Abstract
Chronic graft-versus-host disease (cGVHD) is an increasingly frequent complication of allogeneic stem cell transplantation. Current therapies for cGVHD reduce symptoms but are not cures. The B10.D2→Balb/c (H-2d) minor histocompatibility antigen-mismatched model, which reflects clinical and pathological symptoms of human cGVHD, was used in this study. We demonstrated that a single injection of an agonistic monoclonal antibody (mAb) against CD137, a member of the tumor necrosis factor receptor superfamily, reverses skin fibrosis, ulceration, and alopecia, a dominant feature of cGVHD (cutaneous GVHD), ultimately improving general health conditions. The reversal is associated with markedly reduced CD4+ T-cell cytokines and increased apoptosis of donor CD4+ T cells. The Fas pathway is required for ameliorating cutaneous GVHD by anti-CD137 mAb. Taken together, these data indicate that the anti-CD137 mAb has a therapeutic effect on cutaneous GVHD by removing donor CD4+ T cells that cause cutaneous GVHD. Thus, our study demonstrates an agonistic mAb, specific for a costimulatory molecule, as a possible target for therapeutic intervention in cutaneous GVHD.
Introduction
Chronic graft-versus-host disease (cGVHD) commonly occurs in patients who receive allogeneic stem cell transplants. Even though cGVHD differs among patients, the clinical complications of cGVHD often include fibrosis and scleroderma-like changes.1 cGVHD is mediated by pathogenic donor T cells generated after alloreactivity to host minor histocompatibility (mH) antigens or autoantigens.2 These T cells damage target tissue directly by cytolytic attack, through secretion of proinflammatory and fibrosing cytokines, or via promotion of autoantibody production.2 Treatment of cGVHD, aside from systemic delivery of corticosteroids, has not been established.
A limited number of model systems have been developed to examine cGVHD. Among these, the B10.D2→Balb/c (H-2d) mH antigen-mismatched model of GVHD showed characteristics resembling human cGVHD such as relatively late time of onset, skin fibrosis, ulceration, and alopecia with increased collagen deposition.3-6 This murine model has the following histologic features: lichenoid subepithelial infiltrates, follicular drop-out, loss of subdermal fat, and dermal mononuclear infiltrates (herein, this dominant feature of cGVHD will be referred to as cutaneous GVHD).7 Other features include pulmonary fibrosis,4 inflammation and destruction of salivary and lacrimal glands,8 and hepatic disease characterized by intrahepatic and extrahepatic bile duct mononuclear infiltration followed by fibrous thickening and sclerosis of the bile duct wall.9-11 The pathogenesis of cGVHD requires donor CD4+ T cells.9,12 Interestingly, both donor and host antigen-presenting cells (APCs) mediate CD4+ T-cell–mediated cutaneous GVHD, indicating that endogenous and exogenous host mH antigens can be presented to donor CD4+ T cells in the context of major histocompatibility complex (MHC) class II.13 Since, in the B10.D2→Balb/c model, immunodominant antigens seem to be expressed in the skin14 and microenvironments of the skin tend to be favorable for the development of type 2 CD4+ T helper cells (Th2 cells), it seems that cutaneous GVHD is mediated by Th2 cells particularly in the H-2d genetic background.15 Strong evidence supporting this hypothesis is that severe cutaneous GVHD developed when GVHD in Balb/c mice was induced by cells from signal transducer and activator of transcription 2 (STAT2)–deficient C57BL/6 mice that have markedly reduced Th1 responses and enhanced Th2 responses.16 However, there is no consensus regarding the sole involvement of Th2 in the pathogenesis of cutaneous GVHD, since cutaneous GVHD might be developed by cytokines secreted by Th1 cells,17 Th17 cells,18 and/or autoantibodies.19
