Abstract
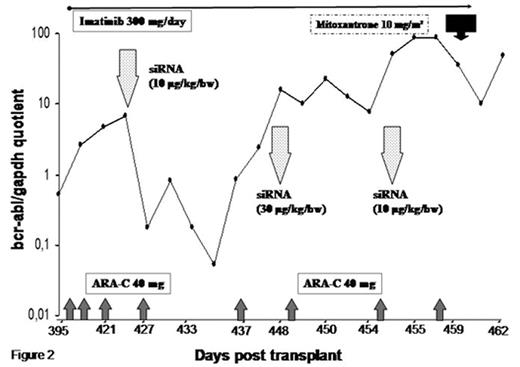
Chronic myeloid leukemia (CML) is a relatively well differentiated myeloproliferative disorder originating from transformed hematopoietic stem cells. However, as patients may relapse after allogeneic stem cell transplantation (SCT) or develop imatinib-resistant disease, additional CML therapies are required. RNA interference is referred to as the recently discovered process of post-transcriptional gene silencing that is initiated by double-stranded RNA molecules known as small interfering RNAs (siRNA). We herein present a first report on the in vivo application of targeted non-virally delivered synthetic bcr-abl siRNA in a 47-year-old female patient with recurrent CML (b3a2 translocation for a p210 oncoprotein) resistant to imatinib (Y253F mutation) and chemotherapy (cytosine arabinoside, ARA-C) after an HLA partially matched unrelated SCT by receiving a myeloablative conditioning therapy with fractionated total body irradiation (10 Gray), cyclophosphamide (120mg/kg b.w.) and anti-thymocyte globulin (30mg/kg b.w.). Transplant associated complications included renal insufficiency and acute graft-versus host disease (GVHD) grade II (skin stage 3, gut stage 1), which developed into an extensive chronic GVHD with mainly skin involvement. Prednisone was continuously tapered to a minimum of 12.5 mg/day post-transplant. The patient developed an histologically relapse of CML. SiRNA treatment was started on day +426 after transplant without discontinuing imatinib mesylate, ARA-C treatment or the immunosuppressive medication. The patient received for the first time 10μg/kg b.w. bcr-abl siRNA intravenously in an anionic dispersion lipid solution (DLS) of 20 ml as an accompanying therapy trial. SiRNA was administered over 1–2 minutes as a bolus injection. In addition, 300μg siRNA in 2 ml DLS were injected directly into a subcutaneous CML node. No side effects were observed during the first hours after administration. Two days after siRNA treatment the patient suffered from moderate dizziness (grade 0–1), this resolved completely after three days. The bcr-abl mRNA level decreased from 6.6% to 0.053% nine days after the first siRNA injection. At day 21 after initial siRNA treatment the bcr-abl expression increased again. We repeated the intravenous application of siRNA with 30μg/kg b.w. in 60 ml DLS (injected over 15 minutes) and 7 days later with another 10μg/kg b.w. in 20 ml DLS (injected over 5 minutes). A transient decrease of the bcr-abl amount was detected after the second - and the third siRNA administration as shown in the figure. After the first siRNA administration we found a transient improvement of blood leucocytes with disappearance of immature cells that defined a complete hematological response. FISH analysis showed an absence of the Philadelphia chromosomes of 200 analyzed interphase nuclei. On day +455 were found again 70% blasts in the blood and a palliative chemotherapy with mitoxantrone (flat 20mg) was initiated according to the patient’s wish. The patient died at day +473 post transplant. Our findings imply that the clinical application of synthetic siRNA is feasible, safe and has real potential for genetic-based therapies using synthetic non-viral carriers.
Author notes
Disclosure: No relevant conflicts of interest to declare.