Abstract
Actin polymerization is crucial in throm-bopoiesis, platelet adhesion, and mega-karyocyte (MK) and platelet spreading. The Wiskott-Aldrich syndrome protein (WASp) homolog WAVE functions downstream of Rac and plays a pivotal role in lamellipodia formation. While MKs and platelets principally express WAVE1 and WAVE2, which are associated with Abi1, the physiologic significance of WAVE isoforms remains undefined. We generated WAVE2−/− embryonic stem (ES) cells because WAVE2-null mice die by embryonic day (E) 12.5. We found that while WAVE2−/− ES cells differentiated into immature MKs on OP9 stroma, they were severely impaired in terminal differentiation and in platelet production. WAVE2−/− MKs exhibited a defect in peripheral lamellipodia on fibrinogen even with phorbol 12-myristate 13-acetate (PMA) costimulation, indicating a requirement of WAVE2 for integrin αIIbβ3-mediated full spreading. MKs in which expression of Abi1 was reduced by small interfering RNA (siRNA) exhibited striking similarity to WAVE2−/− MKs in maturation and spreading. Interestingly, the knockdown of IRSp53, a Rac effector that preferentially binds to WAVE2, impaired the development of lamellipodia without affecting proplatelet production. In contrast, thrombopoiesis in vivo and platelet spreading on fibrinogen in vitro were intact in WAVE1-null mice. These observations clarify indispensable roles for the WAVE2/Abi1 complex in αIIbβ3-mediated lamellipodia by MKs and platelets through Rac and IRSp53, and additionally in thrombopoiesis independent of Rac and IRSp53.
Introduction
Actin polymerization plays essential roles in adhesion, spreading, movement, and pattern formation of cells.1,2 External stimuli to cells, for instance through an integrin, induce de novo actin polymerization that leads to formation of structures with actin cytoskeletons such as filopodia and lamellipodia. The actin-related protein 2/3 (Arp2/3) complex is thought to be essentially involved in the formation of these structures. The Arp2/3 complex is activated by Wiskott-Aldrich syndrome protein (WASp), neural WASp (N-WASp), and WASp-family verprolin-homologous protein; and WAVE1, 2, and 3 of the WAVE/Scar family of proteins.1-4
WASp and N-WASp are regulated via phosphorylation. Direct binding with the complex of small GTPase Cdc42 and phosphatidylinositol (4,5) bisphosphate (PtdInsI (4,5) P2) synergistically activates N-WASp for, in turn, activation of the Arp2/3 complex.5,6 In contrast to WASp and N-WASp, WAVEs play key roles downstream of small GTPase Rac in initiating lamellipodia formation.2,4 Of note is that Rac activates the Arp2/3 complex through a Rac direct effector, IRSp53, and through WAVE in membrane ruffling, lamellipodia, and many other remodeling processes,7-9 where WAVE is a functional component of a large protein complex, including PIR121/Sra1, Nap1/Hem-1, HSPC300, and Abi1.10-14 PIR121/Sra1 binds Rac, providing another linkage between Rac and WAVE. Moreover, Abi1, an Abl kinase–binding protein, is essential for the activation of WAVE2.10,11,15
Terminally differentiated megakaryocytes (MKs), precursors of blood platelets, preserve the unique internal membrane system, called the demarcation membrane system (DMS), that is reportedly important for the generation of platelets through cytoplasmic fragmentation with long and thin extensions, called proplatelets.16-18 The actin cytoskeleton may contribute to initiation of proplatelets.16-18 In MKs, the binding of integrin αIIbβ3 to its major ligand, fibrinogen, favors the generation of proplatelets in vitro and platelet production in vivo,19 and in platelets, fibrinogen ligation to αIIbβ3 promotes actin polymerization necessary for full platelet aggregation, spreading, and stable thrombus formation in a manner dependent on integrin outside-in signaling.20
MKs and platelets express primarily WASp but also N-WASp, although in much smaller quantities.21 Murine WASp-deficient platelets or correspondingly WASp-deficient human platelets exhibit intact Arp2/3 activation in which both normal filopodia and lamellipodia formation are observed.22,23 Although Sabri et al recently demonstrated premature platelet release within the bone marrow due to a defect of podosomes in WASp-null MKs,24 WASp seems dispensable in these processes. Terminally differentiated MKs and platelets express all WAVEs, mainly WAVE1 and WAVE2 (at equal levels), but with a trace amount of WAVE3.23,25 They also may relay signals from integrin αIIbβ3 to the Arp2/3 complex, where both WAVE1 and WAVE2 are associated with Abi1 independently.25 WAVE2 is much more efficiently precipitated by the SH3 domain of IRSp53 than is WAVE1.25 Mice deficient conditionally in both Rac1 and Rac2 upstream of WAVE display normal thrombopoiesis in vivo but a defect in lamellipodial spreading of platelets on fibrinogen or collagen in vitro.26 Although we have proposed that WAVE may mediate Rac-dependent reorganization of the actin cytoskeleton in platelets,25 this hypothesis has not been tested so far.
In this context, we sought to determine the individual roles of WAVE isoforms in MKs and platelets. Since mice deficient in WAVE2 die mostly by embryonic day (E) 11.5 to 12.0, presumably owing to a defect in vascular formation,27 we used the embryonic stem (ES) cell–derived MK as a model cell to examine the integrin-dependent actin cytoskeleton.28,29 Our culture system set the stage for using murine ES cells in order more easily to study in vitro various aspects of MK development, along with functions such as integrin-mediated spreading features.28,29 Signaling pathways in mature MKs focusing on actin cytoskeletal reorganization, as exemplified by spreading on fibrinogen, resemble their counterparts in platelets.28,30 Accordingly, ES cell–derived MKs, together with gene manipulation, prove to be a useful model system for evaluating molecules involved in regulation of the actin cytoskeleton.29 Because a strain of WAVE1-null mouse established by us dies within 7 to 10 days after birth,9 we generated radiation-chimera mice using WAVE1-null E13.5 fetal liver (FL) transplantation for studies of hematopoiesis, including thrombopoiesis, in vivo, and of platelet spreading in vitro. These strategies allowed us to address the hypothesis that WAVE2, but not WAVE1, might have indispensable roles in terminal differentiation of MKs, platelet production, and, through integrin αIIbβ3, in spreading machinery.