CD137 is a member of the TNF receptor superfamily that functions mainly as a strong costimulatory molecule for CD8+ T cells.20,21 In a seminal paper, Mittler et al demonstrated that in vivo ligation of CD137 abrogates T-cell–dependent antibody responses.22 Based on their observations, we have proposed that an agonistic anti-CD137 monoclonal antibody (mAb) can be used to treat CD4+ T-cell–mediated immunologic disorders.23 Indeed, anti-CD137 has been shown to have a preventive/therapeutic effect on experimental autoimmune encephalomyelitis24 and experimental autoimmune uveitis,25 and rheumatoid arthritis26,27 mediated by Th1 T cells and other autoimmune and immunologic diseases that are believed to be mediated by autoantibodies or Th2 cells. These include systemic lupus erythematosus (SLE),28,29 SLE-like cGVHD,30 experimental allergic conjunctivitis,31 and allergic asthma.32-34 The therapeutic effect of anti-CD137 on these diseases seems to be due to the deletion of autoreactive CD4+ T cells by activation-induced cell death (AICD),24,25,30 which may or may not result in CD4+ T-cell tolerance.22,30,33 Anti-CD137 can also induce the deletion of autoreactive B cells by augmenting the production of interferon-γ (IFN-γ) by CD8+ T cells.26,28,30
In this study, we used the B10.D2→Balb/c model of cGVHD to address the therapeutic effect of anti-CD137 on advanced cGVHD. Even though, during the induction phase of cGVHD, anti-CD137 resulted in early death as a consequence of severe acute GVHD, treatment of anti-CD137 between day 30 and day 60 after disease induction markedly reduced the symptoms of cutaneous GVHD. The Fas-mediated AICD of donor CD4+ T cells is required for the therapeutic effect of anti–4–1BB on cutaneous GVHD. These results suggest costimulation as a possible therapeutic intervention in cutaneous GVHD.
Materials and methods
Mice
Male B10.D2 (H-2d) donor mice were purchased from Japan Shizoka Institute for Laboratory Animals (Japan SLC, Hamamatsu, Japan). DBA/2 recipient mice were purchased from Orient (Seoul, Korea). Mice aged 6 to 8 weeks were used for current studies and all mice were maintained in pathogen-free conditions. These studies were approved by the institutional animal care committee.
Antibodies and reagents
Anti-CD137 (3H3)20 and anti-FasL (MFL4)35 mAbs were purified from ascites. Control rat IgG (Ig) was purchased from Sigma-Aldrich (St Louis, MO). The following FITC (fluorescein isothiocyanate)–, PE (phycoerythrin)–, PerCP (peridinin chlorophyll protein)–, or biotin-conjugated mAbs to mouse proteins were purchased from BD Biosciences Pharmingen (San Diego, CA): CD3, CD4, CD8, CD62L, Ly9.1, interleukin-2 (IL-2), IL-4, IL-5, IL-13, and interferon (IFN)-γ. Streptavidin-conjugated horseradish peroxidase (HRP) and FITC-conjugated annexin V were also purchased from BD Biosciences Pharmingen.
Bone marrow T-cell depletion
Bone marrow (BM) cells were collected by flushing femurs and tibias from B10.D2 donor mice into magnetic-activated cell sorting (MACS) buffer (1 × phosphate-buffered saline [PBS], 5 mM EDTA [ethylenediaminetetracetic acid], 3% calf serum). After erythrocyte lysis in hemolysis buffer (144 mM NH4Cl and 17 mM Tris-HCl, pH 7.2), BM cells were incubated with biotinylated anti-CD3 mAb for 20 minutes on ice, washed once, then incubated with streptavidin-conjugated microbeads (Miltenyi Biotech, Auburn, CA) for 20 minutes at 4°C. Cells were depleted of CD3+ cells using MACS (Miltenyi Biotech). Remaining CD3+ cells routinely comprised less than 1% of BM cells. Cells were resuspended in PBS prior to transplantation.
CD4+ T-cell purifications
Single-cell suspensions in PBS were prepared from the spleens and lymph nodes of normal B10.D2 parental donors, filtered through a sterile mesh (BD Falcon, San Diego, CA), and washed. After the erythrocytes were lysed in hemolysis buffer, the remaining cells were resuspended in MACS buffer. CD4+ T cells were purified using anti-CD4–conjugated magnetic beads (Miltenyi Biotech). Positively selected cells routinely contained more than 90% CD4+ T cells.
