Abstract
Background: The severe morbidity and mortality associated with bone marrow transplantation (BMT) is caused by uninhibited immune responses to alloantigen and suppressed immune responses to pathogens. Vasoactive Intestinal Peptide (VIP) is an immunomodulatory neuropeptide produced by T-cells and nerve fibers in peripheral lymphoid organs that suppresses immune responses by induction of tolerogenic dendritic cells. In order to determine the immunoregulatory effects of VIP, we examined T-cell immune responses to allo- and viral-antigens in VIP knockout (KO) mice and mouse BMT recipients of hematopoietic cells from VIP KO donors.
Methods: VIP KO mice and VIP WT littermates were infected with lethal or sub-lethal doses (5 × 104− 5 × 105 PFU) of murine cytomegalovirus (mCMV) and the T-cell response to viral antigen was measured by flow cytometry for mCMV peptide-MHC class 1-tetramer+ CD8+ T-cells. We transplanted 5 × 106 BM plus 1 × 106 splenocytes (SP) either from VIP KO or VIP WT donors in an C57BL/6 to F1(BL/6 × Balb/c) allo-BMT model and assessed survival, GvHD, donor T-cell expansion, chimerism, and response to mCMV vaccination and mCMV infection.
Results: B-cell, αβ and γδ T-cell, CD8+ T-cell, CD11b+ myeloid cell, and dendritic cell numbers were equivalent between VIP KO and WT mice, while VIP KO mice had higher number of CD4+ and CD4+CD62L+CD25+ T-cells. Non-transplanted VIP KO mice survived mCMV infection better compared to VIP WT, with a brisker anti-viral T-cell response in the blood. In the allogeneic BMT setting, recipients of VIP KO BM plus VIP KO SP had more weight loss and lower (40%) 100 day post-transplant survival compared to the recipients of VIP KO BM plus WT SP (80% survival), recipients of WT BM plus KO SP (100% survival), and recipients of WT BM plus WT SP (80% survival). Recipients of VIP KO grafts had a significantly greater anti-mCMV response that peaked four days earlier than the tetramer response of mice transplanted with WT cells. This increased anti-viral response to vaccination correlated with a greater and more rapid T-cell response to secondary viral challenge.
Conclusions: These experiments suggest that the absence of all VIP in the body, or the absence of VIP in a transplanted immune system, enhances anti-viral immunity and allo-immune responses. Modulation of the VIP pathway is a novel method to regulate post-transplant immunity.
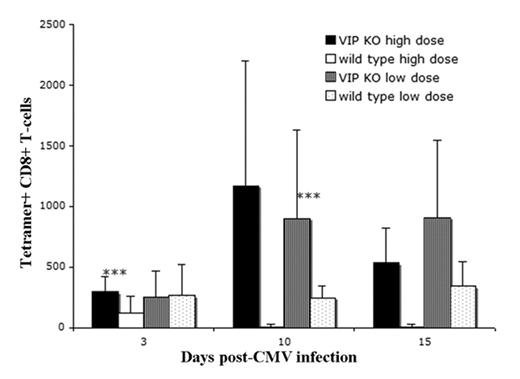
VIP knockout(KO) mice have an increased CMV tetramer response. VIP KO and VIP WT mice were infected (day 0) with either a sub-lethal low dose (5 × 10^4 PFU) or a lethal high dose (5 × 10^5 PFU) of CMV. Peripheral blood was stained for T cell markers and tetramer and analyzed by flow cytometry. On day 3, high dosed VIP KO mice had a higher number of tetramer positive CD8 T cells and better survival than WT mice (all high dose VIP WT died prior to day 10). VIP KO mice had a significant increase in tetramer positive CD8 T cells between days 3 and 10. *** p<0.01, difference between VIP KO and VIP WT littermate at designated dose level and day.
VIP knockout(KO) mice have an increased CMV tetramer response. VIP KO and VIP WT mice were infected (day 0) with either a sub-lethal low dose (5 × 10^4 PFU) or a lethal high dose (5 × 10^5 PFU) of CMV. Peripheral blood was stained for T cell markers and tetramer and analyzed by flow cytometry. On day 3, high dosed VIP KO mice had a higher number of tetramer positive CD8 T cells and better survival than WT mice (all high dose VIP WT died prior to day 10). VIP KO mice had a significant increase in tetramer positive CD8 T cells between days 3 and 10. *** p<0.01, difference between VIP KO and VIP WT littermate at designated dose level and day.
Author notes
Disclosure: No relevant conflicts of interest to declare.