Materials and methods
Plasmid preparation, reagents, and mice
All reagents were from Sigma-Aldrich (St Louis, MO) unless otherwise indicated. All animal and recombinant DNA experiments were approved by the Institutional Animal Care and Use Committee of the Institute of Medical Science, University of Tokyo. WAVE1 knockout mice were prepared as described.9 WAVE3 knockout mice were established by D. Y. and T. T. C57BL/6 mice congenic for the Ly5 locus (B6-Ly5.1) were purchased from Nihon SLC (Shizuoka, Japan). Monoclonal antibody 1B5, a specific blocker for mouse αIIbβ3, was the gift of Dr B. Coller (Rockefeller University, New York, NY). Rabbit anti-WAVE1, anti-WAVE2, anti-WAVE3, and anti-IRSp53 polyclonal antibodies have been described.25,27,31 Mouse anti-Abi1 monoclonal antibody (clone 1B9) was from MBL (Nagoya, Japan). Rabbit anti-Arp3 polyclonal antibody was from Upstate (Lake Placid, NY). Rabbit anti–von Willebrand factor (VWF) polyclonal antibody was from DAKO-Cytomation (Glostrup, Denmark). Rhodamine- and Alexa 647–conjugated phalloidin, Alexa 488–conjugated fibrinogen, and Alexa 488–, Alexa 568–, and Alexa 647–conjugated bovine IgG antibodies were from Invitrogen/Molecular Probes Japan (Tokyo, Japan). Purified human fibrinogen was from American Diagnostica (Greenwich, CT). Fluorescein isothiocyanate (FITC)–conjugated, allophycocyanin (APC)–conjugated, and nonconjugated anti-mouse integrin αIIb and APC-conjugated anti–c-Kit antibodies were from BD/Invitrogen Japan (Tokyo, Japan). Phycoerythrin (PE)–conjugated and nonconjugated anti-mouse GPIbα antibodies were from Emfret (Würzburg, Germany). Mouse leukemia inhibitory factor (LIF; ESGRO) was from Chemicon (Temecula, CA). Human thrombopoietin (TPO), human IL-6, and mouse IL-11 were from Peprotech Japan (Tokyo, Japan). Horseradish peroxidase (HRP)– conjugated secondary antibodies were from Bio-Rad Japan (Tokyo, Japan). Protease inhibitor cocktail, aprotinin, and leupeptin were from Roche Molecular Biochemicals Japan (Tokyo, Japan). pGCDNsam ires-EGFP retroviral vector was from Dr M. Onodera (Tsukuba University, Tsukuba, Japan). cDNA of flag-tagged murine WAVE2 obtained by polymerase chain reaction (PCR) method was inserted into NotI at 5′ and BamHI at 3′ in front of ires-EGFP of the retroviral vector, and sequence was confirmed. The FG12 lentiviral vector was from Drs X. Qin and D. Baltimore (California Institute of Technology, Pasadena, CA).29,32 An efficient sequence in the pSuper vector (Oligo Engine, Seattle, WA) was selected and confirmed by Western blotting as causing complete abolition of protein expression in mouse embryonic fibroblast (MEF) or A431 cells. Determined shRNA sequence was digested with XbaI at 5′ and XhoI at 3′ in the pSuper vector and inserted it into the FG12 transgene vector. We followed published protocols for use of WAVE1 and WAVE2 siRNAs9 and of Abi1 and IRSp53 siRNAs.31 Control small interfering RNA (siRNA) was selected in the pSuper vector with a sequence that causes no reduction of protein expression in MEF cells.31 Viruses were produced by calcium phosphate transfection of 293T cells and viral titers were determined as described.28,29
Cell culture
ES cells (E14.1) were grown on irradiated MEF cells, allowed to differentiate into MKs, and virally infected as described.28,29 Embryoid body (EB) formation was achieved as described.33 Integrin αIIb subunit (αIIb)+c-Kit+ cells derived from EBs of day 5 or day 6 were sorted by flow cytometry (MoFlo; DAKO-Cytomation), and 3 × 104 αIIb+c-Kit+ cells per well were seeded onto subconfluent OP9 stromal cells in a 6-well plate. Fetal liver cells (E13.5) were isolated and washed with PBS twice and seeded (105 cells per well) onto 24-well culture dishes with 100 ng/mL TPO-containing medium. Viral infection was achieved by the multiplicity of infection of 10 for 14 hours.
Quantification of mature MKs and platelets
To determine precise numbers of ES cell–derived MKs and of released platelets, the cells were labeled with APC-conjugated anti-αIIb and PE-conjugated anti-GPIbα antibodies. Cells were washed twice with 3% fetal bovine serum, resuspended with hypotonic propidium iodide (PI) solution (5 μg/mL in 3% fetal bovine serum), and were mixed with True Count Beads (BD/Invitrogen Japan). The number of αIIb+GPIbα+ items (cells and beads) was counted by flow cytometry (Aria; BD Japan, Tokyo), and cell numbers were calculated based upon known bead counts.
Transplantation
WAVE1+/+, WAVE1+/−, or WAVE1−/− (Ly 5.2) fetal liver cells (E13.5) were injected (107 cells) into irradiated (960 cGy) mice (Ly 5.1) aged 7 weeks. Peripheral blood counts were analyzed 10 weeks after transplantation. Percentage of leukocyte chimerism was determined from CD45.2/CD45.1 expression ratio.
Preparation of blood samples
Blood samples were obtained from each mouse under conditions of CO2 exposure. Platelets were prepared as described34,35 for studies of specific fibrinogen binding to integrin αIIbβ3 or on platelet spreading. The platelet sediment was resuspended in an appropriate volume of modified Tyrode-HEPES buffer, pH 7.4 (10 mM HEPES, 12 mM NaHCO3, 138 mM NaCl, 5.5 mM glucose, 2.9 mM KCl, and 1 mM MgCl2) without Ca2+.34 Aliquots of platelet lysates and of spleen lysates were used for Western blotting to confirm genotypes (WAVE1 and WAVE3).
Fibrinogen-binding (activation of integrin αIIbβ3) by various agonists
Image acquisition for platelet and MK spreading
All confocal studies were performed using a confocal system (Radiance 2000; BioRad Lab, Hercules, CA, or Leica TCS SP2, Wetzler, Germany) as described.34,35 A 63×/1.40 NA oil-immersion objective (Leica) was used. Images were assembled with Adobe Photoshop (Adobe Systems, San Jose, CA). Time-lapse images were taken through a phase-contrast microscope (Axiovert S100; Carl Zeiss MicroImaging, Göttingen, Germany). A 40×/1.30 NA FLUAR oil-immersion objective (Carl Zeiss MicroImaging) was used. Analyses were performed with National Institutes of Health [NIH] J-Image software (Bethesda, MD).
Statistical analysis
Experimental differences versus controls were analyzed by Student t test. P values less than .05 were considered significant.
Results
Expression of WAVE1 and WAVE2 in ligand-adherent MKs as a model system relevant to platelet adhesion and spreading
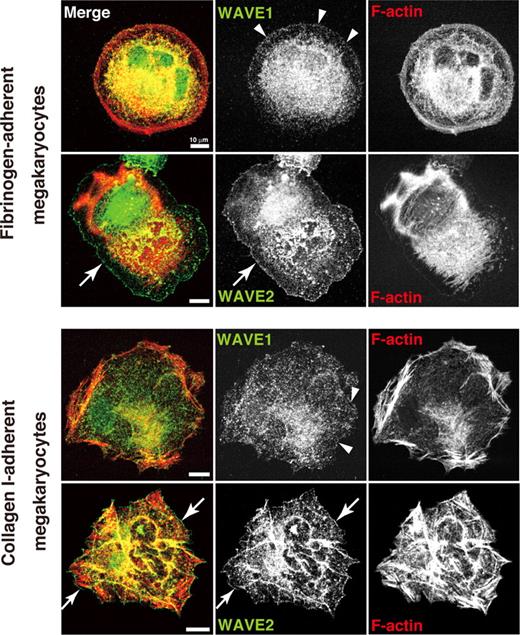
We recently localized WAVEs in spreading human platelets.25 To compare localization pattern between platelets and megakaryocytes, ES cell–derived MKs adherent to their major ligands, fibrinogen or collagen I, were used. WAVE1 was expressed diffusely at both cytoplasm and peripheral edges (arrowheads) in both fibrinogen-adherent MKs and collagen I–adherent MKs (Figure 1). WAVE2, by contrast, was more readily distinguished at the spreading cell fringe (arrows) in MKs adherent to either fibrinogen or collagen I (Figure 1). These results of peripheral fringe were consistent with our results in human platelets.25 These responses depended on integrin αIIbβ3 because MK adhesion to fibrinogen was blocked by antibody 1B5. Our results indicate that WAVE2 may preferentially participate in peripheral Arp2/3 nucleation at lamellipodial protrusion in either an integrin αIIbβ3- or α2β1/GPVI-dependent fashion.25,36
Expression and distribution of WAVE1 and WAVE2 in fibrinogen- and collagen-adherent MKs derived from ES cells. On day 12 of the differentiation protocol, MKs were plated onto 100 μg/mL fibrinogen, or onto 10 μg/mL collagen I, and allowed to stand for 60 minutes. Cells were fixed, permeabilized, and stained with rhodamine-conjugated phalloidin to mark F-actin (red) and with anti-WAVE1 or anti-WAVE2 antibodies followed by Alexa 488 (green), and analyzed by confocal microscopy. Scale bar equals 10 μm. See “Image acquisition for platelet and MK spreading” for complete image acquisition information.