BM transplantation
Recipient Balb/c mice received 750 centigray (cGy) from a cesium irradiator and were reconstituted with 5 × 106 T-cell–depleted BM with or without 1 × 107 purified CD4+ T cells from B10.D2 donors. In some experiments, cGVHD was induced by transferring 5 × 106 donor T-cell–depleted BM and 1 × 107 or 6 × 106 total spleen/lymph node cells. A single injection of anti-CD137 or control Ig (200 μg per mouse) was done intraperitoneally at various time points after BM transplantation. For blocking of the Fas pathway, anti-FasL mAb was administered at days −2, 0, and +2 (200 μg per mouse each time). Administration of anti-CD137 or control Ig was on day 0.
cGVHD clinical scoring system
Evaluation of cGVHD was done as previously described.8 In brief, following BM transplantation, animals were weighed every 3 days and scored for skin manifestations of GVHD beginning on day 15. The following scoring system was used: healthy appearance equals 0; skin lesions with alopecia less than 1 cm2 in area, 1; skin lesions with alopecia 1 to 2 cm2 in area, 2; skin lesions with alopecia more than 2 cm2 in area, 3; additionally, animals were assigned 0.3 point each for skin disease (lesions or scaling) on the ears, tails, and paws. Minimum score was 0; maximum, 3.9. Incidence curves represent all mice that achieved a score of 0.6 or higher, and mean clinical scores were calculated for all mice used for experiments per group.
Histology
Shaved skin from the interscapular region (approximately 2 cm2) was fixed in 10% formalin, embedded in paraffin, sectioned, slide-mounted, and stained with hematoxylin and eosin (H&E) or Masson trichrome.
Flow cytometry
The spleens of cGVHD mice were collected on the indicated days after donor cell transfer. After lysis of the erythrocytes, the splenocytes were preincubated in a blocking buffer (PBS containing anti-CD16/CD32 [2.4G2] mAb/0.2% BSA [bovine serum albumin]/0.1% sodium azide) and then incubated with the relevant mAbs for 30 minutes at 4°C. Finally, they were washed twice with staining buffer (PBS containing 0.2% BSA/0.1% sodium azide) and analyzed by FACscan (BD Biosciences Pharmingen). Cells were stained with annexin V to detect apoptosis, according to the manufacturer's protocol. For the measurement of cytokine-producing CD4+ T cells, cells were isolated from the spleen, draining lymph nodes, or skin of cGVHD mice and intracellular cytokine staining was performed, according to the manufacturer's protocol. In some experiments, before intracellular staining, cells were cultured in the presence of 20 ng/mL phorbol 12-myristate 13-acetate (PMA; Sigma-Aldrich) and 200 ng/mL ionomycin (Sigma-Aldrich) for 4 hours, and protein secretion was blocked by 1 μg/mL Golgiplug (BD Biosciences Pharmingen) added during the culture.
Enzyme-linked immunosorbent assay
Cells (5 × 105 cells/mL) isolated from the spleen and draining lymph nodes of mice with cGVHD were cultured in 24-well plates (Corning Glass Works, Corning, NY) in the presence of 10 ng/mL PMA plus 200 ng/mL ionomycin. Supernatants were harvested 48 hours later. The cytokine contents of each supernatant were measured by enzyme-linked immunosorbent assay (ELISA), according to the manufacturer's instruction.
Statistical analysis
Student t test was used to determine the statistical significance of differences between groups. Error bars represent standard error of mean. The following nonparametric analyses were used: log-rank Mantel-Cox for incidence and survival curves; Mann-Whitney for clinical score data.
Results
Treatment of anti-CD137 during the induction phase of cGVHD induces severe acute GVHD (aGVHD) in mice that are genetically prone to cGVHD
We initially wanted to determine whether anti-CD137 can prevent cGVHD. Anti-CD137 was administered immediately after transfer of donor BM and CD4+ T cells. To our surprise, anti-CD137 induced severe loss of body weight (Figure 1A), ultimately resulting in death within 40 days after antibody treatment (Figure 1B). These results together with other disease symptoms, such as severe diarrhea, hunched back, and ruffling fur texture, suggested that anti-CD137 might shift the disease pattern toward aGVHD (systemic or intestinal GVHD) in mice that were genetically prone to cGVHD. Indeed, histopathological analysis revealed crypt apoptosis and inflammation with ulceration (erosion of the epithelial layer), thus indicating colitis 7 days after the transfer of donor cells and anti-CD137 treatment (data not shown). A majority of the anti-CD137–treated mice were dead before cutaneous GVHD was manifested and a small number of mice that received anti-CD137 exhibited less severe cutaneous GVHD before death, compared with mice that received control Ig (data not shown).
