Abstract
Regulatory T cells can inhibit harmful immunopathologic responses directed against self and foreign antigens and play a major role in controlling autoimmunity. Here we have identified and characterized a subpopulation of CD4 and CD8 T cells in human peripheral blood expressing the immune tolerizing molecule HLA-G. HLA-G–expressing T cells are hypoproliferative, are CD25- and FOXP3-negative, and exhibit potent suppressive properties that are partially mediated by HLA-G. HLA-G–positive (HLA-Gpos) T cells are found at low percentages among CD4 and CD8 single-positive thymocytes, suggesting a thymic origin. The presence of HLA-Gpos T cells at sites of inflammation such as inflamed skeletal muscle in myositis or the cerebrospinal fluid of patients with acute neuroinflammatory disorders suggests an important function in modulating parenchymal inflammatory responses in vivo.
Introduction
The immune system must distinguish between self and nonself structures, but also between harmful and innocuous foreign antigens to prevent nonessential and self-destructive immune responses. Compelling evidence indicates that regulatory T cells play an important role in the maintenance of immune tolerance under nonpathologic as well as under pathologic conditions.1-3 Several subsets of T lymphocytes with the ability to down-regulate the proliferation of effector cells have been described in mice and humans. Different types of regulatory T cells can be differentiated by distinct suppressive mechanisms and by their origin. Naturally occurring CD4+CD25+ regulatory T cells (Tregs) exert their suppressive effects via cell contact, although the nature of the involved molecules is still elusive.4 The suppressive capacity of a second subset, type 1 T regulator (Tr1) cells and Th3 cells, is contact independent and is based mainly on cytokines such as IL-10 and TGF-β.5 It seems that these cells, in contrast to the naturally occurring CD4+CD25+ Tregs, represent altered states of differentiation rather than a unique cell lineage. Furthermore, other T cells with suppressive activity include the antigen-specific CD8+CD28−6 and the TCR αβ double-negative (CD4−CD8−) T-cell subsets.7
HLA-G is a HLA class Ib antigen characterized by a restricted tissue expression, a low polymorphism, and 7 isoforms (HLA-G1 to -G7).8 HLA-G1 has a structure similar to that of classic HLA class I molecules: a heavy chain noncovalently associated with β2-microglobulin and a nonameric peptide. Although capable of acting as a peptide-presenting molecule, its strong immune-inhibitory properties identify HLA-G as a mediator of immune tolerance with specific relevance at immune-privileged sites such as trophoblast or thymus: HLA-G inhibits allogeneic proliferation of T cells,9-12 natural killer cell cytotoxicity,13-16 as well as antigen-specific T-cell cytotoxicity.12,17,18 Its direct modulatory function is mediated via 3 inhibitory receptors, ILT2 = CD85j, ILT4 = CD85d, and KIR2DL4 = CD158d.19-21 Ectopic expression of HLA-G has been identified as one of the mechanisms used by tumor cells to escape immune surveillance17,22-24 and to modulate inflammation in autoimmune disorders.25-27 HLA-G molecules are induced after allograft transplantation28,29 and during mixed lymphocyte reactions.11,30 Under such alloreactive conditions, T cells are capable of secreting soluble HLA-G (sHLA-G), which suppresses alloproliferative responses.30 In nice agreement, soluble HLA-G has been associated with the modulation of graft acceptance outcome.28,29 Expression of membrane-bound HLA-G has also been described on T cells under conditions such as after transplantation and in human immunodeficiency virus (HIV)–infected patients.30,31
We report here that CD4 and CD8 T cells expressing HLA-G are present in human peripheral blood under normal physiologic conditions. These naturally occurring cells show potent suppressive function, can be detected in the thymus and are different from the naturally occurring CD4+CD25+ Tregs. We could detect these cells in target organs of patients with neuroinflammatory disorders, supporting the assumption of their role in modulating peripheral inflammation in vivo. Altogether, our data provide evidence for the identification of a novel subset of naturally occurring regulatory T cells characterized by cell surface expression of HLA-G.
Materials and methods
Blood, CSF, and thymus samples
Phenotypic and functional analysis of HLA-G on T cells was performed with human peripheral blood obtained from healthy donors (n = 28; male-female = 17:11; mean age, 31.5 years; range, 22-55 years). Paired blood and CSF samples were obtained from 34 patients (18 female, 16 male) referred to the Department of Neurology (University of Wuerzburg) for diagnostic lumbar puncture. Fifteen patients with multiple sclerosis (MS), 10 with other inflammatory neurologic diseases (OIND), and 9 with noninflammatory neurologic diseases (NIND) (mean age, 47 years; range, 17-82 years; Table S1, availabel on the Blood website; see the Supplemental Materials link at the top of the online article) were assessed by flow cytometry. Patients with MS were diagnosed according to criteria of McDonald et al.32 Patients included in the study were without corticosteroids for at least 2 months. All patients gave informed consent in accordance with the Declaration of Helsinki and a protocol approved by the review boards of the University of Tuebingen and the University of Wuerzburg. CSF (8-20 mL) was obtained by lumbar puncture from all patients. At the same time, 10 mL peripheral blood was collected by venous puncture.
Normal human thymus tissue was obtained from 6 infants (aged 14 days to 12 months) undergoing corrective cardiac surgery following the institutional review board guidelines of the university clinic of Tuebingen.
Preparation of peripheral blood mononuclear cells (PBMCs), isolation of HLA-Gpos T cells, and generation of dendritic cells (DCs); antibodies and flow cytometry; and immunohistochemistry are described in Document S1.
In vitro culture of CD4 HLA-Gpos T cells
For assessing stability of HLA-G expression over time, CD4 HLA-Gpos and HLA-Gneg T cells were cultured in standard culture medium, RPMI (Gibco, Karlsruhe, Germany) with l-glutamine and 25 mM HEPES, 10% human AB serum (PAA Laboratories, Linz, Austria); 20 μg/mL gentamicin (Biowhittaker, Verviers, Belgium); 100 IU/mL penicillin, 100 μg/mL streptomycin (Gibco); and 2 mM l-glutamine (Biowhittaker), for a period of 3 days. HLA-G expression was assessed by flow cytometry.
T-cell stimulation assays
We stimulated T cells or isolated CD4/8 HLA-Gpos and HLA-Gneg T-cell subsets in standard culture medium with CD3/CD28 beads (Dynal, Oslo, Norway) or glatirameracetate (GA; TEVA, Petach Tikwa, Israel). T-cell proliferation was measured after 3 days in culture and an additional 16-hour pulse with [3H] Tdr (18.5 kBq per well) using a liquid scintillation counter.
For allogeneic stimulation, freshly isolated T cells (whole CD4 T cells, and CD4 HLA-Gpos/neg and CD8 HLA-Gpos/neg T cells; 1–3 × 105 per well in a 96-well plate; triplicates) were cultured in the presence of different numbers of irradiated DCs or in a mix lymphocyte reaction (MLR) using a mix of allogeneic PBMCs from 5 different healthy donors (donor mix). For further information see Document S1.
Suppression assays
Lymphocyte proliferation of CD4 or CD8 T cells in the presence of suppressor cells was assessed by flow cytometry after CFDA-SE labeling of the effector/responder lymphocytes. CD4 or CD8-effector/responder cells (1 × 106) were incubated in 500 μL PBS containing 10 μM CFDA-SE (carboxyfluorescein diacetate, succinimidyl ester; Molecular Probes, Karlsruhe, Germany) and subsequently quenched with medium containing 15% FCS.
To assess the suppressive nature of CD4 and CD8 HLA-Gpos T cells, CFDA-SE–labeled CD4 or CD8 HLA-Gneg T cells (0.3-1 × 105 cells per well) were stimulated in a physiologic manner with 1 μg/mL soluble anti-CD3 (OKT3) in the presence of irradiated (33 Gray) allogeneic cells to provide optimal costimulation, and with increasing numbers of CD4 or CD8 HLA-Gpos T cells as indicated in the figure legends. We measured proliferation on day 4 by flow cytometry. Suppression was calculated after normalization of the values to a maximum given by the proliferation of CD4 or CD8 HLA-Gneg T cells alone. For further information and a description of cytokine assays, see Document S1.
