Abstract
Interleukin 7 (IL-7) plays a crucial role in adult lymphopoiesis, while in fetal life its effect can be partially compensated by TSLP. Whether adult hematopoietic progenitor cells are unresponsive to TSLP or whether TSLP is less available in adult microenvironments is still a matter of debate. Here, we show that increased TSLP availability through transgene (Tg) expression fully restored lymphopoiesis in IL-7–deficient mice: it rescued B-cell development, increased thymic and splenic cellularities, and restored double-negative (DN) thymocytes, αβ and γδ T-cell generation, and all peripheral lymphoid compartments. Analysis of bone marrow chimeras demonstrated that hematopoietic progenitor cells from adult wild-type mice efficiently differentiated toward B- and T-cell lineages in lethally irradiated IL-7 deficient mice provided TSLP Tg was expressed in these mice. In vitro, TSLP promoted the differentiation of uncommitted adult bone marrow progenitors toward B and T lineages and the further differentiation of DN1 and DN2 thymocytes. Altogether, our results show that adult hematopoietic cells are TSLP responsive and that TSLP can sustain long-term adult lymphopoiesis.
Introduction
Lymphopoiesis is regulated by a number of cytokines that control the proliferation, differentiation, and survival of hematopoietic progenitor cells. Among these factors, IL-7 plays an essential role in B- and T-cell development.1-3 IL-7 signals through the IL-7 receptor that is composed of the common γ-chain (γc or CD132) and the IL-7Rα (CD127) chain.4 IL-7Rα can also associate with the thymic stromal lymphopoietin receptor (TSLPR) chain to form the receptor for the cytokine TSLP.5,6 TSLPR signaling occurs independently of γc5-7 and does not rely on the phosphorylation of any known Janus family kinases but leads to the translocation of functional Stat5 to the nucleus.7,8
TSLP was originally cloned from a murine thymic stromal cell line.9 While displaying only 43% protein identity,10,11 human and mouse TSLP share similar biologic functions. Both promote homeostatic expansion of CD4+ T cells,12,13 trigger dendritic cell maturation,14-16 induce TH2 differentiation,14-18 and are important factors in triggering inflammatory allergic responses.14-16,18,19
Several studies have suggested that TSLP may play a role in fetal rather than adult B-cell development. For instance, at 4 weeks of age, γc−/− mice, which are responsive to TSLP but not to IL-7, showed residual B lymphopoiesis. In contrast, in 4-week-old IL-7Rα−/− mice, which are unresponsive to both TSLP and IL-7, B-cell development was absent. However, at 12 weeks of age, both mouse strains were devoid of B lymphopoiesis.20 Moreover, although both adult IL-7−/− and IL-7Rα−/− mice lack γδ T cells, fetal IL-7−/− but not IL-7Rα−/− thymi contained γδ T cells.3,21 It is currently unknown whether these differences reflect distinctive TSLP responsiveness of fetal versus adult hematopoietic cells, or if they are the result of decreased availability of a biologically active form of TSLP in adult mice.
TSLP was shown to support B-cell development in vitro.7,9,22 However, there are conflicting results on the role of TSLP for B-cell development in vivo. TSLPR−/− mice had no defect in B-cell development,12,23 while TSLP Tg expression either promoted24 or inhibited25 B lymphopoiesis.
Experimental data showing a role for TSLP in T lymphopoiesis are limited. TSLP could induce a moderate in vitro proliferation of adult double-negative (DN) thymocytes in synergy with IL-1, but failed to sustain the proliferation of fetal thymocytes.26 While TSLPR−/− mice had no defect in T development, mice lacking both the γc and the TSLPR chains showed lower thymic cellularity than γc−/− mice.12 Moreover, the injection of recombinant TSLP could transiently increase the number of thymocytes in γc−/− mice.12 Although these data suggest that TSLP may be involved in T lymphopoiesis, the developmental stage at which TSLP exerts its biologic function has not been clearly identified.
To understand the function of TSLP on hematopoiesis in vivo, TSLP-transgenic (Tg) mice were generated and backcrossed to an IL-7–deficient background. The TSLP Tg expression was driven by the keratin 14 (K14) promoter that targets gene expression to epithelial cells.27
In this study, we show that TSLP Tg expression rescued B-cell development, increased the thymic cellularity, and rescued the thymic architecture in IL-7−/− animals. DN1 and DN2 thymocytes but also γδ T cells developed in response to TSLP. Moreover, adult WT bone marrow (BM) cells differentiated normally into B and T lineages and restored peripheral compartments when adoptively transferred into lethally irradiated IL-7−/− K14-TSLP Tg recipients. In addition, we observed a strong effect of TSLP overexpression on peripheral myeloid cell expansion. In vitro, TSLP promoted the generation of B and T lineage cells from early lymphoid/myeloid BM progenitors and supported the differentiation of DN1 and DN2 thymocytes. Altogether, we show here that TSLP is a potent cytokine able to support adult B- and T-cell development and to expand both lymphoid and myeloid compartments in peripheral lymphoid organs.
Materials and methods
Mice
All mice were bred and maintained in our animal facility under specific pathogen-free conditions. The animal experiments received the approval of the Cantonal Veterinary Office of the city of Basel, Switzerland.
C57BL/6 mice were purchased from RCC (Itingen, Switzerland). IL-7−/− mice were previously described.1 RAG2−/− γc−/− mice on C57BL/6 background were kindly provided by Jörg Kirberg (MPI, Freiburg, Germany).
For generation of transgenic mice, the murine TSLP open reading frame26 was inserted into the pK14pA construct.28 The transgene DNA was microinjected into fertilized (C57BL/6 × DBA/2) F2 embryos to generate transgenic founders. Progenies of the founder mice were phenotypically identical. One line was backcrossed with C57BL/6 mice for at least 8 generations. The genotype of TSLP Tg mice was identified by polymerase chain reaction (PCR) from genomic DNA with the following primers: 5′-TGCAAGTACTAGTACGGATGGGGC-3′ from the 5′ coding region and 5′-GGACTTCTTGTGCCATTTCCTGAG-3′ from the 3′ coding region. PCR conditions were 94°C for 2 minutes followed by 34 cycles of 94°C for 30 seconds, 62°C for 30 seconds, 72°C for 30 seconds, and finally 72°C for 5 minutes. PCR products were separated on a 1% agarose gel and detection of a 323-bp fragment positively identified TSLP Tg presence.
