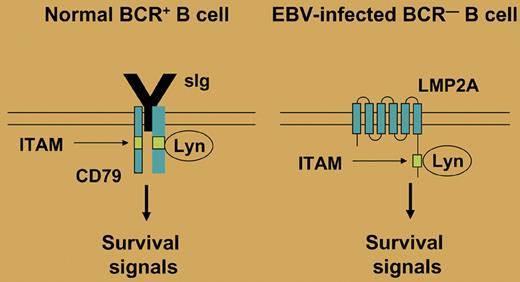
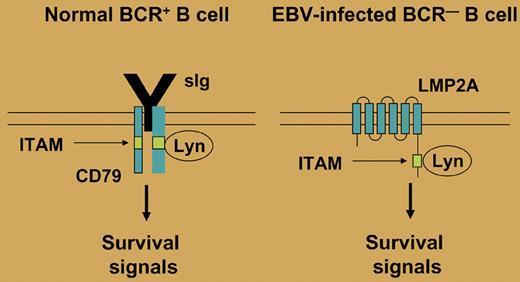
Expression of a B-cell receptor (BCR) is essential for the survival of B cells. In this issue of Blood, Mancao and Hammerschmidt show that the Epstein-Barr virus (EBV)–encoded latent membrane protein 2A (LMP2A) is able to replace the BCR survival signal in BCR-deficient human germinal center (GC) B cells.
More than 95% of humans worldwide are latently infected by EBV. Primary infection is mostly asymptomatic and thereafter, EBV persists in B cells, usually as a harmless passenger. In latently infected B cells, up to 9 virus-encoded proteins are expressed, including the 2 latent membrane proteins LMP1 and LMP2A. Interestingly, LMP1 mimics an active CD40 receptor and LMP2A bears a cytoplasmatic immunoreceptor tyrosine-based activation motif (ITAM), which is also present in the coreceptors of the BCR, mediating BCR signaling (see figure). As CD40 and the BCR play essential roles in the survival and selection of GC B cells, the 2 LMPs may replace these factors and promote the survival of B cells. Initial evidence for a role of LMP2A as a BCR mimic came from mouse models, which showed that in LMP2A transgenic animals, BCR-negative B-cell precursors are generated and can survive in the periphery.1,2 However, these studies did not reveal whether LMP2A could have a similar function in mature B cells. It was indeed demonstrated that BCR-deficient GC B cells can be rescued in vitro from apoptosis by infection with EBV, but these studies did not address the specific function of LMP2A in this process.3-5 An elegant study by Mancao and Hammerschmidt now shows that the rescue function of EBV in mature BCR-deficient GC B cells is indeed dependent on LMP2A. Thus, LMP2A is now established as a BCR mimic and survival factor in human GC B cells.
In rare instances, EBV is involved in the pathogenesis of malignancies, particularly B-cell lymphomas. The findings by Mancao and Hammerschmidt also have important implications for the pathogenesis of 2 EBV-associated B-cell tumors, namely Hodgkin lymphoma and posttransplantation lymphoproliferative disease (PTLD). In classic Hodgkin lymphoma and a fraction of cases of PTLD, the tumor cells are presumably derived from preapoptotic GC B cells that acquired disadvantagous Ig V gene mutations, including BCR-destructive ones.6 As normal B cells are stringently selected for expression of a BCR, GC B cells normally undergo apoptosis upon loss of BCR expression. Notably, in Hodgkin lymphoma, nearly all cases with BCR-destructive mutations are EBV-infected, indicating that precursors of the tumor clone in Hodgkin lymphoma with such mutations can survive and eventually undergo malignant transformation only if infected by EBV.6 The work of Mancao and Hammerschmidt thus assigns a critical role in the development of a fraction of Hodgkin lymphomas and PTLD cases to LMP2A.
The ability of LMP2A to rescue BCR-deficient GC B cells, however, apparently has little or no role in the establishment of the pool of virus-bearing cells and maintenance of viral persistence, because the viral reservoirs are BCR-positive memory B cells, which appear to lack LMP2A expression. Thus, the rescue function of LMP2A may be a side effect of other roles of this protein in viral persistence. One such role is suggested by an observation made by Mancao and Hammerschmidt: that there is a dramatic loss of BCR+ EBV-infected B cells upon induced deletion of LMP2A.
In normal B cells, the BCR provides a tonic survival signal. This signal is mediated through an immunoreceptor tyrosine-based activation motif (ITAM) that is found in the BCR coreceptors CD79a and CD79b. Several tyrosine kinases (eg, Lyn) can bind to the ITAM and initiate a signaling cascade. In EBV-infected B cells, LMP2A may mimic the presence of a functional BCR, as it also contains a cytoplasmatic ITAM, thereby allowing survival of BCR-deficient B cells.
In normal B cells, the BCR provides a tonic survival signal. This signal is mediated through an immunoreceptor tyrosine-based activation motif (ITAM) that is found in the BCR coreceptors CD79a and CD79b. Several tyrosine kinases (eg, Lyn) can bind to the ITAM and initiate a signaling cascade. In EBV-infected B cells, LMP2A may mimic the presence of a functional BCR, as it also contains a cytoplasmatic ITAM, thereby allowing survival of BCR-deficient B cells.
Conflict-of-interest disclosure: The author declares no competing financial interests. ■