months after gene therapy. Shown for each lineage are 2 to 3 independent amplifications for DNA from T (CD3+ lymphocytes), M (CD14+ monocytes), N (CD15+ neutrophils), B (CD19+ lymphocytes), and NK (CD56+CD3−) cells. Note that the unlabeled lane at the far right of gel B contained a sample of the same amplification of T as run in the left-most T lane in gel A to assure that gels A and B had run similarly. Also included in gel B are lanes containing the amplification of control (before gene therapy) blood cell DNA from P2 (C) and a water control (W). All gels contain a lan with molecular weight markers (MW), with the molecular weight indicated in base pairs (bp) at the left margin of gel A. The recurrent bright band at 227 bp (arrow at left) is derived from internal retroviral sequence. Polyclonality is demonstrated by bands of many sizes in T, B, and NK cells and neutrophils at 12 months after gene therapy. Gel C was run at a later time than gels A and B and contains amplifications of DNA from the various blood-cell lineages separated from a single blood sample from subject P2 obtained at 24 months after gene therapy. Shown in gel C are 2 independent LAM-PCR amplifications for the T and M lineages but only 1 amplification for N and NK lineages. Polyclonality of vector inserts is still observed at the 24-month time point.
Polyclonality of vector inserts in blood leukocyte lineages of P2 at 12 and 24 months after gene therapy. Retroviral vector insertions are assessed by the LAM-PCR DNA amplification method22 ; amplified bands of multiple sizes are then separated by gel electrophoresis, where the various bands correspond to different retroviral-genomic DNA junctions. Shown in this figure are 3 electrophoresis gels cropped to show the relevant adjacent lanes. The different gels are separated by a vertical white line. Gels A and B were run at the same time and amplifications of DNA from the various blood-cell lineages separated from a single blood sample from subject P2 were obtained at 12 months after gene therapy. Shown for each lineage are 2 to 3 independent amplifications for DNA from T (CD3+ lymphocytes), M (CD14+ monocytes), N (CD15+ neutrophils), B (CD19+ lymphocytes), and NK (CD56+CD3−) cells. Note that the unlabeled lane at the far right of gel B contained a sample of the same amplification of T as run in the left-most T lane in gel A to assure that gels A and B had run similarly. Also included in gel B are lanes containing the amplification of control (before gene therapy) blood cell DNA from P2 (C) and a water control (W). All gels contain a lan with molecular weight markers (MW), with the molecular weight indicated in base pairs (bp) at the left margin of gel A. The recurrent bright band at 227 bp (arrow at left) is derived from internal retroviral sequence. Polyclonality is demonstrated by bands of many sizes in T, B, and NK cells and neutrophils at 12 months after gene therapy. Gel C was run at a later time than gels A and B and contains amplifications of DNA from the various blood-cell lineages separated from a single blood sample from subject P2 obtained at 24 months after gene therapy. Shown in gel C are 2 independent LAM-PCR amplifications for the T and M lineages but only 1 amplification for N and NK lineages. Polyclonality of vector inserts is still observed at the 24-month time point.
Discussion
There are no standard treatments for XSCID patients who fail to develop or lose T-cell function after haploidentical BM transplantation. We observed unequivocal immunologic improvement in 1 of our 3 patients treated with gene therapy (P2), and all 3 patients had persistence of transduced T and B cells in peripheral blood for at least 12 to 30 months. To date there have been no adverse effects. Thus, consistent with our preclinical studies in human/sheep chimeras,17 our human XSCID trial has demonstrated that mobilized peripheral CD34+ cells can give sustained multilineage marking following ex vivo retroviral transduction and reinfusion. Though myeloid marking waned to less than 1%, reflecting low transduction and/or engraftment rates for HSCs, a significantly higher level of corrected T cells, and to a lesser extent corrected B cells, persisted due to the survival advantage conferred by the γc transgene. Two patients, P2 and P3, maintained T-cell provirus copy numbers of 1.0 and 0.22, respectively, with evidence of new autologous T-cell production; indeed P2 had improvement in several objective measures of T-cell function.
Recently reported gene therapy for 2 XSCID patients at 16 and 20 years of age noted failure to achieve significant T-cell marking.32 This raises the question whether increasing age is a barrier to efficacy of XSCID gene therapy. The protocols for these 2 patients were similar to those successfully used for XSCID infants,1,3 also using bone marrow–derived CD34+ cells. Interestingly, neither these published older XSCID patients nor our P1 had experienced a sustained period of normal thymic output of allogeneic T cells after a BM transplantation earlier in life. In contrast to these patients, our P2 and P3 originally had full T-cell reconstitution for at least 2 years following haploidentical BM transplantation. Older XSCID patients who once had but then lost T-cell production in the thymus might be expected to experience better results following gene therapy than those with a thymus that had never been repopulated, including our patient P1. These observations suggest that success of gene therapy in older children after BM transplantation may relate not only to age per se but also to prior successful T-cell production and retained potential functionality of the thymus. Monitoring of thymic output, as indicated by TREC number or CD45RA+ naive T-cell counts in post–BM transplantation XSCID patients, might permit an early determination regarding whether a previously successful BM transplantation is failing, so that an intervention such as gene therapy can be performed before further loss of thymic potential. We note that our youngest patient, P2, at 10 years of age experienced the most benefit from gene therapy, and it is possible that intervention with gene therapy even earlier may increase efficacy in post–BM transplantation XSCID patients with waning immunity.