RNA extraction, cDNA synthesis, and quantitative real-time PCR
Total RNA extraction and first-strand cDNA synthesis were performed by standard methods. Quantitative analysis of gene expression was performed by reverse-transcription–polymerase chain reaction (RT-PCR) using the ABI prism 7000 Sequence Detection System (Applied Biosystems, Foster City, CA).33 cDNA templates were amplified for IFN-γ, IL-10, TGF-β, and 18S using Sybr Green PCR Master Mix (Eurogentec, Cologne, Germany). For Foxp3 and HLA-G, cDNAs were amplified using qPCR MasterMix Plus (Eurogentec, Cologne, Germany) and a primer pair with probe kit (MGB Probe; Applied Biosystems). Relative quantification was performed with the 2−ΔΔCt-method (Applied Biosystems user bulletin). Samples were normalized to 18S rRNA to account for the variability in the initial concentration of the total RNA and conversion efficiency of the reverse-transcription reaction. For cytokine and FoxP3 expression of CD4 HLA-Gpos and CD4 HLA-Gneg T cells mRNA from unstimulated PBMCs and for the expression of HLA-G mRNA of JEG3 cells were used as calibrators. Oligonucleotides used in this study are as follows: IFN-γ forward, 5′-TTCAGCTCTGCATCGTTTTG and IFN-γ reverse, 5′-CTTTCCAATTCTTCAAAATGCC; IL-10 forward, 5′-GTT TTA CCT GGA GGA GGT GAT and IL-10 reverse, 5′-GGC CTT GCT CTT GTT TTC AC; TGF-β forward, 5′-GCC CTG GAC ACC AAC TAT TG and TGF-β reverse, 5′-CTG GTC CAG GCT CCA AAT; 18S forward, 5′-CGG CTA CCA CAT CCA AGG AA and 18S reverse, 5′-GCT GGA ATT ACC GCG GCT.
Statistical analysis
For statistical analysis of the blood and CSF we used paired t tests. Each paired t test was conducted for CD4 and CD8 HLA-Gpos frequencies in NIND, OIND, and MS patients. For comparison of acute versus stable disease, again paired t test was applied. The P values were calculated 2-tailed and in all cases considered statistically significant if P was less than .05.
Results
A subset of human CD4 and CD8 T cells in peripheral blood expresses the nonclassic MHC molecule HLA-G
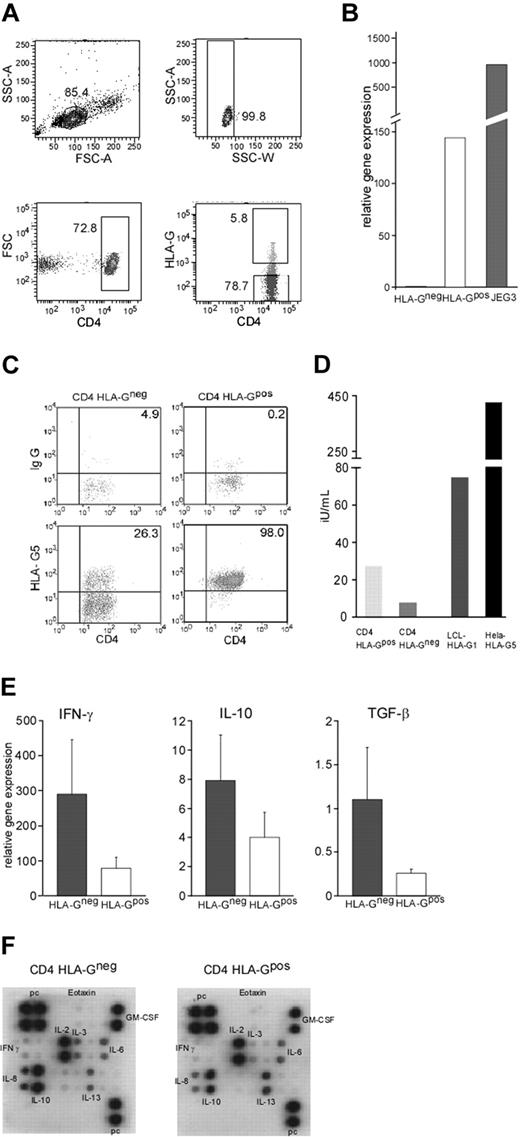
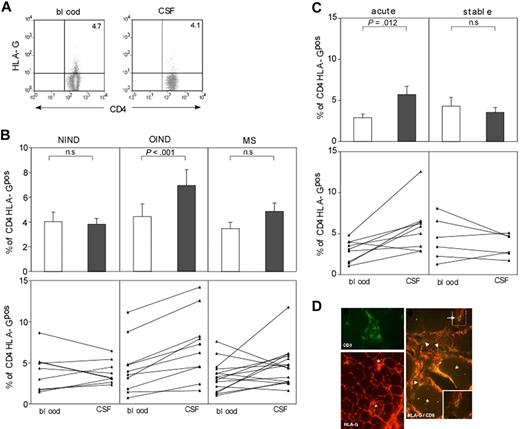
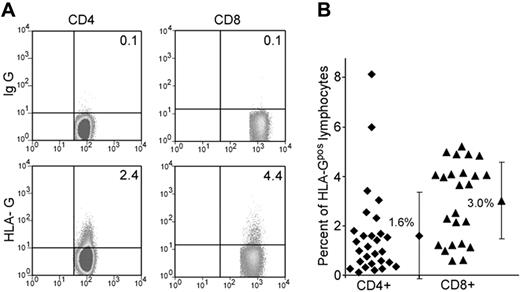
Recent data suggest that the expression and function of HLA-G are broader than initially thought. We first analyzed whether human peripheral blood T cells express HLA-G under physiologic conditions. Peripheral blood from healthy donors (ages 22 to 50 years) was assessed for HLA-G1 expression on CD4 and CD8 T cells by flow cytometry using the monoclonal antibody (mAb) MEM-G9 (Figure 1A). Using mAb MEM/G9 we found that an average of 1.6% (range, 0.1%-8.3%; n = 28) of CD4 T cells and 3.3% (range, 0.6-5.2%; n = 24) of CD8 T cells express HLA-G on the cell surface (HLA-Gpos T cells; Figure 1B). These findings were confirmed using another monoclonal antibody against HLA-G, mAb 87G (data not shown). Neither expression levels nor frequency of HLA-Gpos T cells correlated statistically with age and sex in healthy donors (data not shown). Phenotypical analysis of HLA-Gpos T cells showed a bias toward more naive (CD45RA+, CCR7+) phenotype in CD8 HLA-Gpos T cells (69.3% ± 13.7% naive T cells in CD8 HLA-Gpos versus 39.5% ± 1.7% naive T cells in CD8 HLA-Gneg cells; n = 5). CD4 HLA-Gpos T cells did not differ in their CCR7/CD45RA distribution from their HLA-Gneg counterparts (Figure S1). We next performed a detailed phenotypical analysis of HLA-Gpos T cells. No evidence of activation of the HLA-Gpos subset was revealed by measuring the expression of CD28, ICOS, PD-1, CTLA-4, CD40L (CD154), CD95, or the alpha/beta TCR. The expression of CD31 (PECAM-1) was higher in HLA-Gpos CD8 T cells (MFI CD8 HLA-Gneg = 22.58 ± 16.74, mean MFI CD8 HLA-Gpos = 26.34 ± 17.30; P = .002), possibly reflecting their more naive phenotype (Table S2; Figure S1). Flow cytometric analysis of the TCR Vβ chains did not show repertoire differences between the HLA-Gpos T cells and their HLA-Gneg counterparts, therefore making it unlikely that HLA-Gpos T cells reflect populations with a skewed TCR (data not shown). Thus, we identified a sizable population of HLA-G–expressing T cells both in the CD4 and the CD8 population in the blood of healthy donors. At first glance, these cells show no other obvious phenotypic differences than a more naive phenotype of CD8 HLA-Gpos T cells.