Expression and distribution of WAVE1 and WAVE2 in fibrinogen- and collagen-adherent MKs derived from ES cells. On day 12 of the differentiation protocol, MKs were plated onto 100 μg/mL fibrinogen, or onto 10 μg/mL collagen I, and allowed to stand for 60 minutes. Cells were fixed, permeabilized, and stained with rhodamine-conjugated phalloidin to mark F-actin (red) and with anti-WAVE1 or anti-WAVE2 antibodies followed by Alexa 488 (green), and analyzed by confocal microscopy. Scale bar equals 10 μm. See “Image acquisition for platelet and MK spreading” for complete image acquisition information.
Lack of WAVE2 affects MK development and subsequent platelet production in vitro
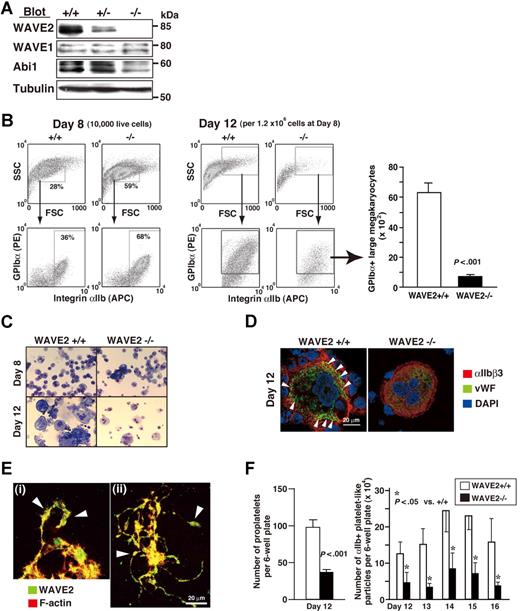
Mice deficient in WAVE2 die within E11.5 to E12.5,27 and hence we generated WAVE2-null (WAVE2−/−) ES cells as described.37 We constructed a novel vector incorporating puromycin resistance based upon an original targeting vector,27 and successfully induced homologous recombination in WAVE2+/− ES cells (E14.1 ES line27 ). As demonstrated on Western blotting, WAVE2 was completely depleted without affecting WAVE1 expression (Figure 2A). Abi1 levels were reduced in lysates from WAVE2−/− EB at day 5 (Figure 2A), indicating that the WAVE2/Abi1 complex, but not WAVE1/Abi1, might be reduced. Results were consistent with our previous demonstration in human platelets that WAVE1 or WAVE2 is bound with Abi1 constitutively.25 Our present results also indicate that the WAVE2/Abi1 complex is not required for growth of ES cells on feeder cells.37 A selected clone no. 3 of WAVE2−/− ES cells showed a normal karyotype (data not shown) and used mainly this clone for further studies. WAVE2−/− ES cell– and WAVE2+/+ ES cell–derived EB formation recapitulated similar early hematopoiesis, as assessed by expression of the hemangioblast marker Flk-1+ and of the hematopoietic markers αIIb+ and CD45+, using flow cytometry (data not shown). In order further to address whether WAVE2 is required for MK development and subsequent proplatelet formation,16,18 we observed megakaryocytopoiesis during coculture with OP9 stromal cells and TPO.28,30 WAVE2−/− ES cells were capable of generating differentiated small- to middle-sized MKs by day 8 (Figure 2B). Unexpectedly, further differentiation with increased cell size after day 8 of culture on OP9 stroma plus TPO, IL-6, and IL-1128 was much impaired in WAVE2−/− MKs compared with WAVE2+/+ or WAVE2+/− MKs, as evidenced by numbers of viable large MKs on flow cytometry (Figure 2B right graph; P < .001). Wright-Giemsa staining of MKs on day 12 (Figure 2C) or expression and localization pattern of VWF in MKs on day 12 (Figure 2D) also suggested that the deletion of WAVE2 might block cytoplasmic maturation.
MKs derived from ES cells deficient in WAVE2. (A) Immunoblots of WAVE2, WAVE1, Abi1, and tubulin from lysates of day-5 embryoid bodies (EBs). To avoid contamination with mouse feeder fibroblasts, day-0 ES cells, were sorted with c-Kit+ cells, applied to EB media,33 and cultured for 5 days. (B) Flow cytometric dotplots of side and forward scatter profiles of 10 000 live cells on day 8, and expression profiles of αIIb and GPIbα stained cells (day 8), as described previously.28,29 On day 12, all cells collected from a 6-well culture plate were stained with anti-αIIb, anti-GPIbα, and propidium iodide and were mixed with True Count Beads. WAVE2+/+, WAVE2+/−, or WAVE2−/− cells and beads were then subjected to flow cytometry. Representative dot plots show all viable cells from WAVE2+/+ and WAVE2−/− plates. Quantification for GPIbα+ cells in an arbitrary gate was performed as described in “Materials and methods.” The graph on the right shows means (± SD; n = 3). (C) On day 8 or day 12, weakly adherent or floating cells28,29 were subjected to cytospin preparation and to staining by Wright-Giemsa technique. WAVE2−/− MKs showed decreased number of large sized cells. (D) On day 12, live cells were fixed following cytospin preparation. Cells were permeabilized, stained with anti-VWF followed by rabbit Alexa 488 (green), and anti-αIIb followed by mouse Alexa 568 (red) with nuclei marked by 4,6-diamino-2-phenylindole (DAPI) staining (blue). WAVE2−/− MK retained VWF expression (diffuse but decreased) in cytoplasm while VWF expression in WAVE2+/+ MK was distributed clearly along the plasma membrane in peripheral cytoplasm (◀). (E) On day 11 (i) or day 12 (ii), proplatelet-exhibiting MKs derived from normal ES cells were fixed and plated on poly-L-lysine–coated cover glasses. Cells were permeabilized and were stained with rhodamine-conjugated phalloidin to mark F-actin (red) and with anti-WAVE2 antibody followed by Alexa 488 (green). Arrowheads indicate swellings. See “Image acquisition for platelet and MK spreading” for complete image acquisition information. (F) The left figure shows means (± SD; n = 3) of total number of proplatelets in a 6-well plate on day 12. The right figure shows means (± SD; n = 3) of number of αIIb+ particles within the same flow cytometry gate as that used for murine platelets. Quantification was performed using True Count Beads as described in “Quantification of mature MKs and platelets.”
