Abstract
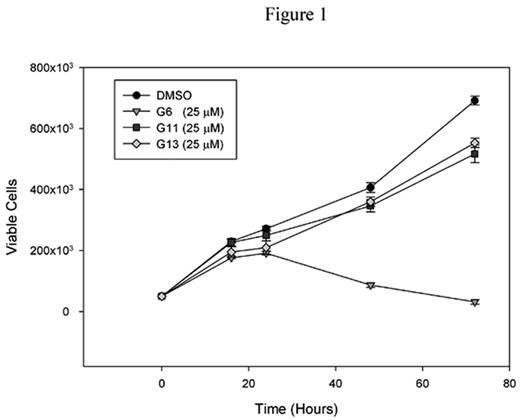
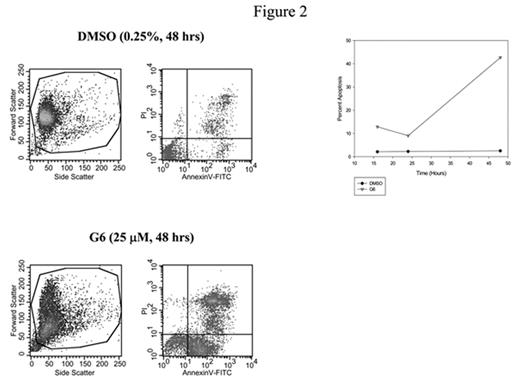
Recent work from our lab has been aimed at identifying novel Jak2 tyrosine kinase inhibitor molecules. Here, we refined our previous Jak2 molecular model using information obtained from the crystal structures of the Jak2 and Jak3 kinase domains. A total of 223,481 compounds within the NIH small molecule database were then screened in silico using FlexX 1.13.2 in order to identify compounds that specifically bind and inhibit Jak2. In what is now our third generation of Jak2 inhibitors, analysis of the highest scoring compounds identified a set of structurally diverse molecules that potently inhibited Jak2 autophosphorylation not only at Tyr 1007, but at all 49 tyrosine residues. These compounds significantly inhibited proliferation of the human erythroleukemia (HEL) cells, which express the Jak2-V617F mutation on both alleles (Fig. 1). One compound in particular, herein designated as G6, was further examined in greater detail. The mechanism by which G6 inhibits Jak2-V617F dependent cell growth is via a marked increase in cellular apoptosis (Fig. 2). We found that G6 does not inhibit c-Src and Tyk2 autophosphorylation at doses that completely inhibit Jak2, therefore suggesting a high degree of specificity. We found that both the G6 and the G13 compounds significantly reduced ex vivo megakaryocyte colony formation of cells taken from a patient diagnosed with essential thrombocythemia and harboring the Jak2-V617F mutation. Finally, we examined the ability of G6 to inhibit red blood cell formation in C57BL/6 mice following acute hemolytic anemia. We found that treatment of animals with G6 (10 mg/kg/day for five days, IP) resulted in a marked inability of these animals to increase their hematocrits following acute anemia when compared to vehicle control animals. Additionally, histological analysis of the bone marrow of G6 injected animals exhibited erythroid hypoplasia resulting in a skewed M:E ratio when compared to vehicle control animals. Collectively, our data demonstrate that these small molecule compounds inhibit Jak2 function in vitro, ex vivo, and in vivo. As such, they may have therapeutic value in treating diseases that are caused by aberrant Jak2 kinase function.
Author notes
Disclosure: No relevant conflicts of interest to declare.