Frequencies and phenotype of HLA-Gpos T cells in peripheral blood. (A) FACS staining of peripheral blood lymphocytes from healthy donors for HLA-G on CD4 and CD8 lymphocytes. Gates for CD4 and CD8 T cells were set on FSC/CD4 and FSC/CD8 dot blots to identify the desired subsets. HLA-G expression (bottom panel) was assessed in comparison with the control isotype staining (top panel). A representative staining is shown. (B) Percentages of HLA-Gpos T cells in the CD4+ or CD8+ subpopulations of healthy volunteers (mean and SD).
Frequencies and phenotype of HLA-Gpos T cells in peripheral blood. (A) FACS staining of peripheral blood lymphocytes from healthy donors for HLA-G on CD4 and CD8 lymphocytes. Gates for CD4 and CD8 T cells were set on FSC/CD4 and FSC/CD8 dot blots to identify the desired subsets. HLA-G expression (bottom panel) was assessed in comparison with the control isotype staining (top panel). A representative staining is shown. (B) Percentages of HLA-Gpos T cells in the CD4+ or CD8+ subpopulations of healthy volunteers (mean and SD).
HLA-Gpos T cells show a reduced proliferation to allogeneic and polyclonal stimuli
To investigate the functional properties of HLA-G–bearing T cells, we established a multistep, magnetic bead–based purification protocol for CD4 HLA-Gpos and CD8 HLA-Gpos T cells. Briefly, CD4 or CD8 T cells are first isolated by negative selection and subsequently the HLA-Gpos cells are labeled with a biotinylated anti–HLA-G antibody (MEM-G9) and pulled down using antibiotin magnetic beads (“Materials and methods”). In all cases, the purity of the selected cells was between 60% and 95% (Figure 2A). To assess stability of HLA-G cell surface expression, purified subsets of CD4 HLA-Gpos and HLA-Gneg T cells were cultured ex vivo and HLA-G expression was determined by serial fluorescence-activated cell sorting (FACS) analysis. Percentages of HLA-G–positive T cells were normalized to HLA-G expression of the ex vivo population (set to 1). Levels of HLA-G expression did not change significantly over 72 hours in culture (Figure 2B). Stimulation of HLA-G–negative T cells using anti-CD3/CD28 beads, PHA, or anti-CD3 alone did not lead to an up-regulation of HLA-G, therefore suggesting that cell surface HLA-G is a stable phenotype rather than an inducible phenomenon (data not shown). Of note, the initial drop of HLA-G observed at 24 hours is most likely due the presence of the antibody used for bead separation in the initial (ex vivo) sample.
CD4 HLA-Gpos T cells show reduced proliferation to allogeneic or polyclonal stimuli. (A) Isolation of CD4 HLA-Gpos T cells by sequential magnetic-activated cell sorting (MACS) bead separation and analysis of the different steps of the isolation by flow cytometry. From top to bottom, dot plots show staining for whole PBMCs before separation, purified CD4 T cells, the CD4 HLA-Gneg T-cell fraction, and the CD4 HLA-Gpos T-cell fraction. Counterstaining for CD14 indicates that there are no contaminating monocytes in the preparation. (B) MACS-sorted CD4 HLA-Gneg and HLA-Gpos T cells were cultured without stimulus for 72 hours to assess HLA-G expression over time. Ex vivo expression of CD4 HLA-Gpos T cells was set as reference. Graph shows summary of 3 experiments. (C) After MACS sorting, whole CD4 (▴), CD4 HLA-Gpos (●), and CD4 HLA-Gneg T cells (■) were stimulated with allogeneic mature DCs at different ratios and proliferation was determined by [3H] incorporation after 4 days. (D) Whole CD4 ( ), CD4 HLA-Gpos (
), CD4 HLA-Gpos ( ), and CD4 HLA-Gneg (
), and CD4 HLA-Gneg ( ) T cells were isolated and stimulated with anti-CD3/CD28 beads. Proliferation was determined after 4 days by [3H] incorporation. (E) CD4 HLA-Gpos (
) T cells were isolated and stimulated with anti-CD3/CD28 beads. Proliferation was determined after 4 days by [3H] incorporation. (E) CD4 HLA-Gpos ( ) and CD4 HLA-Gneg (
) and CD4 HLA-Gneg ( ) T cells were isolated and stimulated with mature allogeneic DCs in the presence or absence of 500 U/mL IL-2. Proliferation was determined after 4 days. (F) CD4 HLA-Gpos (
) T cells were isolated and stimulated with mature allogeneic DCs in the presence or absence of 500 U/mL IL-2. Proliferation was determined after 4 days. (F) CD4 HLA-Gpos ( ) or CD4 HLA-Gneg T cells (
) or CD4 HLA-Gneg T cells ( ) were stimulated for 4 days with allogeneic PBMCs and restimulated again with PBMCs (allo MLR, left) or CD3/CD28 beads (right). Proliferation was determined 3 days later. A representative experiment (1 of at least 3) is shown for panels C-F. Error bars indicate standard error of the mean (SEM).
) were stimulated for 4 days with allogeneic PBMCs and restimulated again with PBMCs (allo MLR, left) or CD3/CD28 beads (right). Proliferation was determined 3 days later. A representative experiment (1 of at least 3) is shown for panels C-F. Error bars indicate standard error of the mean (SEM).
CD4 HLA-Gpos T cells show reduced proliferation to allogeneic or polyclonal stimuli. (A) Isolation of CD4 HLA-Gpos T cells by sequential magnetic-activated cell sorting (MACS) bead separation and analysis of the different steps of the isolation by flow cytometry. From top to bottom, dot plots show staining for whole PBMCs before separation, purified CD4 T cells, the CD4 HLA-Gneg T-cell fraction, and the CD4 HLA-Gpos T-cell fraction. Counterstaining for CD14 indicates that there are no contaminating monocytes in the preparation. (B) MACS-sorted CD4 HLA-Gneg and HLA-Gpos T cells were cultured without stimulus for 72 hours to assess HLA-G expression over time. Ex vivo expression of CD4 HLA-Gpos T cells was set as reference. Graph shows summary of 3 experiments. (C) After MACS sorting, whole CD4 (▴), CD4 HLA-Gpos (●), and CD4 HLA-Gneg T cells (■) were stimulated with allogeneic mature DCs at different ratios and proliferation was determined by [3H] incorporation after 4 days. (D) Whole CD4 ( ), CD4 HLA-Gpos (
), CD4 HLA-Gpos ( ), and CD4 HLA-Gneg (
), and CD4 HLA-Gneg ( ) T cells were isolated and stimulated with anti-CD3/CD28 beads. Proliferation was determined after 4 days by [3H] incorporation. (E) CD4 HLA-Gpos (
) T cells were isolated and stimulated with anti-CD3/CD28 beads. Proliferation was determined after 4 days by [3H] incorporation. (E) CD4 HLA-Gpos ( ) and CD4 HLA-Gneg (
) and CD4 HLA-Gneg ( ) T cells were isolated and stimulated with mature allogeneic DCs in the presence or absence of 500 U/mL IL-2. Proliferation was determined after 4 days. (F) CD4 HLA-Gpos (
) T cells were isolated and stimulated with mature allogeneic DCs in the presence or absence of 500 U/mL IL-2. Proliferation was determined after 4 days. (F) CD4 HLA-Gpos ( ) or CD4 HLA-Gneg T cells (
) or CD4 HLA-Gneg T cells ( ) were stimulated for 4 days with allogeneic PBMCs and restimulated again with PBMCs (allo MLR, left) or CD3/CD28 beads (right). Proliferation was determined 3 days later. A representative experiment (1 of at least 3) is shown for panels C-F. Error bars indicate standard error of the mean (SEM).
) were stimulated for 4 days with allogeneic PBMCs and restimulated again with PBMCs (allo MLR, left) or CD3/CD28 beads (right). Proliferation was determined 3 days later. A representative experiment (1 of at least 3) is shown for panels C-F. Error bars indicate standard error of the mean (SEM).