MKs derived from ES cells deficient in WAVE2. (A) Immunoblots of WAVE2, WAVE1, Abi1, and tubulin from lysates of day-5 embryoid bodies (EBs). To avoid contamination with mouse feeder fibroblasts, day-0 ES cells, were sorted with c-Kit+ cells, applied to EB media,33 and cultured for 5 days. (B) Flow cytometric dotplots of side and forward scatter profiles of 10 000 live cells on day 8, and expression profiles of αIIb and GPIbα stained cells (day 8), as described previously.28,29 On day 12, all cells collected from a 6-well culture plate were stained with anti-αIIb, anti-GPIbα, and propidium iodide and were mixed with True Count Beads. WAVE2+/+, WAVE2+/−, or WAVE2−/− cells and beads were then subjected to flow cytometry. Representative dot plots show all viable cells from WAVE2+/+ and WAVE2−/− plates. Quantification for GPIbα+ cells in an arbitrary gate was performed as described in “Materials and methods.” The graph on the right shows means (± SD; n = 3). (C) On day 8 or day 12, weakly adherent or floating cells28,29 were subjected to cytospin preparation and to staining by Wright-Giemsa technique. WAVE2−/− MKs showed decreased number of large sized cells. (D) On day 12, live cells were fixed following cytospin preparation. Cells were permeabilized, stained with anti-VWF followed by rabbit Alexa 488 (green), and anti-αIIb followed by mouse Alexa 568 (red) with nuclei marked by 4,6-diamino-2-phenylindole (DAPI) staining (blue). WAVE2−/− MK retained VWF expression (diffuse but decreased) in cytoplasm while VWF expression in WAVE2+/+ MK was distributed clearly along the plasma membrane in peripheral cytoplasm (◀). (E) On day 11 (i) or day 12 (ii), proplatelet-exhibiting MKs derived from normal ES cells were fixed and plated on poly-L-lysine–coated cover glasses. Cells were permeabilized and were stained with rhodamine-conjugated phalloidin to mark F-actin (red) and with anti-WAVE2 antibody followed by Alexa 488 (green). Arrowheads indicate swellings. See “Image acquisition for platelet and MK spreading” for complete image acquisition information. (F) The left figure shows means (± SD; n = 3) of total number of proplatelets in a 6-well plate on day 12. The right figure shows means (± SD; n = 3) of number of αIIb+ particles within the same flow cytometry gate as that used for murine platelets. Quantification was performed using True Count Beads as described in “Quantification of mature MKs and platelets.”
MKs elaborate proplatelets before releasing platelets. Proplatelet formation reportedly initiates through actin polymerization.16,18 Our MK culture system permits expansion of the actin-regulated cytosolic membrane (the DMS), and yields proplatelets in ES cell–derived MKs.28 To focus on the relationship between the DMS and WAVE2 localization, we studied costaining of WAVE2 and F-actin in proplatelets generated by OP9 coculture. Coexpression of WAVE2 in the cytosolic membrane and of actin filaments in the subjacent cytoplasm was preserved. WAVE2 was further detected in the swelling (of elongated pseudopods or proplatelets; Figure 2E). These data allowed us to consider the possibility that WAVE2 contributes both to cytoplasmic maturation of MKs and to platelet release initiated by cytoskeletal reorganization.16,18 Hence, we counted proplatelets and αIIb+ platelets during OP9 coculture. In contrast to early megakaryocytopoiesis (by day 8 of culture; Figure 2B), later maturation of MKs was impaired, as evidenced by reduced numbers of proplatelets and platelets in the absence of WAVE2 after day 12 (Figure 2F). These results indicate that impaired maturation in WAVE2−/− ES cell–derived megakaryocytopoiesis may affect reduced thrombopoiesis.
Requirement of WAVE2 for the generation of lamellipodia through αIIbβ3-mediated signaling
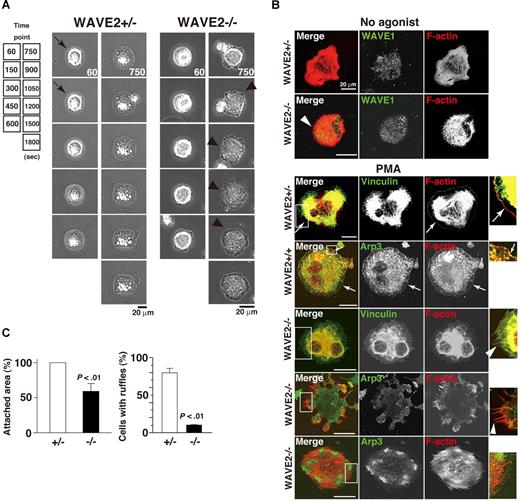
As WAVE2-null mice die in utero, precluding use of WAVE2−/− ES cell–derived platelets, MKs became our model for studies of platelet spreading.28 We examined real-time membrane dynamics during cell adhesion on immobilized fibrinogen. PMA directly stimulates both protein kinase C (PKC) in maintenance of integrin αIIbβ3 activation by inside-out signaling38,39 and facilitation of αIIbβ3 outside-in signaling to the actin cytoskeleton in MKs and platelets.28,34,38,40 In addition, PKCα promotes proplatelet production.41 For these reasons, PMA was primarily used for further experiments. In time-lapse studies of spreading, wild-type control (WAVE2+/+) or WAVE2+/− MKs quickly exhibited filopodia, followed by sustained external extension of peripheral lamellae and rapid spreading upon a fibrinogen matrix (Figure 3A left panel). In sharp contrast, WAVE2−/− MKs persistently extended and retracted filopodia formation without peripheral lamellipodial ruffling (Figure 3A right panel). Similar results were obtained in the presence of the G-protein–coupled receptor agonist, ADP (100 and 500 μM; not shown). Elimination of WAVE2 did not affect the expression and distribution of WAVE1 when MKs spread on fibrinogen (Figure 3B). In Figure 3B's bottom panels, to clearly observe integrin-mediated lamellipodial ruffling, vinculin (integrin-tethered cytoskeletal protein) or Arp3 (component of the Arp2/3 actin complex) was stained. WAVE2−/− MKs displayed sustained filopodial projections over internal stress fibers in the absence or presence of PMA (Figure 3B arrowheads). By contrast, WAVE2+/+ MKs or WAVE2+/− MKs showed peripheral lamellipodia when spread on fibrinogen (arrows; Figure 3B). Analyzed results are shown in Figure 3C. A defect of normal lamellipodia formation by WAVE2−/− MKs was rescued by retroviral transduction of WAVE2 (data not shown). This indicates that WAVE2 is required for normal lamellipodia morphology and wide spreading in αIIbβ3 outside-in–based actin polymerization in ES cell–derived MKs, as in primary fibroblasts.9,27
WAVE2 is required for lamellipodial protrusion in fibrinogen-adherent MKs. (A) Time-lapse record of spreading of fibrinogen-adherent ES cell–derived WAVE2+/− and WAVE2−/− MKs in the presence of 100 nM phorbol myristate acetate (PMA). A WAVE2−/− MK displays enhanced dorsal ruffling and peripheral filopodial projection (arrowheads), while WAVE2+/− MKs transiently shows filopodia within 5 minutes after adhesion (arrows) and lamellipodial formation thereafter. Scale bar equals 20 μm. (B) MKs on day 12 were plated onto fibrinogen for 60 minutes in the absence of an agonist (top 2 cells). In another set, MKs were plated for 120 minutes in the absence or presence of 100 nM PMA (bottom 5 cells). Cells were fixed, permeabilized, and stained with rhodamine-conjugated phalloidin to mark F-actin (red) and with anti-WAVE1, antivinculin, or anti-Arp3 antibodies followed by Alexa 488 (green), and analyzed by confocal microscopy (see “Image acquisition for platelet and MK spreading” for complete image acquisition information). Scale bar equals 20 μm. Arrows indicate peripheral normal lamellipodia formation. Arrowheads indicate filopodia-dominant morphology. (C) A total of 50 cells (WAVE2+/−, □; or WAVE2−/−, ■) were analyzed for attachment area and for the number of cells with lamellipodia-dominant morphology after 120 minutes incubation in the presence of PMA. Data are means (± SD).