For BM chimera, IL-7−/− K14-TSLP Tg mice, IL-7−/− littermates, and C57BL/6 WT mice were lethally γ-irradiated (9 Gy) and intravenously injected with 107 total BM cells from 8-week-old WT mice (CD45.1+). Six weeks or 6 months after reconstitution, BM chimeric mice were analyzed.
Immunization
NP stands for 4-hydroxy-3-nitrophenyl-acetyl. Ten- to 12-week-old IL-7−/− K14-TSLP, IL-7−/−, and C57BL/6 mice were immunized intraperitoneally with 50 μg alum-precipitated NP-ovalbumin (NP-OVA) or intravenously with 100 μg NP-Ficoll. Sera were collected prior to immunization and 10 or 14 days after NP-Ficoll or NP-OVA immunization, respectively.
ELISA
NUNC Immunoplate Maxisorb F96 plates (NUNC, Roskilde, Denmark) were coated with 5 μg/mL NP-BSA at 4°C. Plates were incubated with serial dilutions of sera for 2 hours at room temperature. After washing, alkaline phosphatase–conjugated rat anti–mouse IgM (R6–60.2; PharMingen, San Diego, CA) or goat anti–mouse IgG (Southern Biotechnology Associates, Birmingham, AL) was added to the plates. Plates were developed with FAST pNPP (p-nitrophenylphosphate; Sigma-Aldrich, St Louis, MO) according to the manufacturer's instructions. Serum titers were determined using the end point titer procedure. TSLP enzyme-linked immunosorbent assay (ELISA) was performed with the DuoSet ELISA kit (R&D, Abington, United Kingdom) according to the manufacturer's instructions.
Cell culture
OP929 and OP9-DL1 cells30 were cultured as previously described.31 Semiconfluent cultures of stromal cells were γ-irradiated (30 Gy) before adding sorted DN1, DN2, or early progenitors for lymphoid and myeloid progenitors (EPLMs). Sorted cells (5 × 103)/well of a 24-well plate were cultured in supplemented IMDM on OP9 or OP9-DL1 cells in the presence of either 100 U/mL IL-7 or 500 U/mL TSLP or without additional cytokine. At day 7, cells were harvested, counted, stained, and analyzed by flow cytometry.
Flow cytometry and cell sorting
FITC-, PE-, PE-Cy7–, APC-, or biotin-conjugated α-CD4 (GK1.5), α-CD8α (53–6.7), α-CD19 (1D3), α-CD21 (CR2/CR1), α-CD23 (B3B4), α-CD25 (7D4), α-CD44 (IM7), α-CD45.1 (A20), α-γδ TCR (GL3), α-Vγ3 TCR (536), and α-NK1.1 (PK 136) Abs were purchased from BD Biosciences (Basel, Switzerland). α-CD3 (145–2C11), α-CD11b (M1/70), α-CD24 (M1/69), α-CD62L (MEL-14), α-B220 (RA3–6B2), α-TER119 (TER-119), and α-TCRβ chain (H57–597) were from Biolegend (San Diego, CA). α-CD45 (30-F11), α-CD71 (R17217), α-CD117 (2B8), and α-Gr-1 (RB6–8C5) Abs were from eBioscience (San Diego, CA). α-Vγ1.1-PE (2.11) and α-Vγ2-PE (UC3–10A6) were obtained from A. Wilson (LICR, Epalinges, Switzerland). α-CD93 (PB493) Ab was produced in our laboratory and labeled with biotin by standard methods. As secondary reagent, streptavidin-PE and streptavidin-PE/Cy7 (Biolegend) were used. Flow cytometry acquisition was performed with a FACSCalibur (BD Biosciences) and data were analyzed using Flowjo software (Tree Star, Eugene, OR). EPLM sorting was done as previously described.31 Briefly, erythrocyte-depleted BM cells were sorted as B220+ CD19− CD117+ CD93+ NK1.1− cells. DN1 and DN2 thymocytes were sorted as CD117high CD25− CD44+ and CD117high CD25+ CD44+ cells, respectively.32 Cell sorting was done using a FACS Aria (BD Biosciences) and reanalysis of sorted cells indicated that they were more than 98% pure.
V(D)J rearrangement analysis
B220+ CD19+ double-positive cells (5 × 105) from IL-7−/− K14-TSLP Tg mice (8 weeks old) were fluorescence-activated cell sorting (FACS) sorted, and genomic DNA was isolated by standard protocols. DNA amplification was carried out in 2 rounds of PCR. The first round of PCR amplification contained 5 different 5′ VH primers recognizing the VH families VHJ558, VH7183, VHQ52, VHJ606, VHS107, VHX24, and VHGAM308 together with a nested 3′ JH4 primer. In the second round, 2 μL of the first PCR product was reamplified with a VH family–specific 5′ primer and the same nested 3′ JH4 primer. PCR products were purified on a 1.5% agarose gel by cutting the band with the appropriate size of a V(D)J rearrangement followed by TA cloning. A set of clones was sequenced using the Big Dye Terminator method and the automated DNA sequencer 377 (Applied Biosystems, Weiterstadt, Germany). Sequence analysis was performed with 4Peaks (Apple Computer, http://mekentosj.com/4peaks/), IMGT/JunctionAnalysis (The International Immunogenetics Information System, http://imgt.cines.fr/cgi-bin/IMGTjcta.jv) and IgBLAST (http://www.ncbi.nlm.nih.gov/projects/igblast/).33
Immunofluorescence confocal microscopy
Acetone-fixed thymic sections (8 μm) were incubated with polyclonal α-K5 (PRB-160B; Covalence, Princeton, NJ), α-CD3-biot (145–2C11; eBioscience), and α-K8-Cy5 Abs (TROMA-1; Developmental Studies Hybridoma Bank, University of Iowa) followed by incubation with streptavidin–Alexa 488 (Molecular Probes, Leiden, the Netherlands) and goat α-Rabbit–Alexa 555 (Molecular Probes), and finally embedded in Fluorsave (Calbiochem, San Diego, CA). Images were captured on a Zeiss LSM 510 Meta Laser Confocal Scanning Confocal Microscope System (Carl-Zeiss, Fedbach, Switzerland). Overlays of blue (Cy5), red (Alexa 455), and green (Alexa 488) stainings were colored by computer-assisted management of confocal generated data with Zeiss LSM 510 software version 3.2.
