Abstract
Donor natural killer (NK) cells after haploidentical hematopoietic stem-cell transplantation (HSCT) and infusion of haploidentical NK-cells have demonstrated a therapeutic effect. NK alloreactivity resulting from appropriate Killer cell Ig-like receptor (KIR)-ligand disparity in human-leukocyte-antigen (HLA)-haplotype mismatched HSCT has resulted in improved engraftment and decreased incidence of leukemia relapse. Yet, not all patient-donor pairs benefit for an allogeneic NK-cell effect. To identify NK-cell donors with a suitable KIR-ligand mismatch, we have developed a functional assay to measure NK-cell killing through KIR-ligand interactions. NK-cell lysis of target cells is blocked by inhibitory KIR that recognize classical HLA class I allotypes and HLA mismatches of an altered allelic repertoire, as in haploidentical HSCT, leading to KIR-ligand mismatch and alloreactive NK cell-mediated target killing (Figure 1A). A cytotoxicity assay was developed based on the NK-cell target HLAnull 721.221 cells, and a panel of targets with enforced expression of HLA genes recognized by KIR. After the killing assay was optimized for high throughput and sensitivity, we used the panel of targets to determine whether bulk populations of donor NK cells could be predicted to kill based on KIR and HLA typing. The results demonstrate patterns of target-cell lysis for the KIR repertoires corresponding, for some donors, with predicted donor-versus-recipient NK-cell alloreactivity (Figure 1B). A relative inhibition of HLA+ target-cell lysis of >30% was associated with binding of KIR to introduced HLA class I molecules. The benefit of this assay to transplant physicians is a tool to actually measure phenotype (lysis), rather than relying on predictive models based on genotype. This assay will be combined with typing data to help identify donors with NK-cell killing function for recipients of haploidentical HSCT and infusion of haploidentical NK cells.
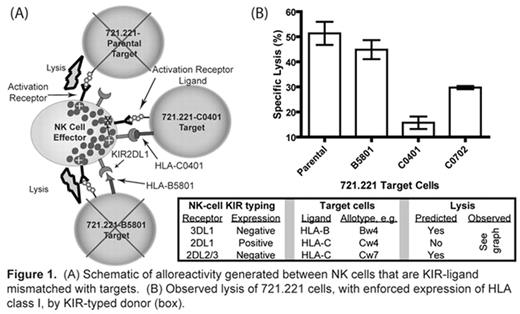
(A) Schematic of alloreactivity generated between NK cells that are KIR-ligand mismatched with targets. (B) Observed lysis of 721.221 cells, with enforced expression of HLA class I, by KIR-typed donar(box).
(A) Schematic of alloreactivity generated between NK cells that are KIR-ligand mismatched with targets. (B) Observed lysis of 721.221 cells, with enforced expression of HLA class I, by KIR-typed donar(box).
Author notes
Disclosure: No relevant conflicts of interest to declare.