WAVE2 is required for lamellipodial protrusion in fibrinogen-adherent MKs. (A) Time-lapse record of spreading of fibrinogen-adherent ES cell–derived WAVE2+/− and WAVE2−/− MKs in the presence of 100 nM phorbol myristate acetate (PMA). A WAVE2−/− MK displays enhanced dorsal ruffling and peripheral filopodial projection (arrowheads), while WAVE2+/− MKs transiently shows filopodia within 5 minutes after adhesion (arrows) and lamellipodial formation thereafter. Scale bar equals 20 μm. (B) MKs on day 12 were plated onto fibrinogen for 60 minutes in the absence of an agonist (top 2 cells). In another set, MKs were plated for 120 minutes in the absence or presence of 100 nM PMA (bottom 5 cells). Cells were fixed, permeabilized, and stained with rhodamine-conjugated phalloidin to mark F-actin (red) and with anti-WAVE1, antivinculin, or anti-Arp3 antibodies followed by Alexa 488 (green), and analyzed by confocal microscopy (see “Image acquisition for platelet and MK spreading” for complete image acquisition information). Scale bar equals 20 μm. Arrows indicate peripheral normal lamellipodia formation. Arrowheads indicate filopodia-dominant morphology. (C) A total of 50 cells (WAVE2+/−, □; or WAVE2−/−, ■) were analyzed for attachment area and for the number of cells with lamellipodia-dominant morphology after 120 minutes incubation in the presence of PMA. Data are means (± SD).
Differential roles for WAVE1 and WAVE2 in MK spreading
To address differences between contributions of WAVE1 and of WAVE2 to αIIbβ3-mediated spreading features, we observed the morphology on immobilized fibrinogen of spreading MKs transduced with short hairpin RNA (shRNA) and, as a marker, with GFP. We have used a lentiviral vector with ubiquitin C–regulated EGFP expression and RNA polymerase III–regulated shRNA to knock-down genes of interest in ES cell–derived MKs as reported.29 The same strategy was used to knock-down WAVE1 or WAVE2 in ES cell–derived MKs. GFP-expressing smaller-sized MKs (on day 9 of culture) were partly sorted by flow cytometry, and a lysate of sorted cells was subjected to SDS–gel electrophoresis and Western blotting to explore whether the expression level of WAVE1 or of WAVE2 was knocked down. GFP-expressing cells transduced with shRNA of WAVE1 or WAVE2, but not with that of a scrambled control, showed reduced WAVE1/WAVE2 expression (Figure 4A right). ES cell–derived MK progenitors not subjected to sorting were further cultured until day 12 as described.28 Scrambled control virus–transduced MKs showed wide spreading with thick actin filaments when stimulated with PMA to promote lamellipodial ruffles (Figure 4A). By comparison, a single administration of WAVE2 shRNA induced “webfoot” formation (filopodial projections with retracted peripheral membranes) even after the addition of PMA (Figure 4Ai,ii arrowheads). The morphologic features of MKs transduced with WAVE2 shRNA exhibited a defect of normal lamellipodia on fibrinogen. However, the knockdown of WAVE1 did not impair lamellipodial ruffling, but led to decreased thickness of lamellae (Figure 4Aiii,iv arrows). In 40 GFP-expressing cells and 40 cells that did not express GFP, transduction with WAVE2 shRNA significantly shifted morphology from lamellipodia rich to cells that are filopodia dominant (Figure 4B). Although a single transduction with WAVE2 shRNA did not promote dissociation from an immobilized-fibrinogen matrix, as assessed by washing, additional transduction of WAVE2−/− MKs with WAVE1 shRNA facilitated dissociation compared with dissociation in untreated WAVE2−/− MKs (P < .01; Figure 4C). Interestingly, fibrinogen-adherent MKs lacking both WAVE2 and WAVE1 formed small filopodia with partial defects in actin filaments, changes distinct from features observed in control MKs or WAVE2−/− MKs (Figure 4D). These results suggest that while WAVE2 is preferentially required to form normal lamellipodial protrusion, WAVE1 plays a role in supporting full spreading on fibrinogen, with αIIbβ3-mediated formation of thick actin bundles in MKs. To address this hypothesis regarding WAVE1, the next study was designed.
Differential roles of WAVE1 and WAVE2 in integrin-mediated lamellipodia formation in ES cell–derived MKs. (A) MK progenitors were infected with lentivirus containing shRNA on day 5 for 14 hours. Sorted αIIb+ c-Kit+ cells were further cultured with OP9 stromal cells. Progenitors expressing GFP on day 9 were sorted and lysed. The lysate was subjected to SDS–gel electrophoresis on Western blotting. On day 12, MKs were plated on fibrinogen for 60 minutes (i, iii) or 120 minutes (ii, iv) in the presence of 100 nM PMA, and fixed and stained with rhodamine-conjugated phalloidin to mark F-actin. (B) GFP+ or GFP− cells transduced with control shRNA, WAVE1 shRNA, or WAVE2 shRNA were assessed by the significance of morphology. A total of 4 independent experiments were performed. (C) MKs on fibrinogen were fixed after washing with PBS once, twice, or 3 times and were stained with αIIb. Increased numbers of washing procedure promoted dissociation of MKs. Data are means (± SD; n = 3). (D) Control MKs or WAVE2−/− MKs transduced with shRNA of scrambled sequence and GFP, and 2 representative fibrinogen-adherent WAVE2−/− MKs transduced with shRNA of WAVE1 and GFP (i,ii) are shown.
Differential roles of WAVE1 and WAVE2 in integrin-mediated lamellipodia formation in ES cell–derived MKs. (A) MK progenitors were infected with lentivirus containing shRNA on day 5 for 14 hours. Sorted αIIb+ c-Kit+ cells were further cultured with OP9 stromal cells. Progenitors expressing GFP on day 9 were sorted and lysed. The lysate was subjected to SDS–gel electrophoresis on Western blotting. On day 12, MKs were plated on fibrinogen for 60 minutes (i, iii) or 120 minutes (ii, iv) in the presence of 100 nM PMA, and fixed and stained with rhodamine-conjugated phalloidin to mark F-actin. (B) GFP+ or GFP− cells transduced with control shRNA, WAVE1 shRNA, or WAVE2 shRNA were assessed by the significance of morphology. A total of 4 independent experiments were performed. (C) MKs on fibrinogen were fixed after washing with PBS once, twice, or 3 times and were stained with αIIb. Increased numbers of washing procedure promoted dissociation of MKs. Data are means (± SD; n = 3). (D) Control MKs or WAVE2−/− MKs transduced with shRNA of scrambled sequence and GFP, and 2 representative fibrinogen-adherent WAVE2−/− MKs transduced with shRNA of WAVE1 and GFP (i,ii) are shown.
Roles of WAVE1 in lamellipodia formation mediated by αIIbβ3 in platelets from chimeric mice
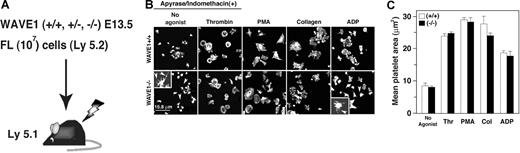
As WAVE1-null mice die soon after birth, precluding their use as a source of WAVE1-null platelets, we generated chimeric mice that had WAVE1-null platelets.9 Sublethally irradiated mice were injected with 107 cells obtained from E13.5 WAVE1+/+, WAVE1+/−, or WAVE1−/− FL. Peripheral blood counts were performed 10 weeks after transplantation. No statistically significant differences among these 3 groups (n = 6 each) were found in leukocyte, erythrocyte, or platelet counts (Figure 5A; Table 1). These results imply that WAVE1 may not participate in vivo in the principal processes of major hematopoiesis, although this has not been studied in separated populations of leukocytes.