Quantitative real-time PCR
RNA extraction was performed with the Nucleospin RNA II kit (Macherey-Nagel, Düren, Germany) followed by DNase digestion with RQ1 RNase-Free DNase (Promega, Madison, WI). RNA (750 ng) was used to perform the Reverse Transcription with Oligo dT (Promega) and dNTPs (Roche, Rotkreuz, Switzerland) with the Superscript III Reverse Transcriptase (Invitrogen, Carlsbad, CA). Real-time PCR was performed with Sensimix (Quantace, Watford, United Kingdom) on a Rotor Gene RG-3000 (Corbett Research, Sydney, Australia). The following primers were used: TSLP FWD: AGGCTACCCTGAAACTGAG, TSLP RVS: GGAGATTGCATGAAGGAATACC, TBP FWD: CGTGAATCTTGGCTGTAAACT, TBP RVS: GTCCGTGGCTCTCTTATTCT. TSLP and TBP primer pairs had identical efficiency. The cycling conditions for both TSLP and TBP amplifications were 10 minutes at 95°C, followed by 40 cycles of 10 seconds at 95°C, 15 seconds at 60°C, and 20 seconds at 72°C. The relative expression of TSLP on TBP was calculated with the comparative CT (ΔΔCT) method.
Isolation of skin-resident lymphocytes
Ears were digested with collagenase IV (Sigma-Aldrich) in HBSS containing 10 mM Hepes, 2.5 mM CaCl2, and 2% FCS for 30 minutes at 37°C. Cell suspensions were filtered, stained, and analyzed by flow cytometry.
Statistical analysis
Statistical significance between the individual groups was analyzed using the unpaired Student t test.
Results
The K14-TSLP Tg rescues B- and T-cell development in IL-7−/− mice
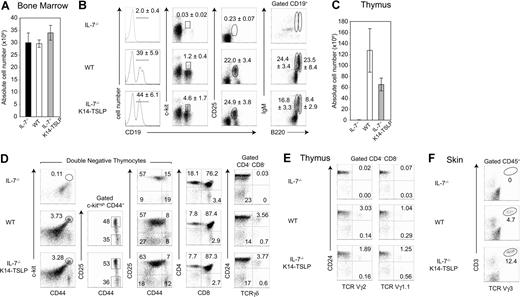
As previously reported,1,2 B lymphopoiesis is dramatically impaired in adult IL-7−/− mice as shown by the absence of CD19+ B-cell progenitors in the BM (Figure 1B). To test if increased TSLP availability could rescue B lymphopoiesis in IL-7−/− mice, K14-TSLP Tg mice were generated and backcrossed to an IL-7−/− background. Absolute numbers of cells in the BM of IL-7−/−, WT, and IL-7−/− K14-TSLP Tg mice were comparable (Figure 1A). In the BM of IL-7−/− K14-TSLP Tg mice, the number of CD19+ B cells was restored to WT numbers (Figure 1B). All B progenitor cell subsets were detectable in IL-7−/− mice overexpressing TSLP. The major effect of TSLP was found in the pro-B/pre-B-I (CD19+ c-kit+) cell compartment (4-fold increase in percentage compared with WT controls) and absolute numbers of pro-B/pre-B-I cells were 4-fold increased (S.C. and D.F., unpublished data, December 2006). Pre-B-II (CD19+ CD25+) cells were present at WT percentages in IL-7−/− K14-TSLP Tg mice. Immature (CD19+ B220+ IgM+) and mature (CD19+ B220++ IgM+) B cells in IL-7−/− K14-TSLP Tg mice were present in lower percentages than in WT mice. These results show that TSLP Tg expression was sufficient to rescue the block of B-cell development in the BM of IL-7−/− animals. In addition, these results suggest that the major target cells for TSLP were, similar to IL-7,34 pro-B/pre-B-I cells.
TSLP Tg expression rescues B- and T-cell development in IL-7−/− mice. IL-7−/−, WT, and IL-7−/− K14-TSLP Tg mice were analyzed at 12 weeks of age. (A) Absolute cell number per 2 femurs. (B) Regions indicate the pro-B/pre-B-I (CD19+ c-kit+), pre-BII (CD19+ CD25+), mature (CD19+ B220high IgM+), and immature (CD19+ B220+ IgM+) B cells in the BM. Numbers are mean and standard deviation of percentage (n = 5). (C) Absolute thymocyte number and (D) thymocyte profiles of DN1 (c-kithigh CD44+ CD25−), DN2 (c-kithigh CD44+ CD25+), DN3 (CD44− CD25+), DN4 (CD44− CD25−), DP (CD4+ CD8+), and SP (CD4+ CD8−, CD4−CD8+) thymocytes as well as of immature (CD24lo/− γδ+) and mature (CD24+ γδ+) γδ T cells are shown. (E) Pregated on CD4− CD8− cells, percentages of Vγ2 and Vγ1.1 T cells in the thymus are shown. (F) Pregated on CD45+ cells, percentages of Vγ3+ CD3+ cells in the skin are shown. Histograms represent the mean and standard deviation from analyzing 5 animals.
TSLP Tg expression rescues B- and T-cell development in IL-7−/− mice. IL-7−/−, WT, and IL-7−/− K14-TSLP Tg mice were analyzed at 12 weeks of age. (A) Absolute cell number per 2 femurs. (B) Regions indicate the pro-B/pre-B-I (CD19+ c-kit+), pre-BII (CD19+ CD25+), mature (CD19+ B220high IgM+), and immature (CD19+ B220+ IgM+) B cells in the BM. Numbers are mean and standard deviation of percentage (n = 5). (C) Absolute thymocyte number and (D) thymocyte profiles of DN1 (c-kithigh CD44+ CD25−), DN2 (c-kithigh CD44+ CD25+), DN3 (CD44− CD25+), DN4 (CD44− CD25−), DP (CD4+ CD8+), and SP (CD4+ CD8−, CD4−CD8+) thymocytes as well as of immature (CD24lo/− γδ+) and mature (CD24+ γδ+) γδ T cells are shown. (E) Pregated on CD4− CD8− cells, percentages of Vγ2 and Vγ1.1 T cells in the thymus are shown. (F) Pregated on CD45+ cells, percentages of Vγ3+ CD3+ cells in the skin are shown. Histograms represent the mean and standard deviation from analyzing 5 animals.