Next, ex vivo isolated CD4 HLA-Gpos T cells were analyzed for their capacity to proliferate to several stimuli. CD4 HLA-Gpos T cells showed a reduced proliferation to allogeneic mature dendritic cells (DCs) and to anti-CD3/CD28 beads compared with the entire CD4 T-cell population or to their HLA-G–negative counterparts (Figure 2C-D). In accordance to the CD4 subsets, we also found a reduced proliferation of CD8 HLA-Gpos T cells to polyclonal or allogeneic stimulus compared with CD8 HLA-Gneg T cells (Figure S2A). Next, we assessed if this hypoproliferative nature could be overcome by addition of IL-2 or repeated stimulation (secondary stimulatory conditions). Therefore we first stimulated CD4 HLA-Gpos and HLA-Gneg T cells with mature DCs in the absence or presence of exogenous IL-2. Although the proliferation of CD4 HLA-Gneg T cells was increased by the addition of IL-2, it had virtually no effects on the proliferation of CD4 HLA-Gpos T cells (Figure 2E). Furthermore, we analyzed the behavior of CD4 HLA-Gpos T cells in secondary proliferation assays. After a first round of allogeneic stimulation, cells were restimulated with either the previous allogeneic stimulus or with CD3/CD28 beads (Figure 2F). In both cases, CD4 HLA-Gpos T cells remained resistant to the induction of proliferation, while CD4 HLA-Gneg cells were responsive. Very similar results were achieved by a second, independent method measuring the proliferative response by CFSE dilution (data not shown). These experiments show that CD4 HLA-Gpos T cells are hypoproliferative and that this low proliferative capacity cannot be overcome by addition of exogenous IL-2 or by secondary stimulation.
Soluble factors produced by CD4 HLA-Gpos T cells
We next investigated the soluble factors produced by HLA-G–expressing CD4 T cells in response to T-cell stimulation. To ensure highest purity, HLA-Gpos and HLA-Gneg CD4 T cells were isolated by cell sorting from prepurified CD3 T cells (Figure 3A). RT-PCR analysis showed that CD4 HLA-Gpos T cells expressed high levels of HLA-G mRNA, whereas their negative counterparts lacked HLA-G transcripts, supporting again the concept of a specific subset rather than transient surface expression (Figure 3B). Next, we investigated the production of the soluble isoform HLA-G5. Therefore, purified CD4 HLA-Gpos and CD4 HLA-Gneg T cells were stimulated for 4 days with CD3/CD28 beads and after fixation stained intracellularly with the HLA-G5–specific antibody 5A6G7. Almost all (98%) of the CD4 HLA-Gpos T cells were positive for HLA-G5. Of note, 26% of the T cells lacking HLA-G1 on the surface also expressed HLA-G5 intracellularly after stimulation (Figure 3C). To further ensure production of soluble HLA-G, we also tested the supernatants of the stimulated cells by enzyme-linked immunosorbent assay (ELISA). CD4 HLA-Gpos T cells showed production of soluble HLA-G, whereas HLA-G–negative T cells had only little sHLA-G in their supernatants, suggesting that HLA-G5 is retained intracellularly in cells not bearing the membrane-bound form of HLA-G at the same time (Figure 3D). As a positive control, we used LCL-HLA-G1 and Hela-HLA-G5 transfectants. To assess production of cytokines, purified CD4 HLA-Gpos or HLA-Gneg T cells were independently stimulated with anti-CD3/CD28 beads for 24 hours and the production of IFN-γ, IL-10, and TGF-β was determined by quantitative real-time PCR (qRT-PCR). Four of 5 experiments showed reduced mRNA levels for IFN-γ in the CD4 HLA-Gpos cells (2.4- to 20-fold; n = 5) compared with their negative counterparts. Similarly, IL-10 mRNA was reduced in 4 of 5 samples of the HLA-Gpos CD4 T cells (2.2- to 8.7-fold). For TGF-β mRNA, only marginal differences were seen (Figure 3E). In addition, supernatants of anti-CD3/CD28–stimulated CD4 HLA-Gpos or HLA-Gneg T cells were tested for cytokine production using a cytokine array. In good correlation with the mRNA data, in all arrays performed CD4 HLA-Gpos T cells showed decreased production of IFN-γ and IL-10 protein. No constant differences were found for other cytokines measured (Figure 3F).
HLA-G production and cytokine profile of CD4 HLA-Gpos and CD4 HLA-Gneg T cells. (A) Separation of CD4 T-cell subpopulation by FACS sorting. After exclusion of aggregated cells (SSC-A versus SSC-W), the gate for CD4 T cells was set on FSC/CD4 dot blot to identify the desired subsets. For sorting, the HLA-G–positive population was assessed compared with the control isotype staining; a second gate was set on HLA-G–negative cells. One representative FACS sort experiment is shown. Values indicate the percentage of cells in gates. (B) Ex vivo expression of HLA-G mRNA in FACS-sorted cells. JEG3 mRNA was used as positive control. A representative experiment (1 of 5) is shown. (C) Intracellular expression of HLA-G5 by intracellular staining for the soluble isoform HLA-G5 in sorted HLA-G–positive and negative cells was performed after 4-day stimulation with CD3/CD28 beads using monoclonal antibody 5A6G7. A gate for CD4 T cells was set on FSC/CD4 dot blot to identify the desired subset. HLA-G5 expression (lower panel) was assessed in comparison with the control isotype staining (top panel). A representative staining is shown. Values indicate the percentage of cells in gates. (D) Production of soluble HLA-G. Supernatants of CD3/CD28-stimulated HLA-G–positive and negative subsets were tested by ELISA for soluble HLA-G. Cell lines transfected with either HLA-G1 or HLA-G5 were used as positive controls. One representative experiment is shown. (E) Cytokines assessed by RT-PCR are IFN-γ (left), IL-10 (middle), and TGF-β (right). Graphs show results of 5 experiments. Error bars indicate standard deviation. (F) Cytokine production of purified subsets. CD4 HLA-Gpos and HLA-Gneg T cells were stimulated with CD3/CD28 beads for 24 hours. Supernatants were assessed for cytokine production using the RayBio array. A representative cytokine profile for CD4 HLA-Gneg (left) and for CD4 HLA-Gpos (right) is shown.
HLA-G production and cytokine profile of CD4 HLA-Gpos and CD4 HLA-Gneg T cells. (A) Separation of CD4 T-cell subpopulation by FACS sorting. After exclusion of aggregated cells (SSC-A versus SSC-W), the gate for CD4 T cells was set on FSC/CD4 dot blot to identify the desired subsets. For sorting, the HLA-G–positive population was assessed compared with the control isotype staining; a second gate was set on HLA-G–negative cells. One representative FACS sort experiment is shown. Values indicate the percentage of cells in gates. (B) Ex vivo expression of HLA-G mRNA in FACS-sorted cells. JEG3 mRNA was used as positive control. A representative experiment (1 of 5) is shown. (C) Intracellular expression of HLA-G5 by intracellular staining for the soluble isoform HLA-G5 in sorted HLA-G–positive and negative cells was performed after 4-day stimulation with CD3/CD28 beads using monoclonal antibody 5A6G7. A gate for CD4 T cells was set on FSC/CD4 dot blot to identify the desired subset. HLA-G5 expression (lower panel) was assessed in comparison with the control isotype staining (top panel). A representative staining is shown. Values indicate the percentage of cells in gates. (D) Production of soluble HLA-G. Supernatants of CD3/CD28-stimulated HLA-G–positive and negative subsets were tested by ELISA for soluble HLA-G. Cell lines transfected with either HLA-G1 or HLA-G5 were used as positive controls. One representative experiment is shown. (E) Cytokines assessed by RT-PCR are IFN-γ (left), IL-10 (middle), and TGF-β (right). Graphs show results of 5 experiments. Error bars indicate standard deviation. (F) Cytokine production of purified subsets. CD4 HLA-Gpos and HLA-Gneg T cells were stimulated with CD3/CD28 beads for 24 hours. Supernatants were assessed for cytokine production using the RayBio array. A representative cytokine profile for CD4 HLA-Gneg (left) and for CD4 HLA-Gpos (right) is shown.