Anti-CD137 induces aGVHD when treated during the induction phase of cGVHD. On day 0, Balb/c recipient mice were sublethally irradiated and reconstituted with 5 × 106 BM cells alone or together with 1 × 107 purified CD4+ T cells from B10.D2 donor mice. Anti-CD137 (200 μg per mouse) was administered immediately after transfer of donor cells (n = 10 per group). (A) Change of body weight. Data indicate percentage of body weight at each time point divided by original body weight at day 0. (B) Percentage of survival. ***P < .001, between the group of mice that received donor BM, CD4+ T cells, and anti-CD137 and the other 3 groups. The results are representative of more than 3 independent experiments.
Anti-CD137 induces aGVHD when treated during the induction phase of cGVHD. On day 0, Balb/c recipient mice were sublethally irradiated and reconstituted with 5 × 106 BM cells alone or together with 1 × 107 purified CD4+ T cells from B10.D2 donor mice. Anti-CD137 (200 μg per mouse) was administered immediately after transfer of donor cells (n = 10 per group). (A) Change of body weight. Data indicate percentage of body weight at each time point divided by original body weight at day 0. (B) Percentage of survival. ***P < .001, between the group of mice that received donor BM, CD4+ T cells, and anti-CD137 and the other 3 groups. The results are representative of more than 3 independent experiments.
Anti-CD137 can reverse advanced cutaneous GVHD
Since anti-CD137 can completely prevent cGVHD without inducing aGVHD in the DBA/2→unirradiated (C57BL/6 × DBA/2) F1 (BDF1) cGVHD model,30 it was possible that irradiation had an influence on the conversion of cGVHD to aGVHD by anti-CD137 in the B10D.2→Balb/c model. Thus, we next wanted to examine the therapeutic effect of anti-CD137 on cutaneous GVHD when the harmful influence of irradiation remains minimal in host mice. To examine this hypothesis, mice with cutaneous GVHD received a single injection of anti-CD137 between month 1 and month 2 after disease induction. In some experiments, anti-CD137 was administered 34 days after disease induction and the changes of clinical score were evaluated thereafter until day 120 after disease induction. As shown in Figure 2Ai, anti-CD137 significantly decreased clinical scores, and a majority of mice gained body weight after receiving anti-CD137 to a larger extent compared with control Ig-treated mice (Figure 2Aii). Not only did anti-CD137 block the progression of disease in mice with a lower clinical score but also anti-CD137 reversed cutaneous GVHD in mice with a maximum clinical score. However, a small number of mice that received anti-CD137 experienced a severe loss of body weight and had intestinal GVHD (data not shown). Despite these variations in clinical score and loss of body weight, all of the mice with ulcers in the skin were cured as early as day 24 after treatment with anti-CD137 (Figure 2B). Regrowth of hairs was observed in the skin lesions of a majority of mice that recovered from cutaneous GVHD after treatment with anti-CD137 (Figure 2C). Histologic examination revealed a thickening of the epithelial layer, loss of hair follicles and subdermal fat, ulcers in the epithelial and dermal layers, and heavy collagen deposition in the skin lesions of control Ig-treated mice (Figure 2D). In marked contrast, the skin of anti-CD137–treated mice had normal architecture and morphology according to the pathological categories mentioned immediately above (Figure 2D). In sum, our results suggest that anti-CD137 can reverse cutaneous GVHD.
Anti-CD137 reverses cutaneous GVHD. Anti-CD137 (200 μg per mouse) was administered one time 34 days after induction of cGVHD and changes of clinical scores and body weight were evaluated thereafter. Data were combined from 3 experiments (n = 14 for control Ig group; n = 18 for anti-CD137 group). (Ai) Changes of mean clinical scores. *P < .05, between the 2 groups. (Aii) Changes of body weight. (B) Comparison of the percentage of mice with ulcers between day 34 and day 58 after disease induction. (C) Gross observation of the skin lesions in the posterior neck area at day 58. (D) Histopathology of the skin at day 58. (Upper column) H&E staining; (lower column) Masson trichrome staining. Images were acquired by a BX51 Olympus light microscope (Tokyo, Japan) 4×/0.10 oil objective, and an Olympus C-3000 zoom camera and processed using Image-Pro Plus, version 4 (MediaCybernetics, Silver Spring, MD). Original magnification, 40 ×.