IL-7−/− mice have reduced thymic cellularity and lack γδ T cells.1,3 The thymus cellularity of IL-7−/− K14-TSLP Tg animals was 60-fold increased compared with IL-7−/− littermates but still remained 2-fold lower than controls (Figure 1C). Contrary to IL-7−/− mice, in which DN1 (c-kithigh CD44+ CD25−) and DN2 (c-kithigh CD44+ CD25+) thymocytes were absent, IL-7−/− K14-TSLP Tg mice showed normal percentages of the DN1 and DN2 subsets (Figure 1D). Moreover, the percentages of DN3 (CD44− CD25+), CD4+ SP, CD8+ SP, and double-positive (DP) thymocyte cells in IL-7−/− K14-TSLP Tg mice were in the range of WT controls (Figure 1D). While γδ T-cell development was undetectable in IL-7−/− mice, IL-7−/− K14-TSLP Tg mice had normal percentages of both mature (CD24lo/−) and immature (CD24high) thymic γδ+ T cells (Figure 1D). Indeed, Vγ2+ T and Vγ1.1+ T cells were generated in IL-7−/− K14-TSLP Tg mice (Figure 1E). Vγ3+ T cells, which develop during fetal life and are later found exclusively in the adult skin,35 were present in the skin of IL-7−/− K14-TSLP Tg but not in IL-7−/− mice (Figure 1F).
Mouse strains in which thymocyte development is impaired beyond the DN1 stage display an abnormal cortex with cortical thymic epithelial cells (cTECs) coexpressing keratin 5 (K5) and keratin 8 (K8).36,37 Consistent with this, the majority of the cTECs in IL-7−/− thymi retained an immature K8+ K5+ phenotype and numerous cysts were found in IL-7−/− thymi (Figure S1, available on the Blood website; see the Supplemental Materials link at the top of the online article). In contrast, IL-7−/− K14-TSLP and WT thymi were devoid of cysts and showed a normal segregation into K8+ K5− cTECs and K8− K5+ mTECs. SP thymocytes, which express high levels of CD3, were localized in the thymic medulla of IL-7−/− K14-TSLP mice. Hence, TSLP overexpression substantially increased thymus cellularity, rescued the generation of αβ and γδ T cells, and corrected the aberrant thymic architecture in IL-7−/− mice.
Peripheral B- and T-cell compartments are normal in IL-7−/− K14-TSLP Tg mice
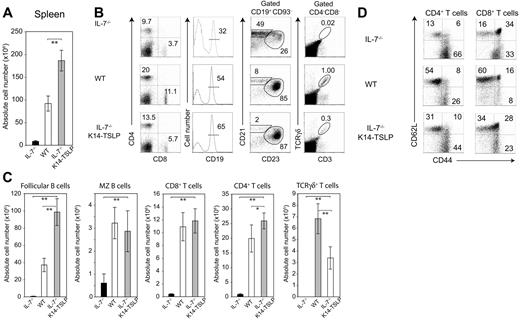
IL-7−/− mice are lymphopenic1 and their spleen cell numbers were 10-fold less than WT controls (Figure 2A). TSLP Tg expression in IL-7−/− mice increased splenocyte numbers by an average of 20-fold. The percentages of splenic CD4+ and CD8+ T cells in IL-7−/− K14-TSLP Tg mice were below WT percentages. However, absolute T-cell numbers in IL-7−/− K14-TSLP Tg mice were normal or, for CD4+ T cells, even beyond WT numbers (Figure 2C). To test if the restoration of peripheral T cells was a result of egress of thymic emigrants or peripheral expansion, we tested CD62L and CD44 expression by splenic CD4+ and CD8+ T cells. The percentage of naive (CD62Lhigh CD44low) T cells was clearly higher in IL-7−/− K14-TSLP Tg mice compared with IL-7−/− percentages but was lower than WT percentages (Figure 2D). These results suggest that the restoration of peripheral T cells in IL-7−/− K14-TSLP Tg mice was a result of both restoration of thymic development and peripheral expansion of T cells. γδ T cells were present in the spleen of IL-7−/− K14-TSLP Tg animals (Figure 2B) in numbers corresponding to half that in WT controls (Figure 2C), indicating that TSLP was less efficient than IL-7 in maintaining the peripheral γδ T-cell pool.
TSLP Tg expression restores splenic lymphocyte compartments in IL-7−/− mice. (A) Absolute splenocyte numbers of IL-7−/−, WT, and IL-7−/− K14-TSLP Tg (12-week-old). (B) Percentages and (C) absolute cell number of CD4+, CD8+, and γδ+CD3+ T cells, and CD19+CD93− mature B cells composed of follicular (CD23+ CD21+) and MZ (CD21high CD23−) B cells are shown. Histograms represent the mean and standard deviation from analyzing 5 animals. *P < .05; **P < .005 (Student t test). (D) Gated on splenic CD4+ and CD8+ T cells, CD62L and CD44 expression is shown.
TSLP Tg expression restores splenic lymphocyte compartments in IL-7−/− mice. (A) Absolute splenocyte numbers of IL-7−/−, WT, and IL-7−/− K14-TSLP Tg (12-week-old). (B) Percentages and (C) absolute cell number of CD4+, CD8+, and γδ+CD3+ T cells, and CD19+CD93− mature B cells composed of follicular (CD23+ CD21+) and MZ (CD21high CD23−) B cells are shown. Histograms represent the mean and standard deviation from analyzing 5 animals. *P < .05; **P < .005 (Student t test). (D) Gated on splenic CD4+ and CD8+ T cells, CD62L and CD44 expression is shown.
In contrast to IL-7−/− mice, the absolute number of marginal zone (MZ) B cells in IL-7−/− K14-TSLP Tg animals was comparable with WT mice (Figure 2C). Follicular B- (FB) cell numbers were even 2.5-fold increased compared with controls (Figure 2C). It has been reported that TSLP promotes the accumulation of myeloid cells in the spleen.25 In agreement with this, we found a 5- to 6-fold increase in the absolute number of Gr-1+ CD11b+ granulocytes (Figure S2). Altogether, these results indicate that TSLP promoted the accumulation of peripheral T, B, and myeloid cells.
Systemic expression of TSLP in IL-7−/− K14-TSLP Tg animals
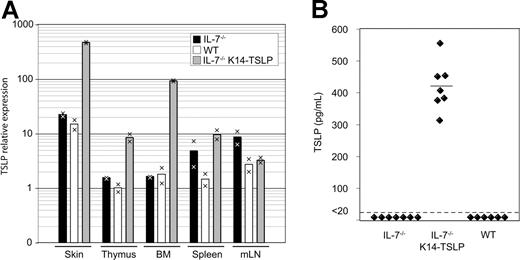
The analysis of IL-7−/− K14-TSLP Tg mice revealed that TSLP was able to support lymphopoiesis. We therefore investigated in which organs TSLP was expressed in Tg mice. Quantitative real-time PCR analysis revealed that, consistently with the K14 promoter specificity, TSLP mRNA was highly expressed in the skin (30-fold relative increase compared with WT) and thymus (8-fold) of Tg mice (Figure 3A). Interestingly, a 50-fold increase in TSLP transcripts was observed in the BM of Tg animals compared with WT controls. The amounts of TSLP transcripts in the spleen and mesenteric lymph nodes were not affected by the Tg expression. In the sera of IL-7−/− K14-TSLP Tg animals, an average concentration of 420 pg/mL TSLP was measured, while TSLP concentrations were less than 20 pg/mL in IL-7−/− and WT controls (Figure 3B). Therefore, the rescue of lymphopoiesis and of peripheral lymphocyte compartments might be the result of Tg-mediated increase of TSLP availability in primary lymphoid organs and in the serum.
