Abstract
AMD3100, Plerixafor (A)+G-CSF (G) have effectively allowed aHSC mobilization in Phase I and II studies. This Phase III, multicenter, randomized, double-blind, placebo controlled study compares the safety and efficacy of A+G Vs. placebo (P)+G to mobilize and transplant patients with MM.
Methods: Adult MM patients requiring an aHSC transplant, in first or second CR or PR were eligible to participate. Patients were declared for single or tandem transplant with the second transplant occurring within 6 months from the first. Patients received G (10μg/kg/day) subcutaneously (SQ) for 4 days; on the evening of Day 4 they received either A (240μg/kg SQ) or P. Patients underwent apheresis on Day 5 after an AM dose of G and 10–11 hours after administration of study treatment. Patients continued to receive the evening dose of study treatment followed by AM dose of G and apheresis for up to a total of 4 apheresis or until ≥6 x 106 CD34+ cells/kg were collected. Patients who failed to collect ≥2 x 106 CD34+ cells/kg were eligible for rescue therapy with A+G, without unblinding of randomized treatment. Only study cells were used for transplant. The primary endpoint was the percentage of patients who achieved ≥6 x 106 CD34+ cells/kg in 2 or less apheresis days. All patients will be followed for ≥12 months post-transplant.
Results: 302 patients were enrolled and randomized into the study. All have completed 100 days follow-up and are included in this intent-to-treat analysis. Baseline characteristics were similar between groups. The primary endpoint was met in 106/128 (72%) patients in the A+G group and 53/154 (34%) patients in the P+G group, p<0.0001.
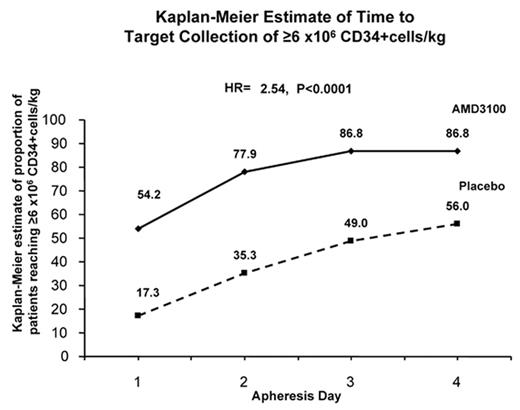
The figure shows that 54% of A+G patients reached target after 1 day of apheresis but 56% P+G patients required up to 4 days of apheresis to reach target. 7 patients in the P+G group required rescue therapy and all collected ≥2 x 106 CD34+ cells/kg after A+G rescue. 142 patients (96%) in A+G group and 136 patients (88%) in the P+G group underwent transplant. Tandem transplants were performed in 32 and 28 patients in A+G and P+G groups, respectively. Median time to engraftment was Day 11 for PMN and Day 18 for platelets in both groups. Grafts were durable in all patients in both group at ≥100 days post-transplant. Patients in the A+G group experienced more GI effects and injection site erythema than patients in the P+G group. These adverse events were generally mild. There were no drug related serious adverse events in either group.
Conclusions: In this study, the addition of AMD3100 to G-CSF is generally safe and well tolerated and is superior to G-CSF alone for aHSC mobilization in MM patients. A+G patients were statistically significantly more likely to achieve target earlier than P+G patients and had successful transplant.
Author notes
Disclosure:Research Funding: Genzyme Corporation. Honoraria Information: AnorMED, Genzyme, MGI Pharma.