Anti-CD137 reverses cutaneous GVHD. Anti-CD137 (200 μg per mouse) was administered one time 34 days after induction of cGVHD and changes of clinical scores and body weight were evaluated thereafter. Data were combined from 3 experiments (n = 14 for control Ig group; n = 18 for anti-CD137 group). (Ai) Changes of mean clinical scores. *P < .05, between the 2 groups. (Aii) Changes of body weight. (B) Comparison of the percentage of mice with ulcers between day 34 and day 58 after disease induction. (C) Gross observation of the skin lesions in the posterior neck area at day 58. (D) Histopathology of the skin at day 58. (Upper column) H&E staining; (lower column) Masson trichrome staining. Images were acquired by a BX51 Olympus light microscope (Tokyo, Japan) 4×/0.10 oil objective, and an Olympus C-3000 zoom camera and processed using Image-Pro Plus, version 4 (MediaCybernetics, Silver Spring, MD). Original magnification, 40 ×.
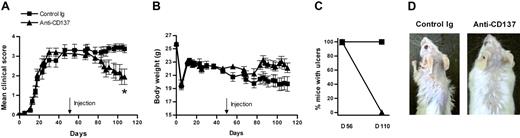
We next examined the therapeutic effect of anti-CD137 on more advanced cutaneous GVHD (about 2 months after disease induction) and observed disease progression from the day of antibody treatment onward. There was no difference in the therapeutic efficacy of anti-CD137 on cutaneous GVHD whether anti-CD137 was administered at either day 34 or day 56 after disease induction (Figure 3). These results suggest that anti-CD137 also can very effectively reverse more severe cutaneous GVHD.
Anti-CD137 has a therapeutic effect in mice with more severe cutaneous GVHD. (A) Anti-CD137 (200 μg per mouse) was administered one time 56 days after induction of cGVHD. Fifty-four days later, clinical scores for each mouse were evaluated. Data were combined from 2 experiments (n = 14 for control Ig group; n = 15 for anti-CD137 group). (A) Changes of mean clinical scores. *P < .05, between the 2 groups. (B) Changes of body weight. (C) Comparison of the percentage of mice with ulcers between day 56 and day 110 after disease induction. (D) Gross observation of the skin lesions in the posterior neck area. The experiment was repeated and similar results were obtained.
Anti-CD137 has a therapeutic effect in mice with more severe cutaneous GVHD. (A) Anti-CD137 (200 μg per mouse) was administered one time 56 days after induction of cGVHD. Fifty-four days later, clinical scores for each mouse were evaluated. Data were combined from 2 experiments (n = 14 for control Ig group; n = 15 for anti-CD137 group). (A) Changes of mean clinical scores. *P < .05, between the 2 groups. (B) Changes of body weight. (C) Comparison of the percentage of mice with ulcers between day 56 and day 110 after disease induction. (D) Gross observation of the skin lesions in the posterior neck area. The experiment was repeated and similar results were obtained.
Anti-CD137 increases apoptosis of donor CD4+ T cells through the Fas pathway
Allostimulation plus CD137 stimulation provides strong AICD signals for donor CD4+ T cells in the DBA/2→BDF1 cGVHD model.30 In a similar context, we wanted to determine whether anti-CD137 can have a therapeutic effect for cutaneous GVHD through the induction of AICD in donor CD4+ T cells. Since the Fas pathway is required for AICD in T cells,36,37 we examined whether Fas/FasL interactions are involved in the inhibition of cutaneous GVHD mediated by anti-CD137. Mice that received donor BM and CD4+ T cells were administered with anti-CD137 on day 45 and a blocking anti-FasL on days 43, 45, and 47 after disease induction. The changes of clinical score were evaluated thereafter until 40 days after treatment with anti-CD137 and mice were killed to harvest splenocytes. As shown in Figure 4A, anti-FasL completely abolished the therapeutic effect of anti-CD137 on the reversal of cutaneous GVHD.
