Abstract
The term hemochromatosis represents a group of inherited disorders leading to iron overload. Mutations in HFE, HJV, and TfR2 cause autosomal-recessive forms of hemochromatosis. Mutations in ferroportin, however, result in dominantly inherited iron overload. Some mutations (H32R and N174I) in ferroportin lead to macrophage iron loading, while others (NI44H) lead to hepatocyte iron loading. Expression of H32R or N174I ferroportin cDNA in zebrafish leads to severe iron-limited erythropoiesis. Expression of wild-type ferroportin or hepcidin-resistant ferroportin (N144H) does not affect erythropoiesis. Zebrafish provides a facile way of identifying which ferroportin mutants may lead to macrophage iron loading.
Introduction
Ferroportin (Fpn), the only identified mammalian cellular iron exporter, functions as a homodimer.1 Mutations in Fpn result in cellular iron overload.2 Fpn-associated iron overload is transmitted as an autosomal-dominant trait. The clinical phenotypes are characterized by either macrophage iron accumulation, low transferrin saturation, and iron-limited erythropoiesis, or by hepatocyte iron accumulation and high transferrin saturation. The 2 different phenotypes are explained by the behavior of Fpn mutant proteins.3 Mutant Fpn proteins that are not trafficked appropriately to the cell surface, or that traffic appropriately but are transport incompetent, give rise to macrophage iron loading. Mutant Fpn proteins that traffic to the cell surface appropriately but are resistant to hepcidin-induced internalization lead to hepatocyte iron accumulation.4-7 The dominant transmission of both forms is due to a dominant-negative effect of the mutant allele on the Fpn dimer.1,6,8 Older patients with mutations in Fpn that should lead to hepatocyte iron loading may also show macrophage iron loading.2,9 All human Fpn mutations are missense mutations, but the mutations do not predict if a mutant Fpn will exhibit defective trafficking, impaired iron export, or hepcidin resistance. Currently, the only way to determine the effect of mutations in Fpn is to follow trafficking of the protein in cultured cells and to examine the effect of the mutant protein on cellular iron accumulation. Here, we show that expression of mutant Fpn in zebrafish offers a rapid approach in identifying mutations that lead to macrophage iron retention. We show that the same human mutations that cause macrophage iron retention lead to iron-limited erythropoiesis in zebrafish
Materials and methods
Maintenance of zebrafish stocks and embryo cultures
Danio rerio (zebrafish) were maintained at 28.5°C on a 14-hour light/10-hour dark cycle. Wild-type zebrafish were bred and raised according to established procedures approved by the University of Utah Institutional Animal Care and Use Committee. Embryos were collected from natural spawnings, cultured, and staged as previously described.10
Injection of Fpn constructs into zebrafish embryos
Mouse Fpn-GFP constructs were generated as previously described6,8 and purified using the EndoFree purification kit (Qiagen, Chatsworth, CA). Approximately 30 to 50 pg DNA was microinjected into zebrafish embryos at the 1-cell stage.10 Embryos at the 1-cell stage were injected with an iron-dextran solution (100 mg/mL, PIGDEX; American Cyanamid Co, Princeton, NJ).
Embryo lysates and Western blot
Embryos were lysed by homogenizing using a dounce homogenizer in lysis buffer.5 Samples were applied to SDS-PAGE using 4% to 20% gels (Tris-Glycine; BioRad Laboratories, Hercules, CA) and transferred in Hybond-ECL (Amersham Biosciences, Piscataway. NJ). Western analysis was performed using rabbit anti-GFP (1:10 000, catalog no. ab6556; Abcam, Cambridge, MA), followed by peroxidase-conjugated goat anti-rabbit IgG (1:10 000; Jackson ImmunoResearch Labs, West Grove, PA).
Other procedures
Staining for erythrocytes was performed by incubating dechorionated (nonfixed) embryos at 48 to 72 hours after fertilization (hpf) in an o-dianisidine solution (Sigma, St Louis, MO).11 Embryonic blood at 48 to 72 hpf was stained with Wright-Giemsa or Prussian blue.12 Peroxidase staining was assayed as an indicator of the endogenous peroxidase activity of hemoglobin. Red blood cells were fixed for 2 minutes with cold methanol and stained with 3,3-diaminobenzidine (DAB) tetrahydrochloride (Sigma, St Louis, MO) solution.13 Slides were examined using an Olympus BX51 microscope (Olympus, Tokyo, Japan) using an Olympus U-CMAD-Z camera and a 10× or a 60×/1.3 NA oil immersion objective lens. Images were acquired using Picture Frame 2.5 software (Olympus America, East Muskogee, OK).
Results and discussion
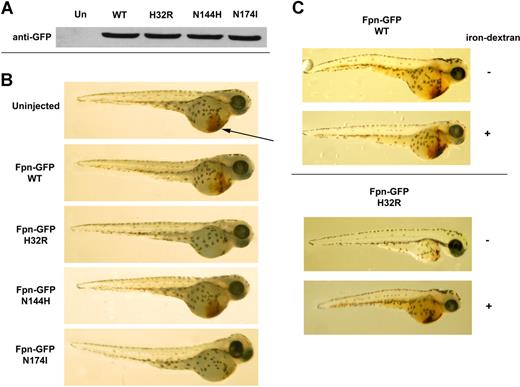
We investigated the effect of expression of Fpn mutants on erythroid development. Wild-type murine Fpn cDNA, as well as Fpn cDNA containing identified human or murine mutations, were injected into 1-cell embryos. Uninjected and injected embryos exhibited similar growth rates and normal morphologic development for the first 2 days. All constructs were expressed at comparable levels as shown by Western blot analysis (Figure 1A). There was, however, a difference in the ability of embryos to generate hemoglobinized red blood cells depending on the injected construct. Expression of wild-type Fpn had no effect on the development of erythrocytes (brown staining denoted by arrow in Figure 1B), but a severe reduction in hemoglobin production was observed in embryos expressing the flatiron mutation (Fpn-GFP H32R; Figure 1B). Mice expressing this Fpn mutation show Kupffer cell iron loading, high serum ferritin, and low transferrin saturation. The H32R mutation gives rise to ferroportin disease because H32R Fpn fails to localize to the cell surface and affects the localization of the wild-type/mutant heterodimer. Patients heterozygous for the Fpn mutant N174I also show Kupffer cell iron loading. This mutant Fpn is targeted to the cell surface but is transport incompetent. Expression of this mutant Fpn in zebrafish also leads to defective hemoglobinization (Figure 1B). In contrast, patients heterozygous for Fpn N144H show hepatocyte iron loading, the result of a transport-competent mutant that is unresponsive to hepdicin-mediated internalization.6 Expression of this mutant Fpn in zebrafish does not impede hemoglobinization (Figure 1B). We injected the embryos with iron-dextran to determine if we could rescue them from the effect of iron limitation due to the expression of the mutant protein. Injection of iron-dextran had no effect on the survival or hemoglobinization in embryos expressing wild type Fpn-GFP (Figure 1C). In contrast, injection of iron-dextran increased hemoglobinization in embryos expressing Fpn-GFP H32R. In addition, there was an increase in survival rate and in the size.
Expression of mutant Fpn in zebrafish affects hemoglobinization of erythrocytes. Zebrafish embryos were injected with wild-type or mutant Fpn-GFP. At 48 hpf, the embryos were homogenized (A), and Fpn-GFP levels were assayed by Western blot analysis or (B) stained with o-dianisidine to detect hemoglobinized cells (brown color denoted by the arrow). The figures are representative of 6 different experiments in which 100 embryos were injected with each construct. The survival rate (n = 600) was 69.6% for embryos injected with wild-type constructs, 50.8% for embryos injected with H32R constructs, 64.7% for embryos injected with N144H constructs, and 48.2% for embryos injected with N174I constructs. (C) Embryos were injected with wild-type or H32R constructs with or without coinjection of iron-dextran. The survival rate was 70% for wild-type embryos injected with or without iron-dextran, 50% for wild-type embryos injected with H32R without iron-dextran, and 62% for wild-type embryos injected with iron-dextran.
Expression of mutant Fpn in zebrafish affects hemoglobinization of erythrocytes. Zebrafish embryos were injected with wild-type or mutant Fpn-GFP. At 48 hpf, the embryos were homogenized (A), and Fpn-GFP levels were assayed by Western blot analysis or (B) stained with o-dianisidine to detect hemoglobinized cells (brown color denoted by the arrow). The figures are representative of 6 different experiments in which 100 embryos were injected with each construct. The survival rate (n = 600) was 69.6% for embryos injected with wild-type constructs, 50.8% for embryos injected with H32R constructs, 64.7% for embryos injected with N144H constructs, and 48.2% for embryos injected with N174I constructs. (C) Embryos were injected with wild-type or H32R constructs with or without coinjection of iron-dextran. The survival rate was 70% for wild-type embryos injected with or without iron-dextran, 50% for wild-type embryos injected with H32R without iron-dextran, and 62% for wild-type embryos injected with iron-dextran.
The reduced hemoglobinization seen in zebrafish embryos expressing Fpn-GFP H32R is not due to the absence of red blood cells. Wright-Giemsa staining showed the presence of erythrocytes that appeared morphologically normal, although the size of the nuclei was larger than that of wild-type animals, as would be expected in animals whose red blood cells might be iron limited (Figure 2A). Similar results were seen in red blood cells obtained from fish that had been injected with Fpn (N174I). Enlarged nuclei were noted in circulating red blood cells in zebrafish with mutations in the ferroportin or DMT1 genes.14,15 To determine if there was a decrease in hemoglobinization, we stained erythrocytes for the presence of heme using diaminobenzidine to assay for hemoglobin peroxidase activity. Wild-type erythrocytes showed abundant staining, while those from zebrafish expressing Fpn-GFP H32R showed less staining (Figure 2B). By visual inspection, all animals appeared to have similar numbers of circulating red blood cells, although with small variations (less than 20% would not have been detected). Previously, we showed that the flatiron mouse had a severe iron-limited erythropoiesis due to a marked reduction in transferrin saturation resulting from defective iron export from macrophages.8 Circulating red blood cells formed in bullfrog larvae, chicken embryos, and mouse embryos contain large amounts of ferritin containing iron acquired in excess of the need for hemoglobin synthesis.16 Prussian blue staining detected iron-containing deposits, presumably ferritin, in erythrocytes from uninjected zebrafish or zebrafish expressing wild-type murine Fpn-GFP (Figure 2C). More than 25% of the red blood cells in wild-type zebrafish contained Prussian blue stainable deposits, while in contrast, no Prussian blue deposits were identified in zebrafish expressing Fpn-GFP H32R. These results suggest that the defect in hemoglobin synthesis results from inadequate delivery of iron to the developing erythrocyte.
Effect of expression of mutant Fpn on erythroid cell development. Blood samples from embryos treated as in Figure 1 were stained with Wright-Giemsa (A), diaminobenzidine (B), or Prussian blue (C). The arrow shows iron accumulation in erythrocytes from embryos expressing wild-type Fpn-GFP, but not in embryos expressing Fpn-GFP H32R.
Effect of expression of mutant Fpn on erythroid cell development. Blood samples from embryos treated as in Figure 1 were stained with Wright-Giemsa (A), diaminobenzidine (B), or Prussian blue (C). The arrow shows iron accumulation in erythrocytes from embryos expressing wild-type Fpn-GFP, but not in embryos expressing Fpn-GFP H32R.
The mammalian placenta and the zebrafish yolk sac provide a homologous function, serving as the site of iron transfer to the embryo. Mutations in zebrafish Fpn lead to decreased iron acquisition by the embryo, resulting in a deficit in red-cell hemoglobin formation.14 Expression of mutant mammalian Fpn in wild-type zebrafish leads to Fpn disease as erythrocytes develop but hemoglobin content is reduced due to iron limitation. Zebrafish homozygous for missense mutations in Fpn show an erythroid hemoglobinization defect, but heterozygotes appear normal.17 Homozygous flatiron mice, or mice with a targeted deletion in Fpn, show early embryonic lethality but heterozygotes are born normally, with iron-limited erythropoiesis developing after birth in the flatiron mice.8,18 These results suggest that yolk sac or placental iron transport is accomplished with a fraction of normal Fpn. When wild-type and mutant alleles are expressed equivalently, cells will still show some transport activity resulting from dimerization of the wild-type protein. Iron transport activity will decrease in the face of higher levels of mutant protein, as the wild-type protein is now captured in a heterodimer. That expression of mutant Fpn in wild-type zebrafish leads to a defect in hemoglobinization provides compelling evidence that ferroportin disease results from a dominant-negative effect rather than haploinosufficiency.
Expression of wild-type Fpn or a transport competent hepcidin-resistant Fpn mutant did not lead to iron-limited erythrocytes. These results demonstrate that expression of Fpn mutants in zebrafish can be used to identify mutations that lead to defective iron transport. The value of this approach is that an unambiguous determination of Fpn functionality can be made within 24 to 48 hours of cDNA injection.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We thank Dr B. Paw (Children's Hospital Harvard, Boston, MA) for providing protocols, Dr S. Perkins (University of Utah, Salt Lake City, UT) for assistance in Prussian blue staining, Dr J. Parant (University of Utah) for advice on injection techniques, and the Zebrafish core facilities at the University of Utah.
This work was supported by National Institutes of Health grant DK 070947 to J.K.
National Institutes of Health
Authorship
Contribution: I.De.D. performed the experiments and wrote the manuscript. M.B.V. performed the experiments. D.Y. captured images and edited the manuscript. J.P.K. analyzed the data and edited the manuscript. D.M.W. analyzed the data and edited the manuscript. J.K. analyzed the data and wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Jerry Kaplan, Department of Pathology, University of Utah School of Medicine, 50 North Medical Dr, Salt Lake City, UT 84132; e-mail:jerry.kaplan@path.utah.edu.