HLA-Gpos T cells are suppressive
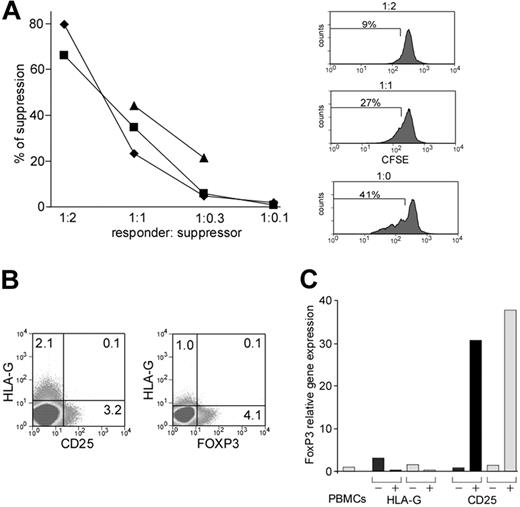
Given the inhibitory role of HLA-G, we wondered if these cells might have a suppressive effect on lymphocyte proliferation. To investigate this possibility, we used a suppression assay where proliferation of CFSE-labeled CD4 HLA-Gneg T cells was assessed by flow cytometry in the presence or absence of different ratios of autologous CD4 HLA-Gpos T cells. This assay was first established and validated using purified CD4+CD25high cells, where autologous suppression was used to evidence their suppressive properties (data not shown). Addition of CD4 HLA-Gpos T cells led to a significant reduction of proliferation of CFSE-labeled CD4 HLA-Gneg T cells (Figure 4A). Suppression was dependent on the suppressor to responder ratio and ranged between 20% and 90% at 1:1 ratio (mean = 58%; n = 8), depending on the donor. To investigate potential suppressive capacity of CD8 HLA-Gpos T cells, we performed identical suppression experiments as described for the CD4 HLAG+ cells, this time using purified CD8 HLA-Gneg T cells as responder cells and CD8 HLA-Gpos T cells as suppressors. Again, we observed a significant suppression of proliferation (Figure S2B). Moreover, when incubating CD8 HLA-Gpos T cells as suppressors with CD4 HLA-Gneg T cells as responders and vice versa, both CD4 and CD8 HLA-Gpos T cells were able to suppress the opposite T-cell subpopulation (data not shown).
CD4 HLA-Gpos T cells have suppressor activity and they do not express CD25 or FoxP3. (A) Suppression assay using CD4 HLA-Gpos T cells. CD4 HLA-Gpos T cells were titrated into CFDA-SE–labeled CD4 HLA-Gneg T cells cultured in the presence of allogeneic PBMCs and soluble anti-CD3. Cell proliferation was quantified by flow cytometric assessment of CSFE dilution histograms on day 4. The left panel compiles suppression capacity of CD4 HLA-Gpos T cells at different ratios in 3 independent experiments. The right panel shows a representative suppression assay with percentages of proliferation stated in histogram. (B) FACS staining for CD25/CD4/HLA-G at the cell surface (left) and intracellular flow cytometry for FOXP3/CD4/HLA-G (right). The dot plots show the staining on gated CD4 T cells; values indicate the percentage of cells in gates. One representative experiment is shown. (C) Expression of FoxP3 mRNA by CD4 HLA-Gpos/neg cells. FACS-sorted cells (HLA-Gpos, HLA-Gneg, CD4+CD25−, and CD4+CD25++) from 2 different healthy donors (light and dark bars) were analyzed for mRNA levels of Foxp3. Expression of Foxp3 in PBMCs from one of the donors was used as calibrator (= 1).
CD4 HLA-Gpos T cells have suppressor activity and they do not express CD25 or FoxP3. (A) Suppression assay using CD4 HLA-Gpos T cells. CD4 HLA-Gpos T cells were titrated into CFDA-SE–labeled CD4 HLA-Gneg T cells cultured in the presence of allogeneic PBMCs and soluble anti-CD3. Cell proliferation was quantified by flow cytometric assessment of CSFE dilution histograms on day 4. The left panel compiles suppression capacity of CD4 HLA-Gpos T cells at different ratios in 3 independent experiments. The right panel shows a representative suppression assay with percentages of proliferation stated in histogram. (B) FACS staining for CD25/CD4/HLA-G at the cell surface (left) and intracellular flow cytometry for FOXP3/CD4/HLA-G (right). The dot plots show the staining on gated CD4 T cells; values indicate the percentage of cells in gates. One representative experiment is shown. (C) Expression of FoxP3 mRNA by CD4 HLA-Gpos/neg cells. FACS-sorted cells (HLA-Gpos, HLA-Gneg, CD4+CD25−, and CD4+CD25++) from 2 different healthy donors (light and dark bars) were analyzed for mRNA levels of Foxp3. Expression of Foxp3 in PBMCs from one of the donors was used as calibrator (= 1).
To exclude that CD4 HLA-Gpos T cells are identical to naturally occurring CD4+CD25+ Tregs, we checked for the coexpression of CD25, the forkhead transcription factor 3 (FOXP3), and HLA-G on freshly isolated PBMCs. CD4 HLA-Gpos T cells show expression of neither CD25 on the surface nor intracellular FOXP3 protein (Figure 4B). Moreover, the levels of FoxP3 RNA did not differ between CD4 HLA-Gpos and HLA-Gneg T cells, though CD4 CD25+ Tregs showed high levels as expected (Figure 4C). Of note, suppressive function of CD4 HLA-Gpos cells was demonstrated by cells isolated both by magnetic bead isolation as well as by flow-cytometry sorting (data not shown). Furthermore, we demonstrated suppressive function of CD4 HLA-Gpos cells also on antigen-specific T cells. CD4 HLA-Gneg T cells were stimulated with GA (glatirameracetate; 40 and 100 μg/mL) in the absence of allogeneic antigen-presenting cells (APCs). Here proliferation was also suppressed by CD4 HLA-Gpos T cells (data not shown).
Suppression by CD4 HLA-Gpos T cells is reduced after neutralization of HLA-G and is not dependent on cell contact
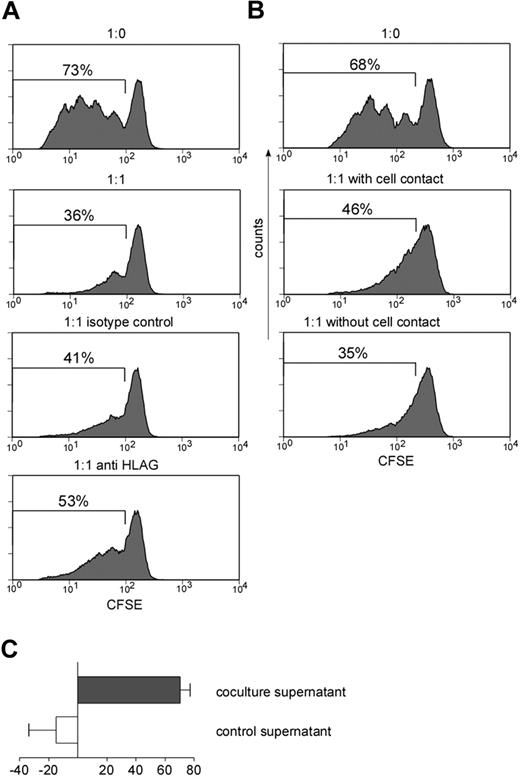
To test the hypothesis that HLA-G is not just the phenotypic marker of a regulatory population but also mediates suppression, we first tried to rescue proliferation of the CD4 HLA-Gneg T cells by neutralizing HLA-G with an anti–HLA-G blocking antibody (mAb87G). mAb87G recognizes transmembraneous HLA-G1 as well as soluble HLA-G5, which represent the 2 major HLA-G isoforms. HLA-G neutralization partially antagonized suppressive effects, leading to a 30% to 70% reduction of suppressive capacity of the CD4 HLA-Gpos T cells (average, 48.9% ± 21.7%; n = 3; Figure 5A). Our data therefore clearly suggest that HLA-G largely contributes to the suppressive effect of the CD4 HLA-Gpos T cells.