TSLP is detectable in the serum of IL-7−/− K14-TSLP Tg mice. (A) TSLP expression in skin, thymus, BM, et al. Each bar displays the mean of values obtained from cDNA from 2 mice. Results are representative of 3 independent experiments. (B) TSLP concentration in sera from IL-7−/−, IL-7−/− K14-TSLP Tg, and WT mice was quantified by ELISA. Each symbol represents the result from an individual mouse. The mean of TSLP concentration is indicated.
TSLP is detectable in the serum of IL-7−/− K14-TSLP Tg mice. (A) TSLP expression in skin, thymus, BM, et al. Each bar displays the mean of values obtained from cDNA from 2 mice. Results are representative of 3 independent experiments. (B) TSLP concentration in sera from IL-7−/−, IL-7−/− K14-TSLP Tg, and WT mice was quantified by ELISA. Each symbol represents the result from an individual mouse. The mean of TSLP concentration is indicated.
In B cells of adult IL-7−/− K14-TSLP Tg mice, the IgH locus contains additional N nucleotides
TSLP was previously shown to support fetal B lymphopoiesis.20 The rescue of B-cell development in IL-7−/− K14-TSLP Tg mice could therefore be due to the effect of TSLP on fetal liver–derived progenitors. One hallmark of fetal lymphopoiesis is the absence of expression of terminal deoxynucleotidyl transferase (Tdt) in lymphocyte precursors,38 leading to the generation of B cells that are completely devoid of N nucleotides at the junctions of their rearranged VDJ immunoglobulin heavy (IgH) chain genes.39 To test whether the B cells generated in IL-7−/− K14-TSLP Tg mice originated from fetal or adult precursors, we examined whether rearranged IgH genes in B cells of 8-week-old IL-7−/− K14-TSLP Tg mice carried N region nucleotide additions. Table 1 shows that of 26 B-cell clone sequences from IL-7−/− K14-TSLP Tg adult spleen, all of them displayed N nucleotide additions. This result indicates that the B lymphopoiesis in adult IL-7−/− K14-TSLP mice was promoted by the action of TSLP on Tdt+ cells and therefore, most likely on adult BM-derived progenitors.
TSLP supports the differentiation of adult BM progenitor cells toward lymphoid lineages
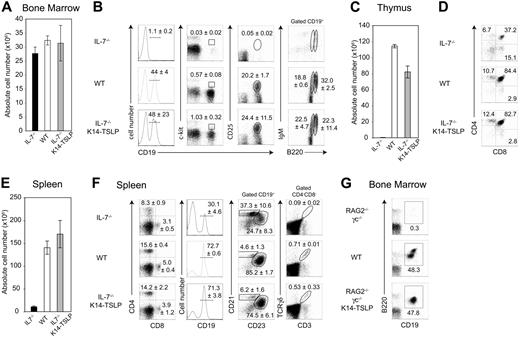
To further assess whether the lymphopoiesis observed in IL-7−/− K14-TSLP Tg mice relied on the differentiation of adult precursors, we generated BM chimeras by adoptively transferring 107 total BM cells from adult WT mice (CD45.1+) into lethally irradiated IL-7−/− K14-TSLP Tg, IL-7−/−, or WT recipients (CD45.2+). Six weeks after transfer, donor chimerism was higher than 95% in all lymphoid organs of each mouse. Donor BM cell numbers were similar for each of the 3 experimental groups (Figure 4A). Donor-derived B-cell precursors were almost undetectable in the BM of IL-7−/− recipients, whereas all stages of B-cell development were present in both IL-7−/− K14-TSLP Tg mice and WT recipients (Figure 4B). Donor-derived thymocyte numbers were increased 270-fold in IL-7−/− K14-TSLP Tg compared with IL-7−/− recipients but remained 1.4-fold below numbers of WT controls (Figure 4C). Similar percentages of donor-derived DN, CD4+ SP, CD8+ SP, and DP were found in WT and IL-7−/− K14-TSLP mice (Figure 4D). The number of donor-derived splenocytes was substantially increased in IL-7−/− K14-TSLP Tg compared with IL-7−/− hosts (Figure 4E). Normal percentage of peripheral CD4+ T cells, CD8+ T cells, and CD19+ B cells was found in reconstituted IL-7−/− K14-TSLP recipients. In agreement with our previous data, splenic B cells in IL-7−/− recipients were mainly composed of MZ B cells, while those found in IL-7−/− K14-TSLP Tg and WT recipients were mainly FB cells (Figure 4F). Donor-derived cells were able to develop into TCRγδ+ T cells in IL-7−/− K14-TSLP Tg mice. These results collectively show that TSLP could efficiently sustain the differentiation of adult BM progenitors toward both B- and T-cell lineages in vivo. Our data contrast in vitro studies showing that in adult BM only pre-BCR+ progenitors are able to respond to TSLP.40 RAG2−/− γc−/− mice are unresponsive to IL-7 and hence unable to generate pro-B cells. In contrast, TSLP Tg expression in adult RAG2−/−γc−/− mice clearly promoted pro-B-cell generation in vivo (Figure 4G).
TSLP sustains the differentiation of WT BM progenitors toward the B- and T-cell lineages in vivo. (A) Absolute cell number per 2 femurs 6 weeks after reconstitution of lethally irradiated IL-7−/−, WT, and IL-7−/− K14-TSLP Tg recipients with 107 BM cells (CD45.1+) is shown. (B) FACS profiles were gated on CD45.1+ donor cells. Regions indicate the pro-B/pre-B-I (CD19+c-kit+), pre-B-II (CD19+CD25+), mature (CD19+B220highIgM+), and immature (CD19+B220+IgM+) B cells. Numbers are mean and standard deviation of percentage (n = 3). (C) Absolute CD45.1+ thymocyte number and (D) percentages of donor-derived SP (CD4+CD8−, CD4−CD8+), DP (CD4+CD8+) thymocytes are shown. (E) Absolute CD45.1+ splenocyte number. Histograms represent the mean and standard deviation from analyzing 3 animals. (F) Regions indicate splenic CD4+, CD8+, and γδ+ T cells, and CD19+ B cells containing follicular (CD23+CD21+) and MZ (CD21high CD23−) B cells. Numbers are mean and standard deviation of percentage (n = 3). (G) RAG2−/− γc−/−, WT, and RAG2−/− γc−/− K14-TSLP Tg mice were analyzed at 10 weeks of age. Regions indicate the committed (CD19+B220+) B cells in the BM. Representative FACS analyses of 1 of 3 mice.