Unlike P2, subjects P1 and P3 have not had improvement in immunologic parameters that can be attributed to gene therapy despite the persistence of marked T cells. Several factors may contribute to their poorer responses. For P1, the low numbers of corrected T cells may be a consequence of the same circumstances that caused his 4 previous BMT rejections. His unusual IL2RG mutation at the poly-A addition signal causes destabilization of mRNA but allows residual trace expression of normal γc protein and development of small numbers of autologous T cells. Prior to gene therapy of P1, his autologous T cells were identified histochemically and by DNA fingerprinting in lymphocytic infiltrates of skin and hair follicles. These sites of rash and alopecia, reminiscent of Omenn syndrome, resolved following gene therapy. P3, treated with gene therapy at 14 years of age, has had persistently higher gene marking of T cells than P1, but lower than seen in P2, consistent with the hypothesis of an effect from age-associated thymic senescence. P3 also had the most restricted pretreatment T-cell repertoire of our 3 patients, consistent with very poor thymic function (spectratyping not shown).
The dose of transduced CD34+ cells is likely to affect the level and persistence of transduced T cells, which in turn influences degree of immune reconstitution. The 2 XSCID gene therapy clinical trials in infants suggested that greater than 3 × 106 transduced bone marrow CD34+ cells/kg were needed for successful immunologic reconstitution.3,15 Moreover, in the published report of unresponsive older XSCID patients, 1 received only 0.7 × 106 transduced CD34+ cell/kg, whereas the other patient received 4.5 × 106 CD34+ cells/kg but with a transduction efficiency of only 13%.32 We obtained significantly larger numbers of peripheral-blood CD34+ cells from apheresis and thus treated each patient with approximately 30 × 106 transduced CD34+ cells/kg at approximately 40% transduction efficiency. The higher corrected cell dose may have led to higher and more persistent T-cell marking in all 3 of our patients than in the 2 previously reported older patients.
Though a high dose of transduced cells might confer an increased risk of insertional mutagenesis, it also might be necessary for achieving the level and persistence of marking we have observed. To date we have found no instances of genomic insertion of retrovirus near LMO2 in 463 unique validated junction fragments sequenced from samples obtained before and after infusion of transduced cells. Possible explanations for our different insertion profiles include the following, in addition to ascertainment differences from methods of isolating junction fragments: (i) our MFGS murine leukemia virus vector backbone had 3 engineered stop codons and lacks the mutation in the stem cell–specific repressor binding site16 of the vector in the XSCID gene therapy trial in France; (ii) we used a GALV-pseudotyped vector (as did the XSCID gene therapy trial in England, in which LMO2 insertions and leukemia have also not been reported) that may target a more immature spectrum of CD34+ cells than the amphotropic vector used in France3 ; (iii) a 12-fold lower concentration of IL-3 during transduction culture than the French trial; (iv) older patients rather than infants; and (v) CD34+ cells derived from GCSF-mobilized peripheral blood rather than bone marrow. These differences, particularly age, retroviral envelope, and source of CD34+ cells, may have resulted in a different composition and/or ratio of transduced early progenitors and true stem cells. To date, the risk factors that allowed leukemias to develop in 1 of the 2 gene therapy trials in XSCID infants are not fully defined. Further follow-up of clinical, immunologic, and molecular parameters in our patients will establish the long-term safety and efficacy of this approach to gene therapy for preadolescents with XSCID who have failed to achieve or maintain immune reconstitution after BM transplantation.
An Inside Blood analysis of this article appears at the front of this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This work was supported by the Divisions for Intramural Research, NIAID, and NHGRI, NIH.
We thank our patients and their families; Drs R. H. Buckley, T. Frazier, and H. M. Rosenblatt for referral, advice, and excellent patient care; and K. Hines and other staff of the Department of Transfusion Medicine (DTM), Clinical Center (CC), NIH, for preparation of gene-modified cells. We also thank Takara Bio, Inc (Shiga, Japan) for providing clinical-grade RetroNectin under a Clinical Supply Agreement. This paper is dedicated to the memory of Charlie Carter (DTM, CC, NIH).
National Institutes of Health
Authorship
Contribution: J.C., H.L.M., and J.M.P. designed the study and performed the majority of work in carrying out the clinical trial; J.D., S.S.D.R., B.N.H., and J.U. provided clinical care support; E.Y.H.N. provided regulatory support; S.S.D.R., A.P.H., G.F.L., N.N., C.S., and N.L.W.-T. provided basic laboratory support; and J.C., H.L.M., and J.M.P. wrote the paper.
H.L.M. and J.M.P contributed equally as senior authors to this study.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Harry L. Malech, Laboratory of Host Defenses, NIAID, NIH, Building 10-CRC 5-West Labs, Room 5–3750, 10 Center Drive MSC 1456, Bethesda, MD 20892-1456; e-mail: hmalech@nih.gov.