HLA-G–mediated suppression is reversed after neutralization of HLA-G and independent of cell-cell contact. (A) Suppression assay at a ratio of 1:1 HLA-Gpos to CFDA-SE–labeled HLA-Gneg CD4 T was performed in the absence of antibody (second panel), in the presence of an IgG isotype control (third panel), and in the presence of anti–HLA-G1/5 blocking antibody (87G; fourth panel). Proliferation was measured by flow cytometry at day 4. (B) Suppression assay at a ratio of 1:1 HLA-Gpos to CFDA-SE–labeled HLA-Gneg CD4 T was performed either conventionally or in a transwell chamber: CFDA-SE–labeled CD4 HLA-Gneg T cells were placed in the lower chamber while CD4 HLA-Gpos were added to the upper chamber with no cell-cell contact to CD4 HLA-Gneg responder cells (ratio 1:1). Proliferation of CFDA-SE–labeled cells was measured by FACS. Graphs in panels A-B show representative experiments reproduced at least 3 times. (C) Supernatants of suppression assays with a CD4 HLA-Gneg/CD4 HLA-Gpos ratio of 1:1 (coculture) and 1:0 (control) were transferred to new proliferation assays of CFDA-SE–labeled CD4 HLA-Gneg T cells. Suppressive activity of supernatant was measured after 4 days by FACS analysis. Graph shows summary of 6 independent experiments. Error bars indicate standard deviation.
HLA-G–mediated suppression is reversed after neutralization of HLA-G and independent of cell-cell contact. (A) Suppression assay at a ratio of 1:1 HLA-Gpos to CFDA-SE–labeled HLA-Gneg CD4 T was performed in the absence of antibody (second panel), in the presence of an IgG isotype control (third panel), and in the presence of anti–HLA-G1/5 blocking antibody (87G; fourth panel). Proliferation was measured by flow cytometry at day 4. (B) Suppression assay at a ratio of 1:1 HLA-Gpos to CFDA-SE–labeled HLA-Gneg CD4 T was performed either conventionally or in a transwell chamber: CFDA-SE–labeled CD4 HLA-Gneg T cells were placed in the lower chamber while CD4 HLA-Gpos were added to the upper chamber with no cell-cell contact to CD4 HLA-Gneg responder cells (ratio 1:1). Proliferation of CFDA-SE–labeled cells was measured by FACS. Graphs in panels A-B show representative experiments reproduced at least 3 times. (C) Supernatants of suppression assays with a CD4 HLA-Gneg/CD4 HLA-Gpos ratio of 1:1 (coculture) and 1:0 (control) were transferred to new proliferation assays of CFDA-SE–labeled CD4 HLA-Gneg T cells. Suppressive activity of supernatant was measured after 4 days by FACS analysis. Graph shows summary of 6 independent experiments. Error bars indicate standard deviation.
To assess whether direct cell to cell contact is essential for the suppressive function of the CD4 HLA-Gpos T cells, we performed the suppression assays in a transwell system, where responder and suppressor cells were not in direct contact. Basically, suppression was maintained when comparing proliferation rates with or without cell-cell contact (Figure 5B). To further investigate if suppressive capacity of CD4 HLA-Gpos T cells is dependent on soluble factors, we collected supernatants from suppression assays and transferred them to new proliferation assays of CFSE-labeled CD4 HLA-Gneg T cells. Supernatants from assays performed in the presence of CD4 HLA-Gpos T cells clearly suppressed T-cell proliferation (mean = 71.0% ± 17.40%), while control supernatants even enhanced proliferation compared with fresh medium only (Figure 5C).
HLA-Gpos T cells are found in the human thymus
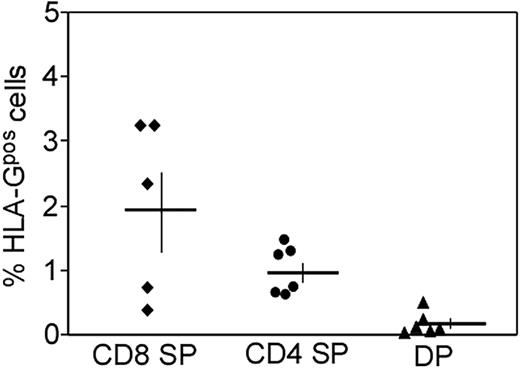
Our experiments show that HLA-G in HLA-Gpos T cells is stably expressed on the protein and mRNA level and cannot easily be induced by T-cell stimulation. However, this does not necessarily imply that HLA-G expression is constitutive on a cell population, since it still could be transiently induced by unknown stimuli in vivo. However, an important hint that our population of HLA-Gpos T cells represents a distinct cell population came from thorough analysis of HLA-G expression on thymocyte subsets. FACS analysis of HLA-G expression in freshly isolated thymocytes (6 donors, ages 14 days to 1 year) showed that a small fraction of single-positive CD4+ (average, 1.0%; range, 0.6% to 1.5%) and CD8+ thymocytes (average, 2.0%; range, 0.4% to 3.3%) had a consistent and distinct expression of HLA-G (Figure 6), which was comparable with the intensity of HLA-G expression on T cells in peripheral blood. Double-positive thymocytes were devoid of HLA-G, indicating that HLA-G expression is acquired after positive selection and transition to the CD4 and CD8 single-positive state, respectively. Thus, detection of HLA-G on thymocytes strongly supports our assumption of HLA-Gpos T cells representing a subset of cells belonging to the natural repertoire of T cells.
HLA-Gpos T cells are present in the human thymus in mature CD4 and CD8 subsets. FACS analysis of HLA-G expression in thymocyte subsets: Thymocytes were stained for CD4, CD8 and HLA-G. Gates were set on CD4+CD8− and CD4−CD8+ single-positive thymocytes as well as on double-positive thymocytes. The percentage of HLA-Gpos cells was assessed in comparison with a negative control. The summary of 6 independent stainings is shown. Horizontal bars indicate the mean of 6 samples; vertical bars show SEM.
HLA-Gpos T cells are present in the human thymus in mature CD4 and CD8 subsets. FACS analysis of HLA-G expression in thymocyte subsets: Thymocytes were stained for CD4, CD8 and HLA-G. Gates were set on CD4+CD8− and CD4−CD8+ single-positive thymocytes as well as on double-positive thymocytes. The percentage of HLA-Gpos cells was assessed in comparison with a negative control. The summary of 6 independent stainings is shown. Horizontal bars indicate the mean of 6 samples; vertical bars show SEM.
HLA-Gpos T cells are present at sites of inflammation
Until now our data demonstrate the presence of HLA-Gpos T cells with strong regulatory properties in peripheral blood of healthy donors and in thymocytes. To investigate their putative role in vivo, we looked for the presence of HLA-Gpos T cells at sites of inflammation, such as the target organs in the inflamed nervous system and in muscle disorders. We stained the cells in the cerebrospinal fluid (CSF) and peripheral blood of patients with various inflammatory disorders (multiple sclerosis, MS, n = 15; other inflammatory neurologic diseases, OIND, n = 10) and patients with noninflammatory neurologic diseases (NIND, n = 9) (Table S1). Patients with MS were separated in those having stable disease and those experiencing an acute relapse. HLA-Gpos T cells were detectable in the CSF of all 3 patient groups (Figure 7A; Figure S3A). We observed significantly higher frequency of CD4 HLA-Gpos T cells in the CSF of patients with acute inflammatory disorders when compared with peripheral blood, while no statistically significant differences were found in NIND and MS patients for CD4 HLA-Gpos T cells (Figure 7B). No differences were observed with regard to CD8 HLA-Gpos T cells in all 3 groups (Figure S3B). No differences were observed in the frequencies of HLA-G–positive CD4 and CD8 T cells in the blood of all 3 patient groups; no statistical differences was found in comparison to healthy donors. Interestingly, when MS patients were divided according to their disease status, CD4 HLA-Gpos T cells were slightly but significantly increased in the CSF of patients with acute relapse (Figure 7C). Again, no differences were found in the CD8 HLA-Gpos compartment (Figure S3C). Of note, the frequency of HLA-Gpos T cells did not correlate with absolute cell counts in the CSF. We next investigated muscle biopsy specimens from patients with various inflammatory myopathies. Polymyositis (PM) and inclusion body myositis (IBM) are putative autoimmune disorders, where muscle fibers are under attack of mainly cytotoxic T lymphocytes.34 Muscle biopsy specimens from patients with inflammatory myopathies (polymyositis, inclusion body myositis, dermatomyositis, DM, n = 5 each) were assessed for the coexpression of HLA-G and T-cell markers by double immunofluorescence staining. As expected, neither HLA-G immunoreactivity nor T cells infiltrates were detectable in sections of nonpathologic muscle (data not shown). In inflammatory specimens, HLA-G was up-regulated as described previously for muscle cells.26 Importantly, some of the infiltrating or invading CD8 T cells showed expression of HLA-G (Figure 7D), indicating that HLA-Gpos regulatory T cells may play a role in modulating muscle inflammation. Numbers of detectable HLA-Gpos T cells correlated with the amount of cellular infiltrates. No quantitative differences in frequency or topologic distribution were found between PM, IBM, and DM.