TSLP sustains the differentiation of WT BM progenitors toward the B- and T-cell lineages in vivo. (A) Absolute cell number per 2 femurs 6 weeks after reconstitution of lethally irradiated IL-7−/−, WT, and IL-7−/− K14-TSLP Tg recipients with 107 BM cells (CD45.1+) is shown. (B) FACS profiles were gated on CD45.1+ donor cells. Regions indicate the pro-B/pre-B-I (CD19+c-kit+), pre-B-II (CD19+CD25+), mature (CD19+B220highIgM+), and immature (CD19+B220+IgM+) B cells. Numbers are mean and standard deviation of percentage (n = 3). (C) Absolute CD45.1+ thymocyte number and (D) percentages of donor-derived SP (CD4+CD8−, CD4−CD8+), DP (CD4+CD8+) thymocytes are shown. (E) Absolute CD45.1+ splenocyte number. Histograms represent the mean and standard deviation from analyzing 3 animals. (F) Regions indicate splenic CD4+, CD8+, and γδ+ T cells, and CD19+ B cells containing follicular (CD23+CD21+) and MZ (CD21high CD23−) B cells. Numbers are mean and standard deviation of percentage (n = 3). (G) RAG2−/− γc−/−, WT, and RAG2−/− γc−/− K14-TSLP Tg mice were analyzed at 10 weeks of age. Regions indicate the committed (CD19+B220+) B cells in the BM. Representative FACS analyses of 1 of 3 mice.
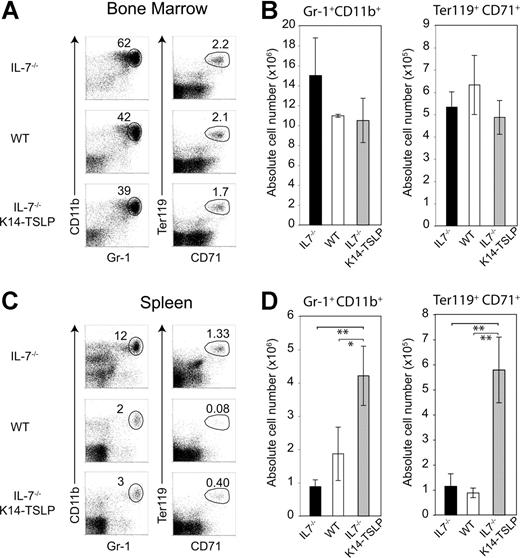
To test whether the K14-TSLP Tg had an additional effect on erythroid and myeloid compartments, we analyzed BM chimeras 6 months after reconstitution. Percentages and absolute numbers of donor-derived granulocytes (Gr-1+ CD11b+) and erythroblasts (Ter119+ CD71+) were similar in the BM of IL-7−/− K14-TSLP Tg and WT recipients (Figure 5A-B). In contrast, a significant increase in granulocyte and erythroblast numbers was found in the spleen of IL-7−/− K14-TSLP Tg mice (Figure 5C,D). Altogether, our results show that adult BM progenitors efficiently differentiated toward B and T lineages in response to TSLP and that TSLP promoted the accumulation of myeloid cells in the spleen.
TSLP Tg expression leads to the accumulation of granulocytes and erythroid precursors in the spleen. Six months after reconstitution of lethally irradiated IL-7−/−, IL-7−/− K14-TSLP, or WT recipient mice with 1 × 107 BM cells from CD45.1+ mice, recipients were analyzed. All FACS profiles were gated on CD45.1+ donor cells. (A) Granulocytes (Gr-1+ CD11b+) and erythroblasts (Ter119+ CD71+) in the BM are shown. (B) Absolute number of granulocytes and erythroblasts in BM. (C) Granulocytes (Gr-1+ CD11b+) and erythroblasts (Ter119+ CD71+) in the spleen are shown. (D) Absolute number of granulocytes and erythroblasts in spleen. *P < .05; **P < .005 (Student t test). Histograms represent the mean and standard deviation from analyzing 3 animals.
TSLP Tg expression leads to the accumulation of granulocytes and erythroid precursors in the spleen. Six months after reconstitution of lethally irradiated IL-7−/−, IL-7−/− K14-TSLP, or WT recipient mice with 1 × 107 BM cells from CD45.1+ mice, recipients were analyzed. All FACS profiles were gated on CD45.1+ donor cells. (A) Granulocytes (Gr-1+ CD11b+) and erythroblasts (Ter119+ CD71+) in the BM are shown. (B) Absolute number of granulocytes and erythroblasts in BM. (C) Granulocytes (Gr-1+ CD11b+) and erythroblasts (Ter119+ CD71+) in the spleen are shown. (D) Absolute number of granulocytes and erythroblasts in spleen. *P < .05; **P < .005 (Student t test). Histograms represent the mean and standard deviation from analyzing 3 animals.
TSLP promotes B- and early T-cell development from EPLMs in vitro
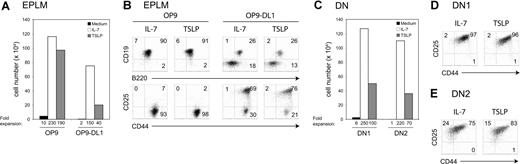
The in vivo effect of TSLP prompted us to study whether TSLP could promote lineage commitment from a lymphoid/myeloid BM progenitor cell. EPLMs have been identified as B220+ CD117+ CD19− NK1.1− BM cells that can give rise to both myeloid and lymphoid lineages.31 To test if TSLP promoted the development of B and T cells from EPLMs, we plated 5 × 103 FACS-sorted EPLMs on either OP9 or OP9 stromal cells expressing the Notch ligand Delta-like-1 (OP9-DL1) and added either TSLP or IL-7. EPLMs cultured on OP9 cells without additional cytokine did not substantially expand (Figure 6A). In contrast, cells cultured with IL-7 or TSLP underwent a 230- and a 190-fold expansion, respectively. The majority of EPLMs cultured with either TSLP or IL-7 had differentiated along the B-cell lineage, as 90% coexpressed CD19 and B220 (Figure 6B). When placed on OP9-DL1, EPLMs cultured with IL-7 or TSLP expanded 150- and 40-fold, respectively (Figure 6A). TSLP promoted the differentiation of EPLMs toward the T-cell lineage when cultured on OP9-DL1 cells (Figure 6B). Indeed, after 7 days of coculture, 76% showed a DN2 (CD25+ CD44+) phenotype, whereas 26% had entered the B lineage (CD19+ B220+). Comparably, cocultures supplemented with IL-7 gave rise to 69% DN2 cells and 26% B-committed cells. These results show that TSLP efficiently supports both B- and T-cell differentiation from adult lymphoid/myeloid BM progenitors.
