Abstract
Purpose: This trial aimed to investigate the efficacy and safety of sequential treatment with rituximab and CHOP-21 in patients with PTLD unresponsive to reduction of immunosuppression.
Methods: An ongoing prospective multicenter phase II trial was initiated in January 2003. Patients were treated sequentially with rituximab at days 1, 8, 15 and 22 followed by four cycles of CHOP-21 combined with G-CSF support starting 4 weeks after the last dose of rituximab.
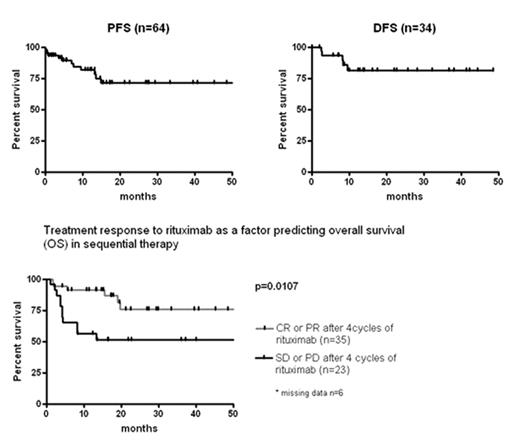
Results: In this 3rd interim analysis after enrolment of 75 patients 64 patients have finished the protocol. The median follow up is 19.6 months. 58 patients were diagnosed with monomorphic PTLD, 6 with polymorphic PTLD. 23 patients were kidney, 15 liver, 12 heart, 3 lung, 2 heart+lung, 3 kidney+pancreas, 1 bone marrow transplant recipients (5 others). Median age was 53 years (range 16 to 74). 59% had stage III or IV disease. 48% of tumors were EBV positive. 79% of patients had late PTLD (i.e. later than 1 year after transplantation). LDH was elevated in 67% of patients. The overall response rate of sequential therapy was 90% (CR 65%, PR 25%). Progression free survival (PFS) and disease free survival (DFS) were 71.4% and 81.2% at two years, respectively. Treatment response to rituximab (CR/PR versus SD/PD) was a significant factor predicting overall survival (OS) with OS rates of 91.3% and 56.5% at 1 year, respectively (p=0.0107). Following chemotherapy, WHO °3/4 leukopenia was observed in 38% of cycles and 16% of patients suffered from WHO °3/4 infections. There were four early therapy-associated deaths due to infections (7%). Fatal bleeding complications occurred in 3% and 5% of patients died from primary refractory disease.
Conclusions: This is one of the largest prospective studies in PTLD. Sequential treatment with rituximab and CHOP-21 + G-CSF is well tolerated and highly effective. Treatment response to rituximab is predictive for overall survival. As compared to rituximab monotherapy more patients achieve a CR with sequential therapy and PFS is very much prolonged.
Author notes
Disclosure:Employment: From 1 July 2005 Dr. Oertel is an employee of Roche Pharma AG - Germany. He has no stock ownership or any current financial benefit or potential future financial gain due to the publication. Research Funding: Support of investigator initiated clinical studies and/or research grants have been received from several pharmaceutical companies including Roche Pharma AG by Dr. Choquet, Prof. Leblond, Prof. Reinke, Prof. Riess and Dr. Trappe. Dr. Lehmkuhl has received research grants from Novartis Pharma GmbH, Roche Pharma AG, Astellas Pharma GmbH and Genzyme GmbH. Honoraria Information: Honoria for scientific and educational presentations have been received from several pharmaceutical companies including Roche Pharma AG by Dr. Choquet, Prof. Leblond, Dr. Lehmkuhl, Prof. Reinke, Prof. Riess and Dr. Trappe.