HLA-G T cells are present in sites of neuroinflammation and inflammatory myopathies. (A) FACS analysis of paired blood and CSF specimens from patients with and without neuroinflammatory diseases was performed. Gates for CD4+ lymphocytes were set on CD4/FSC dot blots, and the percentage of HLA-Gpos cells was assessed in comparison with a negative control. A representative staining of 1 patient is shown. (B) Summary of paired samples of blood and CSF specimens for CD4 HLA-Gpos T cells from NIND (left), OIND (middle), and MS (right) patients. Mean with SEM for each patient group is shown in upper panel; single pairing of blood-CSF samples for each patient is shown in lower panel. (C) Comparison of CD4 HLA-Gpos T-cell percentages of MS patients in acute or stable phase of the disease. Again mean with SEM is shown in upper panel; lower panel shows single patient pairing. (D) Consecutive cryosections of muscle from patients with polymyositis were stained with the HLA-G–specific mAb 87G, the CD8 mAb B9.11 either as single staining (left column) or as double immunofluorescence. Besides some muscle fibers (asterisk), part of the CD8 T cells (arrowhead and arrows), found to surround or even invade muscle fibers, also stains positive for HLA-G. Original magnifications: HLA-G single staining: (magnification × 250); CD8 single staining and CD8/ HLA-G double staining: (magnification × 500). Sections were analyzed using an Axiophot 2 microscope (Zeiss, Oberkochen, Germany).
HLA-G T cells are present in sites of neuroinflammation and inflammatory myopathies. (A) FACS analysis of paired blood and CSF specimens from patients with and without neuroinflammatory diseases was performed. Gates for CD4+ lymphocytes were set on CD4/FSC dot blots, and the percentage of HLA-Gpos cells was assessed in comparison with a negative control. A representative staining of 1 patient is shown. (B) Summary of paired samples of blood and CSF specimens for CD4 HLA-Gpos T cells from NIND (left), OIND (middle), and MS (right) patients. Mean with SEM for each patient group is shown in upper panel; single pairing of blood-CSF samples for each patient is shown in lower panel. (C) Comparison of CD4 HLA-Gpos T-cell percentages of MS patients in acute or stable phase of the disease. Again mean with SEM is shown in upper panel; lower panel shows single patient pairing. (D) Consecutive cryosections of muscle from patients with polymyositis were stained with the HLA-G–specific mAb 87G, the CD8 mAb B9.11 either as single staining (left column) or as double immunofluorescence. Besides some muscle fibers (asterisk), part of the CD8 T cells (arrowhead and arrows), found to surround or even invade muscle fibers, also stains positive for HLA-G. Original magnifications: HLA-G single staining: (magnification × 250); CD8 single staining and CD8/ HLA-G double staining: (magnification × 500). Sections were analyzed using an Axiophot 2 microscope (Zeiss, Oberkochen, Germany).
Taken together, our data demonstrate that HLA-G–positive T cells with putative regulatory function are found in the target organs in patients with inflammatory disorders of the CNS or muscle. The correlation of HLA-G T cells with acute CNS inflammation (eg, MS relapse) supports the assumption of their putative immunoregulatory role in modulating parenchymal inflammatory responses.
Discussion
In this study, we identified and characterized 2 subsets of T cells that constitutively express the immune-tolerogenic molecule HLA-G on the cell surface. Both, CD4 HLA-Gpos and CD8 HLA-Gpos T cells exist in the peripheral blood of healthy volunteers as small subsets, which are able to suppress immune responses in vitro and were found at sites of inflammation in vivo. Regulatory T cells, also referred to as suppressor T cells, are important mediators of immune tolerance and play a critical role in the control of autoimmunity, transplantation, and tumor immune surveillance.4,35 Several populations of regulatory cells can be distinguished, most importantly the naturally occurring CD4+CD25+ Tregs, which are constitutively present in humans under normal conditions.36,37 Distinct from those are the induced regulatory T cells, which appear under certain conditions of tolerization, such as Tr1 cells.5 Furthermore, cells with regulatory properties are found within the populations of CD8+CD28− cells, TCR γδ cells, and CD4−CD8− T cells.7,38,39 Albeit the importance of these populations is evident, their study is difficult due to the lack of cell surface marker(s) sufficient to deduce an attributed immune-suppressive or regulatory function per se. Our population of T cells is characterized by the expression of the immune-tolerogenic molecule HLA-G on the cell surface. Phenotypical analysis of the HLA-Gpos CD4 T cells revealed a cytokine profile that differs from Th1, Th2, Th3, or Tr1 cells.40,41 CD4 HLA-Gpos T cells, in contrast to most other regulatory T cells, do not produce increased levels of IL-10 or TGF-β, the cytokines that have been mostly attributed with regulatory functions. Reduced production of IFN-γ, however, could indicate a nonproinflammatory nature of the CD4 HLA-Gpos T cells (Figure 3). Similar to the CD4+CD25+ Tregs,36,37 HLA-Gpos T cells show reduced proliferation to several stimuli. However, in contrast to CD4+CD25+ Tregs, this hypoproliferation cannot be overcome by the addition of exogenous IL-2 (Figure 2).
CD4 HLA-Gpos as well as CD8 HLA-Gpos T cells exert strong suppression (20%-90% at 1:1 ratio; Figure 4 and Figure S2B), which could partially be antagonized by blocking HLA-G with a neutralizing antibody (mAb 87G). Suppression of HLA-Gpos T cells was not depending on cell contact, as we could show using transwell experiments and transfer of supernatants in the suppression experiments (Figure 5). It is therefore assumed that HLA-G is a key molecule characterizing phenotype and function of the HLA-Gpos T-cell population (Figure 5). HLA-G has potent immune-suppressive properties, which are exerted by interaction with different inhibitory receptors either as transmembraneous or soluble isoforms.42,43 The identification of a small subpopulation of T cells expressing HLA-G now allows the identification of a regulatory T-cell subset by a well-defined cell surface marker that at the same time seems to represent the tolerogenic “player.” This allows the ad hoc identification of a regulatory T-cell subset by an unambiguous marker indicating suppressive function. Our experiments show that expression of the main transmembraneous isoform HLA-G1 is virtually always accompanied by the expression of soluble isoforms, namely HLA-G5. Furthermore, HLA-G1 can be shed and therefore can act as soluble HLA-G. However, both isoforms have similar suppressive functions.17,44 Thus, it is reasonable to argue that both transmembraneous as well as soluble HLA-G (iso) forms contribute to the suppression. The exact cellular and molecular mechanisms how HLA-Gpos T cells exert autologous immune suppression remain elusive at present. One hypothesis is that suppressive function is mediated by HLA-G that directly interacts with respective receptors on HLA-G–negative T cells. The only described HLA-G receptor present on T cells is ILT-2 (CD85j, LIR-1), thus making this immunoglobulin-like transcript a possible candidate underlying the molecular interaction of HLA-G–mediated T-cell suppression. HLA-G–positive cells could induce or trigger HLA-G–negative cells to promote anti-inflammatory or antiproliferative cytokine milieu. These changes might contribute to the production of “suppressive” soluble factors, which might include more than HLA-G.