Analysis of chimeric mice generated by liver transplantation from WAVE1−/− embryos. (A) EDTA-treated peripheral blood was obtained from live chimeric mice. (B) Washed platelets from WAVE1+/+, WAVE1+/−, or WAVE1−/− mice were plated onto fibrinogen-coated coverslips for 45 minutes in the presence of apyrase (2 U/mL) and indomethacin (10 μM) with or without 1 U/mL thrombin. Some preparations included 100 nM PMA, 10 μg/mL collagen I, or 100 μM ADP. Cells were fixed, permeabilized, and stained with rhodamine-conjugated phalloidin to mark F-actin and analyzed by confocal microscopy (see “Image acquisition for platelet and MK spreading” for complete image acquisition information. Scale bar equals 15.8 μm. This experiment is representative of 4 thus performed (from 2 independent transplantations). (C) Platelet spreading was quantified by computer analysis of platelet surface areas. Each bar represents at least 100 platelets analyzed as described in “Image acquisition for platelet and MK spreading.” The results shown are means (± SD).
Analysis of chimeric mice generated by liver transplantation from WAVE1−/− embryos. (A) EDTA-treated peripheral blood was obtained from live chimeric mice. (B) Washed platelets from WAVE1+/+, WAVE1+/−, or WAVE1−/− mice were plated onto fibrinogen-coated coverslips for 45 minutes in the presence of apyrase (2 U/mL) and indomethacin (10 μM) with or without 1 U/mL thrombin. Some preparations included 100 nM PMA, 10 μg/mL collagen I, or 100 μM ADP. Cells were fixed, permeabilized, and stained with rhodamine-conjugated phalloidin to mark F-actin and analyzed by confocal microscopy (see “Image acquisition for platelet and MK spreading” for complete image acquisition information. Scale bar equals 15.8 μm. This experiment is representative of 4 thus performed (from 2 independent transplantations). (C) Platelet spreading was quantified by computer analysis of platelet surface areas. Each bar represents at least 100 platelets analyzed as described in “Image acquisition for platelet and MK spreading.” The results shown are means (± SD).
Recently, McCarty et al have demonstrated that in platelets, Rac1 is a primary participant in assembly of lamellipodia, but not in these agonist-induced integrin activation (inside-out signaling).26 WAVE1, as well as WAVE2, has been shown to function downstream of Rac1 in primary fibroblasts.9 The same signaling cascade may also exist in platelets.25 To elucidate whether the absence of WAVE1 affects the appearance of lamellipodia in platelet spreading on fibrinogen, or agonist-induced integrin activation in platelets, we initially performed confocal microscopic examination. In order to avoid stimulation by released ATP/ADP/thromboxane A2, some experiments were performed in the presence of apyrase and indomethacin.26 The mean spreading area on immobilized fibrinogen in the absence or presence of thrombin (1 U/mL), ADP (100 μM), or PMA (100 nM) in WAVE1−/− platelets was comparable with that in WAVE1+/+ platelets (Figure 5B,C). When stimulated with collagen I (10 μg/mL), WAVE1−/− platelets appeared to spread less on fibrinogen than did control platelets, but no statistically significant difference was found (Figure 5B,C). Lamellipodia formation also developed normally when WAVE1−/− platelets were stimulated with thrombin, PMA, or collagen. Interestingly, WAVE1−/− platelets formed abnormally long filopodia, as demonstrated in most “no-agonist” and partial “ADP-stimulated” platelets that were allowed to spread on fibrinogen (Figure 5B arrowheads). On the other hand, integrin activation by such “inside-out signaling” was completely normal in WAVE1−/− platelets (data not shown). In platelets, then, WAVE1 may not play a major role in integrin activation and in αIIbβ3-mediated actin reorganization in the absence or presence of major activators during spreading.26
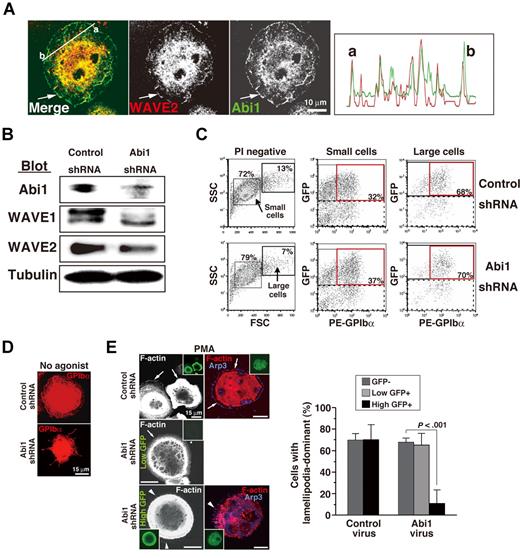
Roles of Abi1, the WAVE-associated complex, in MK terminal differentiation and in spreading by MKs on immobilized fibrinogen
A sequential pathway composed of Rac, IRSp53, and the WAVE2/Abi1 complex is essential for generation of lamellipodia in epithelial cells that predominantly express WAVE2.31 This signaling cascade may be the same as that in MKs and platelets.25 In other cells, the large protein complex consisting of PIR121 (Sra1), Nap1 (or, in hematopoietic cells and their descendants only, Hem-1), HSPC300, and Abi1 has been proposed to stabilize WAVE.10-14 Among these, Abi1 and HSPC300 bind directly to the WHD domain of WAVE2, with Abi1 determining recruitment of WAVE2 into the lamellipodial leading edge42 and the activation of WAVE2.14 Abi1 and WAVE2 consistently are colocalized in peripheral lamellae (Figure 6A arrow left and right panels) and in cytosol (and possibly nuclei) of fibrinogen-adherent MKs that have undergone spreading on fibrinogen.25 RNAi-mediated knockdown of Abi1 by virus transduction in ES cell–derived progenitors led to reduced expression of Abi1, along with predominantly reduced expression of WAVE1 rather than of WAVE2 (Figure 6B). Interestingly, even though deregulated WAVE2 expression was not prominent following transduction of Abi1 shRNA, the knockdown of Abi1 led to a decrease of live MKs, as demonstrated by reduced numbers of large-sized MKs that were GFP+GPIbα+ PI− (P < .01; Figure 6C). In addition, the transduction of Abi1 shRNA showed impaired lamellipodial ruffles on fibrinogen in the absence (Figure 6D) or presence (Figure 6E) of PMA. These phenotypes appeared to recapitulate those of WAVE2−/− MKs or MK-transduced WAVE2 siRNA, although WAVE2 expression was not completely ablated. We noticed that most cells that expressed GFP with high intensity began to stop growing 48 hours after transduction of Abi1 shRNA but not of control shRNA, suggesting that complete abolition of Abi1 expression may impair cell growth. To eliminate Abi1 expression completely in viable blood cells may be impossible; we could only reduce Abi1 expression enough to reduce WAVE1 expression compared with WAVE2 reduction. This is somewhat surprising in view of previous studies using HeLa cells and Drosophila S2 cells, in which the expression of WAVE2 depends upon that of Abi1.11,12 Zipfel et al had demonstrated, however, that knockdown of Abi1 (even with Abi2) in Jurkat T cells did not significantly reduce the expression of WAVE2.43 The mechanisms in T cells or MKs remain to be examined and discussed.