TSLP promotes the expansion and the differentiation of EPLMs, and DN1 and DN2 cells in vitro. (A) Absolute cell numbers harvested after a 7-day culture period of EPLMs (B220+ CD19− NK1.1− CD117+ CD93+) on either OP9 or OP9-DL1 cells in the presence of TSLP or IL-7, or without additional cytokine. (B) The percentage of CD19+ B220+ B cells, DN1 (CD25− CD44+), DN2 (CD25+ CD44+), and DN3 (CD25+ CD44−) T-cell precursors is shown. Data shown here are from 1 representative experiment of 2. (C) Sorted DN1 (CD25− CD44+ CD117high) or DN2 (CD25+ CD44+ CD117high) cells were plated on OP9-DL1 in the presence of TSLP or IL-7, or without additional cytokine. Absolute cell numbers were calculated after 7 days of culture. Flow cytometric analysis of (D) DN1 or (E) DN2 cells cultured for 7 days on OP9-DL1 in the presence of TSLP or IL-7. Data shown here are from 1 representative experiment of 2.
TSLP promotes the expansion and the differentiation of EPLMs, and DN1 and DN2 cells in vitro. (A) Absolute cell numbers harvested after a 7-day culture period of EPLMs (B220+ CD19− NK1.1− CD117+ CD93+) on either OP9 or OP9-DL1 cells in the presence of TSLP or IL-7, or without additional cytokine. (B) The percentage of CD19+ B220+ B cells, DN1 (CD25− CD44+), DN2 (CD25+ CD44+), and DN3 (CD25+ CD44−) T-cell precursors is shown. Data shown here are from 1 representative experiment of 2. (C) Sorted DN1 (CD25− CD44+ CD117high) or DN2 (CD25+ CD44+ CD117high) cells were plated on OP9-DL1 in the presence of TSLP or IL-7, or without additional cytokine. Absolute cell numbers were calculated after 7 days of culture. Flow cytometric analysis of (D) DN1 or (E) DN2 cells cultured for 7 days on OP9-DL1 in the presence of TSLP or IL-7. Data shown here are from 1 representative experiment of 2.
DN1 and DN2 thymocytes are responsive to TSLP
We investigated which T-cell progenitors were directly responsive to TSLP. DN1 (Figure 6C,D) or DN2 (Figure 6C,E) cells were FACS sorted and cultured on OP9-DL1 stromal cells either alone or in the presence of TSLP or IL-7. Sorted DN1 cells cultured for 1 week with IL-7 underwent a 250-fold expansion, whereas cells cultured with TSLP expanded 100-fold (Figure 6C). Most of the cells had progressed to the DN2 stage after a culture period of 7 days (Figure 6D). DN2 cells grown in the presence of IL-7 or TSLP expanded 220- and 70-fold, respectively (Figure 6C). After 7 days in medium supplemented with TSLP, 15% of the originally sorted DN2 cells had a DN3 (CD25+ CD44−) phenotype, whereas 83% retained the DN2 phenotype (Figure 6E). Similar results were obtained from culturing DN2 cells with IL-7. Taken together, these results show that TSLP is able to promote the expansion and differentiation of adult DN1 and DN2 thymocytes in vitro.
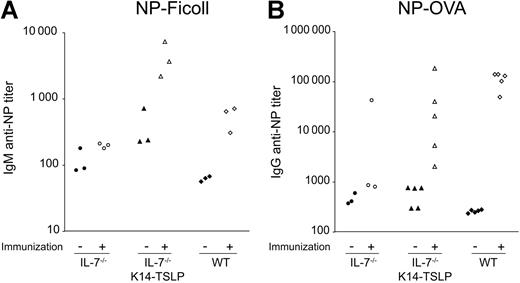
IL-7−/− K14-TSLP Tg mice efficiently mount Ab responses to T-independent and T-dependent Ags
The functionality of B and T cells developing in IL-7−/− K14-TSLP Tg mice was assessed by immunization with the T-independent Ag NP-Ficoll or the T-dependent Ag NP-OVA. Ab titers in preimmune sera from IL-7−/− K14-TSLP Tg mice and IL-7−/− mice were higher compared with preimmune sera from WT mice (Figure 7A). Ten days after intravenous immunization with 100 μg NP-Ficoll, IL-7−/− mice failed to mount a significant anti–NP IgM response. In contrast, IL-7−/− K14-TSLP Tg animals mounted a clear anti–NP IgM response reflected by a 10-fold increase in specific Ab titer (Figure 7A), an increase similar to that seen in WT controls.
NP-specific Ab titers in the sera of immunized mice. Ten- to 12-week-old IL-7−/− K14-TSLP Tg, IL-7−/−, and C57Bl/6 mice were immunized and sera were analyzed as indicated. Each symbol represents the result from an individual mouse. Filled symbols represent titers prior to immunization; empty symbols represent titers after immunization. (A) Titers of NP-specific IgM prior and 10 days after intravenous NP-Ficoll immunization. (B) Titers of NP-specific IgG titers prior and 15 days after intraperitoneal NP-OVA immunization.
NP-specific Ab titers in the sera of immunized mice. Ten- to 12-week-old IL-7−/− K14-TSLP Tg, IL-7−/−, and C57Bl/6 mice were immunized and sera were analyzed as indicated. Each symbol represents the result from an individual mouse. Filled symbols represent titers prior to immunization; empty symbols represent titers after immunization. (A) Titers of NP-specific IgM prior and 10 days after intravenous NP-Ficoll immunization. (B) Titers of NP-specific IgG titers prior and 15 days after intraperitoneal NP-OVA immunization.
