Abstract
Background: PR1 peptide has been established as a human myeloid leukemia-associated antigen. We studied PR1 peptide vaccine in a phase I/II clinical trial in HLA-A2 + patients with AML, MDS and CML. To address whether prior HSCT or prior use immunosuppressive drugs would prevent PR1-induce cytotoxic T lymphocyte (PR1-CTL) immunity after vaccination with PR1 peptide vaccine, we studied the outcome in 20 patients with a prior HSCT, who were treated on the PR1 vaccine trial.
Methods: Twenty patients (13 with AML or MDS, 7 with CML) were vaccinated at a median time of 9.5 months (range: 1–220) after HSCT. Sixteen patients had received a prior allogeneic HSCT (12 had allogeneic related, 3 had allogeneic unrelated, and 1 had syngeneic) and 4 patients had a prior autologous HSCT. At the time of vaccination, 5 patients were in CR, and 15 had measurable disease. Patients could not receive systemic immunosuppressive therapy for at least 4 weeks prior to vaccination, and had to be free of acute or chronic GVHD requiring systemic therapy. The vaccine was given subcutaneously every 3 weeks for a total of 3 injections at one of three dose levels: 0.25, 0.5 and 1.0 mg. GM-CSF at a dose of 75 mg was injected subcutaneously into the same site. Immune response to the vaccine (IRV) was defined as > 2-fold increase in PR1-CTL by PR1/HLA-A2 tetramer assay.
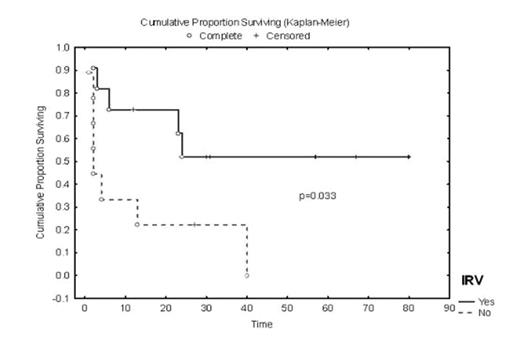
Results: After a median follow up of 56.5 months (range: 27–89), toxicity was limited to grade I/II injection site reactions in 7 (35%) patients. IRV were observed in 11/20 (55%) patients. Nine of 11 (82%) IRV+ patients versus 1 of 9 (11%) IRV- patients had clinical responses (p = .005). Median event-free survival (EFS) was 23.8 months in IRV+ patients versus 1.9 months in IRV- patients (p=0.03). Median overall survival (OS) in IRV+ patients has not yet been reached vs. 40 months in IRV- patients (p = 0.08). Only 2 patients with pre-existing, limited chronic GVHD experienced a mild exacerbation within 3 months of vaccination, which was controlled with topical steroids. PR1-CTL were enriched in central memory phenotype (TCM) that persisted up to 4 years in clinical responders. Univariate and multivariate Cox proportional hazards analyses showed a low pre-vaccine bone marrow blast count (<10%) was associated with a lower risk of progression (p=0.001 and 0.001, respectively). Type of HSCT, interval between HSCT and PR1 vaccine, PR1 dose level and disease status at HSCT did not have a significant impact on EFS or OS.
Conclusion: PR1 vaccine produced PR1-CTL in 11/20 (55%) patients after HSCT. IRV was associated with significantly better clinical response and longer EFS.
Author notes
Disclosure:Research Funding: Jeffrey J Molldrem: The Vaccine Company. Honoraria Information: Muzaffar H. Qazilbash: The Vaccine Company.