Involvement of Abi1 in megakaryocytopoiesis and spreading. (A) Colocalization of WAVE2 and Abi1 in a fibrinogen-adherent ES cell–derived MK in the presence of 100 nM PMA (arrows). Right graph shows the intensities of WAVE2 expression (red) or Abi1 expression (green) between “a” and “b” by NIH J-Image. (B) Immunoblots of Abi1, WAVE1, WAVE2, and tubulin from lysates during early megakaryocytopoiesis. (C) Flow cytometric dot plots of side and forward scatter profiles of 10 000 viable cells staining with anti-GPIbα (PE) antibody on day 12. Large MKs transduced with Abi1 shRNA appeared fewer than the number of large control MKs. (D,E) On day 12, ES cell–derived MKs were plated on fibrinogen for 60 minutes in the absence (D) or presence (E) of 100 nM PMA and were analyzed as depicted. (D) GPIbα expression by MKs in which Abi1 shRNA or scrambled control shRNA was transduced. (E) Arrows indicate peripheral normal lamellipodia. Arrowheads indicate filopodia-rich regions. GFP+ or GFP− cells transduced with control shRNA or Abi1 shRNA were assessed for lamellipodia-dominant morphology in the presence of PMA. A total of 3 independent experiments were performed. See “Image acquisition for platelet and MK spreading” for complete image acquisition information.
Involvement of Abi1 in megakaryocytopoiesis and spreading. (A) Colocalization of WAVE2 and Abi1 in a fibrinogen-adherent ES cell–derived MK in the presence of 100 nM PMA (arrows). Right graph shows the intensities of WAVE2 expression (red) or Abi1 expression (green) between “a” and “b” by NIH J-Image. (B) Immunoblots of Abi1, WAVE1, WAVE2, and tubulin from lysates during early megakaryocytopoiesis. (C) Flow cytometric dot plots of side and forward scatter profiles of 10 000 viable cells staining with anti-GPIbα (PE) antibody on day 12. Large MKs transduced with Abi1 shRNA appeared fewer than the number of large control MKs. (D,E) On day 12, ES cell–derived MKs were plated on fibrinogen for 60 minutes in the absence (D) or presence (E) of 100 nM PMA and were analyzed as depicted. (D) GPIbα expression by MKs in which Abi1 shRNA or scrambled control shRNA was transduced. (E) Arrows indicate peripheral normal lamellipodia. Arrowheads indicate filopodia-rich regions. GFP+ or GFP− cells transduced with control shRNA or Abi1 shRNA were assessed for lamellipodia-dominant morphology in the presence of PMA. A total of 3 independent experiments were performed. See “Image acquisition for platelet and MK spreading” for complete image acquisition information.
Novel signaling regulates MK terminal differentiation/maturation and proplatelet production
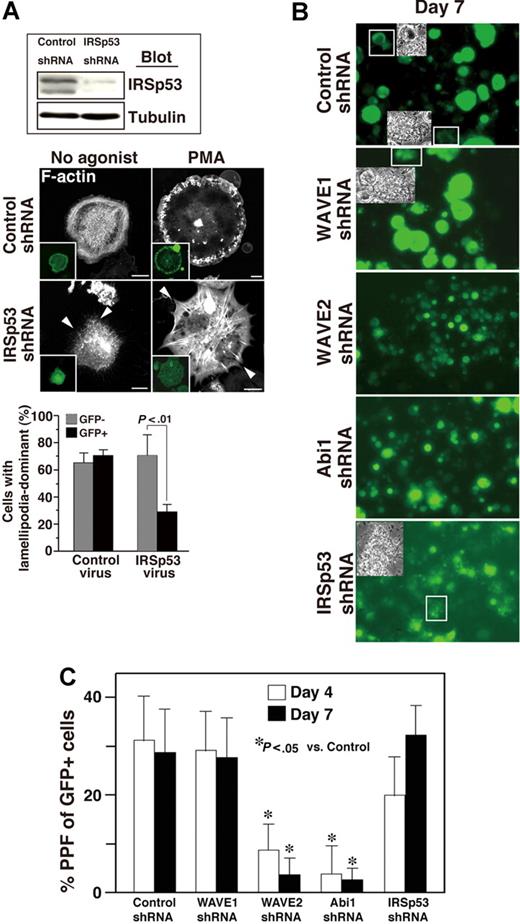
ES cell–derived MKs in which expression of IRSp53 was reduced by shRNA spread poorly and appeared filopodia-rich on fibrinogen-coated surfaces irrespective of PMA stimulation (Figure 7A), suggesting that both IRSp53 and the WAVE2/Abi1 complex are required for αIIbβ3-based normal lamellipodia morphology in these cells.
IRSp53 may be associated with Rac-dependent lamellipodia formation in adherent MKs but not with Rac-independent MK maturation. (A) ES cell–derived progenitors expressing GFP via control or IRSp53 knockdown were sorted. The lysate was subjected to SDS–gel electrophoresis on Western blotting. On day 12 of the differentiation protocol, ES cell–derived MKs transduced with shRNA of IRSp53 and GFP were plated onto 100 μg/mL fibrinogen for 60 minutes. IRSp53 knockdown cells exhibited filopodia-dominant features in the absence or presence of 100 nM PMA (arrowheads), while control megakaryocytes displayed peripheral lamellipodial ruffles. Scale bar equals 20 μm. Summarized results are the same as those in Figure 6E. Data are means (± SD). (B,C) Cells were prepared from E13.5 FL as described in “Cell culture.” Cells were exposed for 14 hours to a lentivirus encoding both several shRNAs and GFP on day 0. When studied on day 4, approximately 40% of the cells exposed to virus were successfully transduced, as assessed by GFP expression (not shown). The transduction of WAVE2 or Abi1 shRNA reduced (B) MK size and (C) proplatelet numbers throughout observation until day 7 of culture. Insets in panel B depict the morphology of proplatelets expressing GFP. This experiment is representative of 3 performed. Magnification, ×40. Numbers of proplatelets from FL-derived primary MKs on day 4 and day 7 (C). Data are means (± SD). *P < .05 versus control for day 4 or day 7. See “Image acquisition for platelet and MK spreading” for complete image acquisition information.
IRSp53 may be associated with Rac-dependent lamellipodia formation in adherent MKs but not with Rac-independent MK maturation. (A) ES cell–derived progenitors expressing GFP via control or IRSp53 knockdown were sorted. The lysate was subjected to SDS–gel electrophoresis on Western blotting. On day 12 of the differentiation protocol, ES cell–derived MKs transduced with shRNA of IRSp53 and GFP were plated onto 100 μg/mL fibrinogen for 60 minutes. IRSp53 knockdown cells exhibited filopodia-dominant features in the absence or presence of 100 nM PMA (arrowheads), while control megakaryocytes displayed peripheral lamellipodial ruffles. Scale bar equals 20 μm. Summarized results are the same as those in Figure 6E. Data are means (± SD). (B,C) Cells were prepared from E13.5 FL as described in “Cell culture.” Cells were exposed for 14 hours to a lentivirus encoding both several shRNAs and GFP on day 0. When studied on day 4, approximately 40% of the cells exposed to virus were successfully transduced, as assessed by GFP expression (not shown). The transduction of WAVE2 or Abi1 shRNA reduced (B) MK size and (C) proplatelet numbers throughout observation until day 7 of culture. Insets in panel B depict the morphology of proplatelets expressing GFP. This experiment is representative of 3 performed. Magnification, ×40. Numbers of proplatelets from FL-derived primary MKs on day 4 and day 7 (C). Data are means (± SD). *P < .05 versus control for day 4 or day 7. See “Image acquisition for platelet and MK spreading” for complete image acquisition information.
