In this issue of Blood, Jamaluddin and colleagues report important observations showing that DNA hypomethylation is a key biochemical mechanism responsible for elevated levels of plasma homocysteine (Hcy) called hyperhomocysteinemia (HHcy), which induces cyclin A suppression and growth inhibition in endothelial cells (ECs). These observations unveil a novel mechanistic link between HHcy and accelerated cardiovascular disease (CVD).
Hcy has been established as an independent risk factor for CVD.1 There are large bodies of experimental evidence supporting the hypothesis that HHcy causes atherosclerotic phenotypes in established cell-culture and animal models. Nonetheless, the causal role of Hcy in atherosclerosis remains to be proven in controlled clinical trials. The mechanistic link between HHcy and CVD, however, is poorly understood and is under active investigation worldwide.
Vascular disease is initiated by endothelial injury resulting from chemical, microbiological, immunological, or mechanical insults to the vessel wall. Endothelial injury is a key feature of arteriosclerosis, leading to platelet aggregation, activation of coagulation, vascular smooth muscle cell (VSMC) proliferation, and atherosclerosis.2 It was suggested3 that endothelial injury, the initial feature of atherosclerosis, may account for Hcy pathology. Earlier work from this group3 found that Hcy has distinct cell-type–specific proatherogenic effects in vascular cells: at a physiologically relevant concentration (10-50 μM), Hcy inhibits EC growth and promotes VSMC proliferation. Subsequently, they found that the distinct growth-inhibitory effect of Hcy in ECs is explained by reduced cyclin A transcription and associated methylation inhibition.4 In this issue of Blood, Jamaluddin and colleagues further characterize how Hcy causes DNA hypomethylation and reduces cyclin A transcription in ECs, and also reveal that Hcy reduces DNA methyltransferase 1 (DNMT1) activity and demethylates cyclin A promoter, leading to cyclin A chromatin remodeling. They further demonstrate that adenovirus-transduced DNMT1 gene expression reverses the inhibitory effect of Hcy on cyclin A expression and EC growth inhibition.
Another novel observation made in the current work is that DNA methylation of a repressor site on a promoter can activate gene transcription. Jamaluddin and colleagues have identified a CpG island that functions as a cyclin A core promoter, wherein 2 CpG sites are demethylated. One of these CpG sites is located on the cell-cycle–dependent element (CDE). Mutation of the CG sequence on the CDE results in a 6-fold increase in promoter activity. These data support the notion that the CDE is a repressor of the cyclin A promoter in ECs, and that the CDE CpG site is responsive to Hcy hypomethylation in a pathophysiologically relevant model.
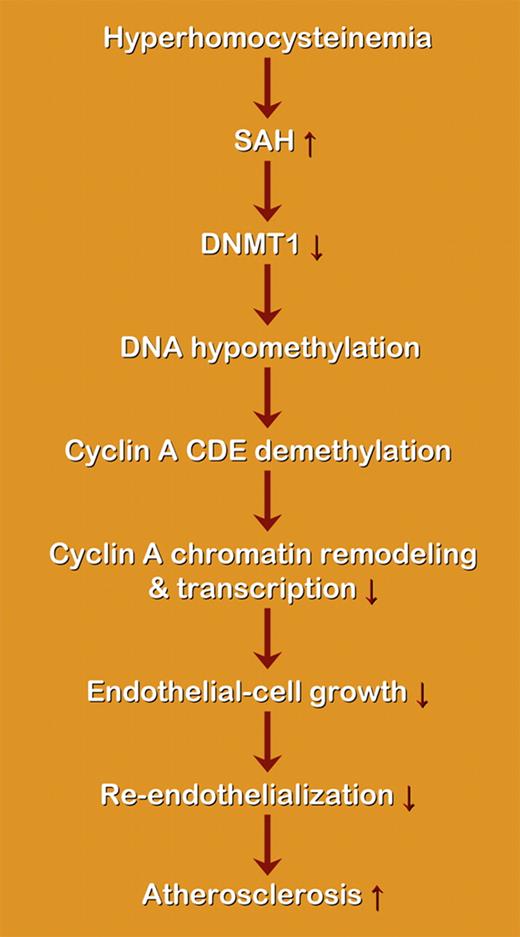
Schematic representation of the proposed effect of hyperhomocysteinemia on DNA methylation and atherosclerosis. SAH indicates S-adenosyl-homocysteine; DNMT, DNA methyltransferase. Hcy may induce the accumulation of SAH, a potent inhibitor of cellular methylation, as well as subsequent cellular hypomethylation, including DNA hypomethylation. Hcy-induced DNA demethylation is mediated by DNMT1 inactivation, which leads to demethylation of the cyclin A promoter CDE, and also leads to cyclin A promoter remodeling. Cyclin A chromatin remodeling may facilitate the function of CDE suppressor and result in cyclin A suppression. The activity of the suppressor element is increased due to DNA hypomethylation, which promotes chromatin remodeling and the access of suppressors to the chromatin, leading to transcriptional inhibition.
Schematic representation of the proposed effect of hyperhomocysteinemia on DNA methylation and atherosclerosis. SAH indicates S-adenosyl-homocysteine; DNMT, DNA methyltransferase. Hcy may induce the accumulation of SAH, a potent inhibitor of cellular methylation, as well as subsequent cellular hypomethylation, including DNA hypomethylation. Hcy-induced DNA demethylation is mediated by DNMT1 inactivation, which leads to demethylation of the cyclin A promoter CDE, and also leads to cyclin A promoter remodeling. Cyclin A chromatin remodeling may facilitate the function of CDE suppressor and result in cyclin A suppression. The activity of the suppressor element is increased due to DNA hypomethylation, which promotes chromatin remodeling and the access of suppressors to the chromatin, leading to transcriptional inhibition.
Overall, the current study provides new evidence pointing to a conclusion regarding the potential for hypomethylation to decrease gene expression by promoting chromatin remodeling and the access of suppressors to the chromatin, leading to transcriptional inhibition (see figure). Future research on DNA methylation in other target genes in Hcy signaling in vascular cells and other cell types might help to dissect the underlying mechanism and to provide suggestions for future therapeutic strategies.
Conflict-of-interest disclosure: The author declares no competing financial interests. ■
Acknowledgments:
Dr Zou's Laboratory is supported by National Institutes of Health (NIH) grants HL079584, HL074399, and HL080499; a research award from the American Diabetes Association; a research award from the Juvenile Diabetes Research Foundation; a research award from the Oklahoma Center for Advancement of Science and Technology; and the Travis Endowed Chair in Endocrinology, University of Oklahoma Health Sciences Center.
National Institutes of Health

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal