Abstract
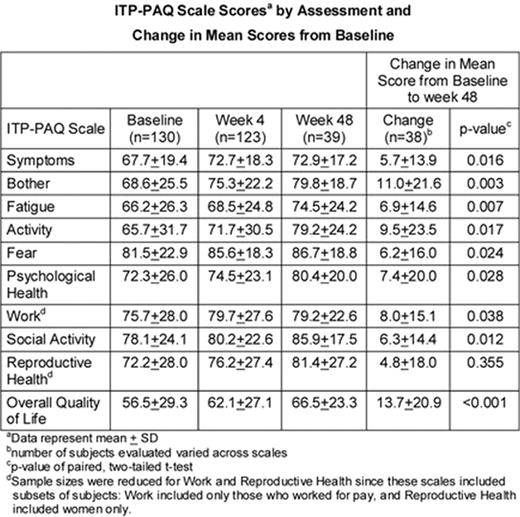
The ITP Patient Assessment Questionnaire (ITP-PAQ) was developed to complement clinical data by assessing multiple facets of disease-specific HRQoL in adult patients with chronic ITP (Mathias et al., Health Qual Life Outcomes, 2007). Here we present interim results from an ongoing, open-label extension study that will evaluate the ability of AMG 531 to improve their HRQoL in the long-term. AMG 531 is a novel thrombopoiesis-stimulating peptibody that is being studied for its ability to increase platelet production by stimulating the thrombopoietin receptor. Adult chronic ITP patients receiving AMG 531 completed the ITP- PAQ at baseline, and weeks 4, 12, 24, and every 12 weeks thereafter. The ITP-PAQ consists of 44 items and 10 scales. Data have been assessed for this interim analysis through week 48, a time-point with reasonable patient numbers at the time of the data cut-off. Mean scores at each assessment, and changes in mean scores from baseline to week 48 were calculated, with positive changes in mean score indicating improvement in HRQoL. Compliance rates for the ITP-PAQ were 95% at baseline, 93% at week 4, and 83% at week 48. Over the 48-week period, results demonstrated an upward trend for the scale scores (see Table). The greatest improvements were reported in the Symptoms, Bother, Activity, Reproductive Health, and Overall Quality of Life scales, particularly during the first 4 weeks of the study. Changes in mean score from baseline to week 48 were significantly increased for Symptoms, Bother, Fatigue, Activity, Fear, Psychological Health, Work, Social Activity, and Overall Quality of Life. ITP patients receiving AMG 531 had an initial, yet sustained, improvement in ITP-PAQ scores. Subsequent analyses of results collected in this ongoing study will be used to substantiate our findings, and the therapeutic benefit of AMG 531 on HRQoL in patients with chronic ITP will be provided by correlations with clinical data.
Author notes
Disclosure:Employment: RD, KN, JN: Amgen. Consultancy: MT: Biovitrum, Sumphogen; SM: Amgen. Ownership Interests:; RD, KN, JN: Amgen. Research Funding: MT: Amgen, Baxter; DH, RG, FL, AL, HT, JV. Honoraria Information: MT: Baxter, Biovitrum. Membership Information: MT: Baxter, Biovitrum, Nabi; AL: Amgen.