What might be the role of HLA-G regulatory cells in vivo? Although much knowledge has been accumulated concerning the generation and functional properties of Tregs, less attention has been paid to the key question of where the immune regulation by Tregs may take place in vivo. Regulatory T cells can inhibit both the priming and the effector phase of an immune response, so suppression might occur both within lymphoid tissues and at peripheral sites during immune reactions. Therefore HLA-Gpos regulatory T cells could play a role in the mechanisms of central and peripheral tolerance. In the thymus thymic epithelial cells are capable of expressing HLA-G.45 We demonstrate here HLA-G expression on a subset of single-positive CD4 and CD8 thymocytes, but not in their double-positive precursors. Although we cannot discard that some peripheral HLA-Gpos T cells recirculate into the thymus, given the small percentages present in the periphery of healthy humans and the lack of specific migration markers, we favor the hypothesis that this population originates in the thymus and the expression of HLA-G is acquired during maturation. Our HLA-G–positive T cells express HLA-G protein and RNA, indicating endogenous HLA-G production. Together with our finding of HLA-G regulatory T cells in the periphery of healthy adult volunteers under physiologic conditions, it is reasonable to postulate a role of HLA-Gpos T cells in the maintenance of peripheral tolerance. However, HLA-Gpos regulatory T cells might also play an important role in the regulation of peripheral inflammation. Ectopic HLA-G expression or induction of HLA-G on immune cells has been documented in a variety of pathologic conditions, namely autoimmune or infectious inflammation,26,31,34,46,47 transplantation, and neoplastic transformation.22,23,48-51 We found HLA-Gpos T cells in the target organs of patients with neuroinflammatory disorders, as well as in the muscle in idiopathic myositis (Figure 7; Figure S3). The frequency of CD4 HLA-G regulatory T cells was significantly elevated in the CSF from patients with acute MS relapses and in patients with acute neuroinflamamtory disorders such as vasculitis, encephalitis, or meningitis, whereas CD8 HLA-Gpos T cells were found at sites of muscle inflammation. Similarly, HLA-G–expressing T cells had been found in patients who underwent transplantation and in HIV-infected patients.30,31 It is therefore conceivable that these suppressive T-cell populations migrate to or are selectively attracted to the sites of inflammation to combat the destructive events, and thus the study of HLA-G–positive T cells and their relevance at target organs of inflammation become interesting. Of note, HLA-Gpos T cells are detectable at low frequencies also in CSF from patients even without an accompanying inflammatory or structural damage (eg, patients with headache or pseudotumor cerebri). Thus it is tempting to speculate that HLA-Gpos T cells may play a role in CNS immune surveillance. It remains to be determined how the population of HLA-Gpos regulatory T cells contributes to lesion pathogenesis in acute and chronic MS or to muscle damage in myositis. However, the idea of local immunosuppression by functional regulatory T cells modulating disease activity and progression is appealing from an immunopathogenetic as well as from a therapeutic view.52 There is evidence that T cells including regulatory cells in the CNS may also provide beneficial effects in neuroinflammation. This view evolved from work demonstrating the expression of neurotrophic factors by T cells and in the CNS,53,54 the protective function of autoreactive T cells in neuroinflammation,55 and the contribution of CD4+CD25+ Tregs in recovery and protection from autoimmune encephalomyelitis.56 This could indeed be the case in humans, because HLA-G–expressing T cells tend to accumulate in the cerebrospinal fluid of patients with inflammatory neurologic disease or migrate to points of muscle inflammation in PM to counteract detrimental immune responses. Not mutually exclusive to the hypothesis of migration or attraction of HLA-G regulatory cells to sites of peripheral inflammation,57 HLA-Gpos regulatory T cells could also be generated at the sites of inflammation, for instance after acquiring HLA-G via trogocytosis from donor cells,58 or as it has been shown for CD4+CD25hi FOXP-3 regulatory T cells in EAE (experimental autoimmunce encephalomyelitis).59 Hereby they would balance levels of peripheral immune reactions. Concerning their putative role in maintaining peripheral immune tolerance, it is yet to be determined whether the function of our HLA-G T regulatory cells is impaired in autoimmune conditions, as it has been described for CD4+CD25+ Tregs in MS.60,61 Of note, no orthologues of HLA-G have been identified in other species, prohibiting in vivo experiments on the role of HLA-G regulatory T cells in animals.
The identification of a novel population of CD4 and CD8 T cells characterized by the cell surface expression of HLA-G with regulatory properties may have implications for the mechanisms of inflammation, autoimmunity, and also tumor immune surveillance. We believe that these cells belong to the physiological repertoire of suppressor T cells, which putatively are important to maintain immune tolerance.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This work was supported by grants from the German Research Foundation (Wi 1722/3–1 to H.W.), the IZKF Wuerzburg (48–0 to H.W.), the SFB 685 (to E.T. and A.M.), and the Hertie Foundation and the fortüne program of the Medical Faculty of the University of Tuebingen (to H.W. and A.M.). E.T. is supported by a Margarete von Wrangell fellowship from the Land Baden-Wuerttemberg.
We are grateful to all our blood donors and patients. We thank Profs T. Hünig, K. Toyka, and M. Weller for helpful discussions on the paper.
Authorship
Contribution: U.F., E.T., A.M., and H.W. designed research, analyzed data and wrote the paper; Y.-H.H., A.W., and T.B. designed research and analyzed data; U.F., E.T., and Y.-H.H. contributed equally to this work.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Heinz Wiendl, Department of Neurology, Julius-Maximilians-University Wuerzburg, Josef-Schneider-Strasse 11, D-97080 Wuerzburg, Germany; e-mail: heinz.wiendl@klinik.uni-wuerzburg.de.

![Figure 2. CD4 HLA-Gpos T cells show reduced proliferation to allogeneic or polyclonal stimuli. (A) Isolation of CD4 HLA-Gpos T cells by sequential magnetic-activated cell sorting (MACS) bead separation and analysis of the different steps of the isolation by flow cytometry. From top to bottom, dot plots show staining for whole PBMCs before separation, purified CD4 T cells, the CD4 HLA-Gneg T-cell fraction, and the CD4 HLA-Gpos T-cell fraction. Counterstaining for CD14 indicates that there are no contaminating monocytes in the preparation. (B) MACS-sorted CD4 HLA-Gneg and HLA-Gpos T cells were cultured without stimulus for 72 hours to assess HLA-G expression over time. Ex vivo expression of CD4 HLA-Gpos T cells was set as reference. Graph shows summary of 3 experiments. (C) After MACS sorting, whole CD4 (▴), CD4 HLA-Gpos (●), and CD4 HLA-Gneg T cells (■) were stimulated with allogeneic mature DCs at different ratios and proliferation was determined by [3H] incorporation after 4 days. (D) Whole CD4 (), CD4 HLA-Gpos (), and CD4 HLA-Gneg () T cells were isolated and stimulated with anti-CD3/CD28 beads. Proliferation was determined after 4 days by [3H] incorporation. (E) CD4 HLA-Gpos () and CD4 HLA-Gneg () T cells were isolated and stimulated with mature allogeneic DCs in the presence or absence of 500 U/mL IL-2. Proliferation was determined after 4 days. (F) CD4 HLA-Gpos () or CD4 HLA-Gneg T cells () were stimulated for 4 days with allogeneic PBMCs and restimulated again with PBMCs (allo MLR, left) or CD3/CD28 beads (right). Proliferation was determined 3 days later. A representative experiment (1 of at least 3) is shown for panels C-F. Error bars indicate standard error of the mean (SEM).](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/110/2/10.1182_blood-2006-11-057125/4/m_zh80130703080002.jpeg?Expires=1769281591&Signature=MBNKNUcn0dFR8zNk3yZzAsGFYlSvL2SoVhO--e32fx4eoJbkYDKCZmt0YfE5cA2n6KgQw2dPEQb8AtpRCFeU-TsSNE77mWLFDCuxdbJAofZWpAnkOFYJGZsyu~jl2ot6vl5x5lzGWcN7o5gSVs9zunglihu6OGRb39bY95atfzDGW3mVppt4hIs8gXKVHTNyhpByXSeiqxdZN-ArfGFkSku1-idKJjGqwJzt~NOrlDkqFs487tKg2LZik4RZsqVxniYGAPSoErroWxV1aWvYykaO0Za7fb4RL60UgFxRbCaQcm5E7BDsemSHPiOQwkzRXZf0-BwW97yibY2qNFXBWQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)