When immunized with 50 μg of the T-dependent Ag NP-OVA, IL-7−/− K14-TSLP Tg mice showed an increase in anti–NP IgG titers (Figure 7B). These results indicated that both B and T cells generated in IL-7−/− K14-TSLP Tg mice were functional and could effectively collaborate in mounting an Ab response to a T-dependent Ag. Further investigation will be required to understand the mechanisms underlying the high variation in anti–NP IgG titers observed in IL-7−/− K14-TSLP Tg immunized mice. Taken together, our results show that B and T cells generated in IL-7−/− K14-TSLP Tg mice were functional.
Discussion
Mouse models for studying the function of TSLP have left several questions unanswered regarding its role in adult B- and T-cell differentiation and myeloid cell expansion. We show here a so-far-unappreciated capacity of TSLP to promote B- and T-cell development in adult mice. BM lymphoid/myeloid progenitors as well as DN thymocytes were responsive to TSLP. In the absence of IL-7, TSLP overexpression was able to restore central and peripheral lymphoid compartments and to amplify myeloid cells in the periphery.
TSLP mRNA is expressed at low levels in primary lymphoid organs26 and TSLP concentrations are low (< 20 pg/mL) in the serum of WT and IL-7−/− mice41,42 (Figure 3). These endogenous TSLP levels fail to overcome IL-7 deficiency. Here, we show that TSLP serum concentrations of 420 pg/mL were sufficient to compensate for the lack of IL-7. While even lower levels of TSLP affect B-cell development in a WT background,24 the minimal concentration that is required locally or in the serum to sustain lymphopoiesis in absence of IL-7 remains to be determined.
TSLP overexpression restored all stages of B-cell differentiation in the BM of IL-7−/− mice (Figure 1B). Adoptively transferred adult WT BM progenitors replenished all B-cell compartments in BM and spleen of IL-7−/− K14-TSLP Tg recipient (Figure 4). Together with the finding that IgH junctions of B cells displayed N regions (Table 1), our data indicate that adult BM progenitors were responsive to TSLP. This is further supported by the fact that TSLP induced the differentiation of adult EPLMs toward CD19+ B cells in vitro (Figure 6). In addition, pro-B cells developed in RAG2−/− γc−/− mice in response to TSLP Tg expression (Figure 4G). These results contrast a previous study showing that in vitro, pre-BCR expression was required for adult B-cell precursors to be TSLP responsive.40 The differences in our findings might be a result of different requirements for B-cell development in vitro and in vivo.
This study further shows that TSLP promotes the differentiation of adult BM progenitors toward T-cell lineage in vitro (Figure 6A,B) and that DN1 and DN2 cells are directly responsive to TSLP (Figure 6D-E). TSLP Tg expression restored DN1 and DN2 thymocyte compartments in IL-7−/− mice (Figure 1D) and normalized the organization of the thymus (Figure S1). Since DN thymocytes play a crucial role in cTEC maturation,36,37 our results suggest that the TSLP-driven generation of DN1 and DN2 cells corrected cTEC differentiation in IL-7−/− mice. TSLP Tg expression also rescued the generation of γδ T cells. In IL-7−/− fetal thymus, Vγ3+ T cells are generated but do not persist in the adult.3 The presence of Vγ3+ T cells in the skin of IL-7−/− K14-TSLP Tg mice shows that TSLP could replace IL-7 in maintaining the dendritic epidermal Vγ3+ T cells.
The effect of TSLP on in vitro and in vivo thymocyte expansion was lower compared with IL-7. This could be due to a less stimulatory activity of TSLP on thymocytes. Indeed, TSLP has a weaker effect on α-CD3 stimulated SP thymocytes than IL-7.12 Alternatively, it is possible that the frequency of TSLP-responding precursors might be lower than those of IL-7–responding precursors.
In the spleen, however, absolute T-cell numbers were restored and CD4+ T cells were even significantly increased in IL-7−/− K14-TSLP mice compared with WT controls. A substantial percentage of CD4+ and CD8+ T cells in IL-7−/− K14-TSLP Tg mice showed a naive CD62L+ CD44− phenotype, indicating that they were recent thymic emigrants. On the other hand, 44% of CD4+ and 23% of CD8+ T cells in IL-7−/− K14-TSLP mice showed a CD44high activated phenotype (Figure 2D), suggesting that TSLP-driven peripheral T-cell expansion could compensate for the relatively low thymic output. This result might explain the high variation of NP titer observed in T-dependent Ab response (Figure 7B), as activated T cells might be less efficient in their helper function.
The robust accumulation of FB cells in response to TSLP overexpression confirms previous results in K5-TSLP Tg mice24 and is reminiscent of the effect of IL-7.43 In addition, TSLP promoted the accumulation of myeloid precursors in the spleen. Whether this is a direct or indirect in vivo effect of TSLP remains to be clarified. High systemic concentration of TSLP in β-actin–TSLP Tg mice leads to myeloid hyperplasia in the spleen and, surprisingly, impairs lymphopoiesis.25 In contrast, we find that TSLP can sustain T and B lymphopoiesis. This discrepancy might be a result of significantly lower amounts of TSLP Tg expressed in our mouse model. It will be important to elucidate the mechanism by which high amounts of TSLP inhibit lymphopoiesis.
Altogether, our data demonstrate that TSLP can promote adult B and T lymphopoiesis, restore peripheral lymphocyte compartments, and induce peripheral accumulation of myeloid cells. The compromised lymphopoiesis found in IL-7−/− mice might therefore not be due to the inability of adult hematopoietic cells to respond to TSLP but is rather a consequence of limited TSLP availability.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This work was supported by the Swiss National Science Foundation (SNF) grant PPOOA-68855, the Gottfried und Julia Bangerter-Rhyner foundation, and the Swiss life foundation (D.F.). S.C. was supported by a Roche Research Foundation Fellowship. A.G.R. is holder of the chair of Immunology endowed by Hoffman-La Roche Ldt Basel. The AGR laboratory is financially supported by the SNF.
We thank R. Ceredig, D. van Essen, S. Infantino, and H. Acha-Orbea for helpful discussions and comments on the paper; J. Kirberg for providing RAG2−/− γc−/− mice; A. Wilson for the generous Ab gift; and J. Gill for valuable help with confocal microscopy.
Authorship
Contribution: D.F. designed research; S.C. and A.G.R. participated in designing research; S.C. performed research; A.G.F., L.F., and A.G.R participated in performing research; A.G.F. contributed vital reagent; S.C., D.F., and A.G.R. analyzed and interpreted data; S.C. and D.F. wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Daniela Finke, Developmental Immunology, Center for Biomedicine, Department of Clinical and Biological Sciences, University of Basel, Mattenstrasse 28, 4058 Basel, Switzerland; e-mail: daniela.finke@unibas.ch.