Abstract
Background: Single autologous hematopoietic cell transplantation (AHCT) is considered standard therapy for patients with multiple myeloma (MM). Several observational studies and some RCTs have shown superior outcomes with tandem transplants; others have shown conflicting results. The objective of this study is to present the totality of evidence by conducting a systematic review to assess the efficacy of double ASCT in previously untreated MM patients.
Methods: A systematic and comprehensive search of the literature was performed using MEDLINE, and Cochrane library databases from 1966–2007 for all phase III randomized controlled trials (RCT) comparing single versus tandem AHCT in previously untreated MM patients with previously. Additionally, we searched ASCO, ASH and European Society for hematology meeting abstracts between 2003–2007 years. Data was extracted and pooled on benefits and harms as per the methods recommended by the Cochrane Collaboration.
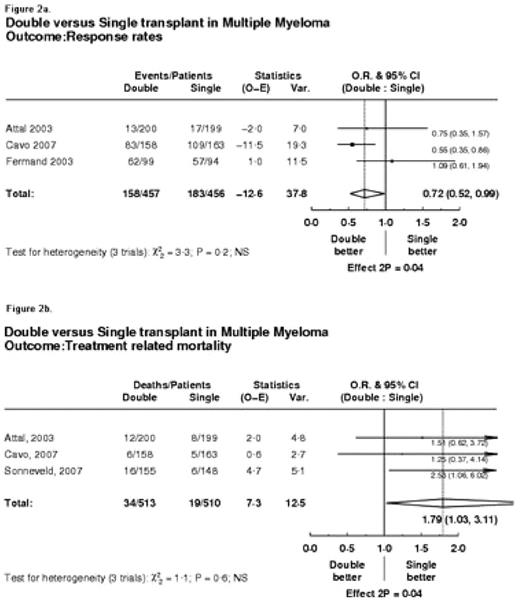
Results: Altogether 5 RCTs enrolling 1569 patients met the inclusion criteria. Data were available from all trials for overall survival, and for the remaining outcomes data were extractable from 3 RCTs. As shown in figure 1a. overall survival was not significantly different between tandem and single transplant (hazard ratio [HR]=0.91, 95% CI 0.81 to 1.02, p=0.09). However, there was a significant improvement in event free survival (HR=0.74, 95%CI 0.64, 0.85; p=0.00002, see figure 1b.) and response rates (HR=0.72, 95%CI 0.52 to 0.99; p=0.04, see figure 2a.) favoring tandem AHCT, but at the expense of significant increase in treatment related mortality (HR=1.79, 95%CI 1.03 to 3.11; p=0.04, see figure 2b.). There was no statistically significant heterogeneity between trials (see figure 1 and 2).
Conclusion: The available existing evidence shows that, in patients with previously untreated MM, tandem AHCT does not result in improved survival. Tandem AHCT is associated with improved event-free survival and response rates but at the cost of significant increase in fatal side effects (see figure). However, the latter conclusion can also be attributed to “outcome reporting bias” since data for non-survival outcomes were only available in 3 out of 5 RCTs.
Double versus Single transplant in Multiple Myeloma Outcome: Overall Survival
Figure 1b.
Double versus Single transplant in Multiple Myeloma Outcome: Event-free survival
Double versus Single transplant in Multiple Myeloma Outcome: Overall Survival
Figure 1b.
Double versus Single transplant in Multiple Myeloma Outcome: Event-free survival
Double versus Single transplant in Multiple Myeloma Outcome: Response rates
Figure 2b.
Double versus Single transplant in Multiple Myeloma Outcome: Treatment related mortality
Double versus Single transplant in Multiple Myeloma Outcome: Response rates
Figure 2b.
Double versus Single transplant in Multiple Myeloma Outcome: Treatment related mortality
Author notes
Disclosure: No relevant conflicts of interest to declare.