We also asked if deprivation of WAVE2, Abi1, or IRSp53 individually (using shRNA) affects terminal differentiation of CB57/BL6 mouse E13.5 FL–derived primary MKs.16,18 Cells that had been successfully transduced with scramble control or specific shRNA to WAVE1, WAVE2, Abi1, or IRSp53 expressed GFP. Transduction with shRNA of WAVE2 or of Abi1 decreased MK size (Figure 7B) and significantly reduced the generation of proplatelets at day 4 and day 7 (Figure 7C). By comparison, MKs which underwent transduction with IRSp53 shRNA, although they were relatively smaller than control- or WAVE1 shRNA–transduced MKs at day 7, formed many GFP+ proplatelets in culture (Figure 7C). These results suggest that IRSp53, a direct effector of Rac that lies upstream of the WAVE2/Abi1 complex25 and optimizes activity of the complex,31 may not be involved in thrombopoiesis in primary MKs.
Discussion
Integrin-based reorganization of the actin cytoskeleton is recognized to have a pivotal role in both megakaryocytopoiesis and proplatelet formation.16-18,36,44 Following terminal differentiation of platelets, actin polymerization is required for platelet spreading on surfaces, for platelet transformation in suspension from disks to spheres with spiny protrusions, and for stable thrombus formation under flow conditions.23,25,40,44,45 Platelets from Rac1 and Rac2 double-knockout mice exhibit intact adhesion but impaired lamellipodia formation during full spreading on fibrinogen; however, “shape change” in suspension proceeds normally in such plate-lets.26 In platelets, accordingly, actin polymerization required for the development of lamellipodia and for stable thrombus formation is obviously Rac dependent.
We have postulated that WAVE is a Rac effector that mediates actin polymerization in platelets.25 However, this hypothesis has never been tested because, first, platelets deficient in WAVE2 are not available and, secondly, platelets deficient in WAVE1 are difficult to obtain in numbers sufficient for study. We therefore used ES cell–derived MKs28 and CB57/BL6 mouse E13.5 FL–derived primary MKs.16,18 This approach enabled us to examine adhesion and spreading of MKs in addition to developmental aspects of thrombopoiesis. With respect to adhesion and spreading, herein we show that WAVE1 and WAVE2 synergistically regulate both adhesion and full spreading, while WAVE2 alone can be dispensed with for adhesion (Figure 4). WAVE1 has previously been suspected to participate in MK development because WAVE1 expression gradually increases during MK enlargement in culture.23 According to our results, however, WAVE1 is not required for MK development and thrombopoiesis (Figures 5A, 7B,C).
WAVE1−/− platelets demonstrated no major defects in spreading, and generation of lamellipodial protrusion on fibrinogen appeared normal when the platelets were stimulated with an agonist. Of note is that WAVE1−/− platelets likely formed abnormally long filopodia even without activation by strong agonists such as thrombin or PMA (Figure 5B high magnification). Calaminus et al have shown that WAVE1 specifically functions only downstream of GPVI, a platelet receptor for collagen, but not of the G-protein–coupled receptor or αIIbβ3 integrin, in platelets.46 Our results are consistent with this report. They proposed that pathways other than GPVI-mediated stimulation may relay signals to Arp2/3, possibly through WAVE2.46 In addition, both spreading and integrin activation appeared normal in platelets deficient in WAVE3, obtained from newly established homozygous WAVE3-null mice (data not shown). Our results, including those in MKs in which expression of Abi1 or IRSp53 was reduced, permit the conclusion that the WAVE2/Abi1 complex is essential for integrin αIIbβ3-mediated and agonist-stimulated lamellipodia formation, presumably excepting GPVI-stimulated actin polymerization,46 and that the function of the complex depends on Rac and IRSp53.
Our findings with respect to spreading in WAVE2−/− MKs (Figures 3–4) intriguingly resemble those in yolk sac–derived PIP5Kγ-deficient MKs; these formed spike-like filopodial projection and generated no PtdInsI (4,5) P2.47 PtdInsI (4,5) P2 production reportedly is required for membrane ruffling and proplatelet formation in fully differentiated MKs.18 WAVE2 activation, however, has been shown to depend upon the production of PtdInsI (3,4,5) P3 rather than that of PtdInsI (4,5) P2–mediated signaling31,48 or Abi1, per se.42 Given that in platelets lamellipodia formation requires D3 polyphosphoinositides, the transition factor from PtdInsI (4,5) P2 to PtdInsI (3,4,5) P3,38 both phosphoinositides may be associated with WAVE2/Abi1 complex–mediated cellular functions.
The data in Figure 7C show no significant effect on proplatelet formation by IRSp53 siRNA. By contrast, reduced expression of WAVE2 or of Abi1 impaired both growth of MKs and subsequent generation of proplatelets in both models using ES cell– and FL-derived MKs (Figures 2,6,7). In view of the effect of the WAVE2/Abi1 complex on cell growth, our results are likely consistent with a requirement for the WAVE/Abi complex (involving WAVE2 and Abi1/2) in T-cell receptor–mediated proliferation of T lymphocytes.43 The effects of deprivation of WAVE2, Abi1, and IRSp53 on MK enlargement and proplatelet formation thus are distinct. We infer that the WAVE2/Abi1 complex, but not the WAVE1/Abi1 complex, may have a hitherto undescribed and essential role in terminal differentiation of MK for subsequent thrombopoiesis, a role that could be distinct from the canonical Rac-dependent pathway.7,31 This is in complete agreement with the presence of normal numbers of platelets exhibiting specific functional defects in mice deficient in Rac1 and Rac2.26 Studies in 32D cells that overexpress p210BCR-ABL or in the Meg-01 MK cell line that endogenously expresses p210BCR-ABL have shown that p210BCR-ABL increases integrin-dependent cell adhesion capacity, a function independent of Abl kinase.49 Moreover, the Abl kinase inhibitor STI-571 (10 or 25 μM) did not inhibit proplatelet generation (K.E. and S. J. Shattil, February 2003, unpublished observations). Because Annexin V+PI− populations after day 8 of culture were comparable in WAVE2−/− MKs and in WAVE2+/+ MKs (data not shown), apoptosis is seemingly not a major mechanism in impaired enlargement of WAVE2−/− MKs or of MKs in which RNAi-mediated knockdown of Abi1 has occurred. An Abl-mediated but kinase-independent signaling pathway might contribute to MK terminal maturation. In addition, we cannot rule out the possibility that the Abi1 protein alone may regulate cellular proliferation of blood cells. That Abi1 is part of the Myc/Max complex of transcriptional factors and is essential for dendrite and synapse formation50 is intriguing. Because Myc/Max heterodimers are known to regulate cycling, transformation, and development of the cell, the function of Abi1 alone or of the WAVE2/Abi1 complex might be related with cell-cycle mechanisms in terminal differentiation/maturation of MKs. These possibilities remain to be elucidated. Whether WAVE2-mediated lamellipodia formation can initiate proplatelet generation also remains to be investigated.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Dr A. Knisely for manuscript review and Drs D. Baltimore, B. Coller, X. Qin, and M. Onodera for providing reagents. We are also very grateful to H. Tsukui and A. Yamasaki for their excellent help.
This work was supported by grants from the Ministry of Education, Culture, Sport, Science and Technology of Japan, and by Mitsubishi Pharma Research Foundation (Osaka, Japan).
Authorship
Contribution: K.E., S.S., and A.O. designed the research. K.E., H. Nishikii, T.O., S.S., T.K., and A.O. performed experiments. K.E., H. Nishikii, and A.K. analyzed data. D.Y. and T.T. made WAVE3-null mice. K.E. and H. Nakauchi wrote the paper and organized this research. K.E., H. Nishikii, and T.O. contributed equally to this work.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Koji Eto and Hiromitsu Nakauchi, Laboratory of Stem Cell Therapy, Center for Experimental Medicine The Institute of Medical Science, University of Tokyo, 108-8639 Japan; e-mail:keto@ims.u-tokyo.ac.jp, nakauchi@ims.u-tokyo.ac.jp.