Both pregnancy and essential thrombocytosis (ET) are hypercoagulable states.
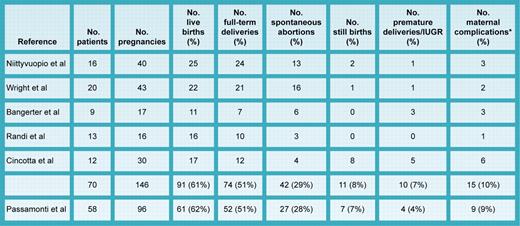
When they coexist, the complication risk for both mother and fetus is magnified, but which complications, how magnified, and for whom are still unresolved questions. In this issue of Blood, Passamonti and colleagues report the largest series yet of pregnant ET patients with special reference to JAK2 V617F expression. When compared with other representative series (see table; citations available in Griesshammer et al1 ), the live birth rate (64%), full-term delivery rate (51%), spontaneous abortion (28%) and stillbirth (7%) rates were identical. Importantly, complication risk did not correlate with maternal age, parity, thrombophilia, leukocyte or platelet counts, thrombosis history, or antiplatelet therapy; JAK2 V617F expression was the only significant risk factor. However, at the same time, the fall in platelet count during pregnancy was not different for JAK2 V617F–negative versus–positive patients, and notably, despite the apparent adverse effect of JAK2 V617F expression, there was no correlation of pregnancy outcome with that of a prior pregnancy. Furthermore, aspirin therapy was not only ineffective in JAK2 V617F–positive patients, but was actually deleterious in JAK2 V617F–negative patients; similarly, prior cytoreductive therapy was also harmful.
Summary of pregnancy outcomes in ET. *Maternal complications included hypertension, eclampsia, preeclampsia, placental abruption, ectopic pregnancy, and hemorrhage. IUGR indicates intrauterine growth retardation.
Summary of pregnancy outcomes in ET. *Maternal complications included hypertension, eclampsia, preeclampsia, placental abruption, ectopic pregnancy, and hemorrhage. IUGR indicates intrauterine growth retardation.
How should we interpret these seemingly contradictory observations? First, as usual with ET, both small patient numbers and ascertainment bias provide numerators without adequate denominators. Second, given the disparate assays used in this report, the definition of JAK2 V617F positivity is a moving target, and 3.9% expression would be considered negative by some investigators. On the other hand, JAK2 V617F platelet expression was not analyzed in patients lacking neutrophil expression.2 Third, it has been claimed that JAK2 V617F–positive ET patients are polycythemia vera (PV)–like with respect to hemoglobin, leukocyte counts, and thrombosis proclivity.3 Yet, in the present study, no hazard ratio was provided for the hemoglobin level. Given the range of hemoglobin levels cited, in light of the discredited World Health Organization (WHO) PV diagnostic criteria,4 it is simply not sufficient to state that no patient had PV. Most importantly, however, the authors have encountered the statistical phenomenon of regression toward the mean,5 which explains the lack of concordance between successive pregnancy outcomes, and which can only be avoided by a randomized, placebo-controlled trial.
How then should these data be used clinically? First, ET is a journey, not a destination; isolated thrombocytosis is no more sufficient for a diagnosis of ET than leukocytosis is for chronic myeloid leukemia (CML). In approximately 10% of patients, ET transforms to PV, and in many PV patients, the disease masquerades as ET because in women with PV, an elevated plasma volume frequently masks the increase in red cell mass.6 A normal hematocrit level in a pregnant ET patient is a warning sign and needs to be reduced by phlebotomy. Second, prior cytoreductive therapy with hydroxyurea should be avoided if at all possible, particularly since it is only protective against transient ischemic attacks and not arterial or venous thrombosis.7 Indeed, based on all studies to date, treating the platelet count during pregnancy in the absence of bleeding or microvascular symptoms is inappropriate. Third, patient education is essential for informed decision making, and finally, given the statistical issues, a registry of pregnant ET patients together with randomized, controlled clinical trials is our only hope for obtaining truly useful knowledge about this important clinical problem.
Conflict-of-interest disclosure: The author declares no competing financial interests. ■