The Fas pathway is required for the therapeutic effect of anti-CD137 on cutaneous GVHD. (A,B) Anti-CD137 (200 μg per mouse) was administered 45 days after disease induction. Anti-FasL (200 μg per mouse) treatment occurred on days 43, 45, and 47. Forty days later, clinical scores for each mouse were evaluated and mice were killed for preparation of splenocytes (n = 6-7 for each group). (A) Changes of mean clinical scores. *P < .05 between the group of mice that received anti-CD137 and the group of mice that received anti-CD137 and anti-FasL. (B) Splenocytes were prepared and stimulated for 2 days in the presence of PMA and ionomycin. Culture supernatants were collected and levels of IL-5, IL-13, and IFN-γ were analyzed by ELISA. IFN-γ was undetectable. **P < .01 and **P < .001 between the group of mice that received anti-CD137 and the group of mice that received anti-CD137 and anti-FasL. (C) Anti-CD137 (200 μg per mouse) was administered 30 days after disease induction. Anti-FasL (200 μg per mouse) treatment occurred on days 28, 30, and 32. Splenocytes were prepared 5 days after treatment with anti-CD137 and triple-stained with anti-CD4, anti-Ly9.1, and annexin V. Ly9.1−CD4+ T cells were gated and analyzed for annexin V staining. *P < .05, between the group of mice that received anti-CD137 and the group of mice that received anti-CD137 and anti-FasL (n = 6-7 per group).
The Fas pathway is required for the therapeutic effect of anti-CD137 on cutaneous GVHD. (A,B) Anti-CD137 (200 μg per mouse) was administered 45 days after disease induction. Anti-FasL (200 μg per mouse) treatment occurred on days 43, 45, and 47. Forty days later, clinical scores for each mouse were evaluated and mice were killed for preparation of splenocytes (n = 6-7 for each group). (A) Changes of mean clinical scores. *P < .05 between the group of mice that received anti-CD137 and the group of mice that received anti-CD137 and anti-FasL. (B) Splenocytes were prepared and stimulated for 2 days in the presence of PMA and ionomycin. Culture supernatants were collected and levels of IL-5, IL-13, and IFN-γ were analyzed by ELISA. IFN-γ was undetectable. **P < .01 and **P < .001 between the group of mice that received anti-CD137 and the group of mice that received anti-CD137 and anti-FasL. (C) Anti-CD137 (200 μg per mouse) was administered 30 days after disease induction. Anti-FasL (200 μg per mouse) treatment occurred on days 28, 30, and 32. Splenocytes were prepared 5 days after treatment with anti-CD137 and triple-stained with anti-CD4, anti-Ly9.1, and annexin V. Ly9.1−CD4+ T cells were gated and analyzed for annexin V staining. *P < .05, between the group of mice that received anti-CD137 and the group of mice that received anti-CD137 and anti-FasL (n = 6-7 per group).
At day 85 after disease induction, the spleens were almost completely reconstituted with donor T cells (Table 1). Analysis of cytokines secreted by the harvested splenocytes after polyclonal stimulation showed that anti-CD137 significantly decreased the production of the Th2 cytokines, IL-5 and IL-13, but anti-FasL completely restored the production of Th2 cytokines that were reduced by anti-CD137 (Figure 4B). We observed basal levels of IFN-γ in culture supernatants in all of the 4 groups (data not shown). We next wanted to examine whether apoptosis of donor CD4+ T cells induced by anti-CD137 was associated with the inhibition of Th2 cytokine production. For this purpose, mice with cutaneous GVHD were administered with anti-CD137 on day 30 after disease induction and anti-FasL at days 28, 30, and 32. Donor CD4+ T cells harvested from the spleens 5 days after anti-CD137 treatment showed increased annexin V staining when compared with donor CD4+ T cells derived from control Ig-treated recipients (Figure 4C). However, the increased levels of apoptosis of donor CD4+ T cells induced by anti-CD137 were significantly diminished by anti-FasL both in percentage and absolute number of apoptotic donor CD4+ T cells (Figure 4C). These results suggest that apoptosis of donor CD4+ T cells induced by anti-CD137 was associated with the inhibition of Th2 cytokine production. Taken together, our results suggest that anti-CD137–mediated therapy of cutaneous GVHD requires AICD of donor CD4+ T cells through the Fas pathway.
Anti-CD137 blocks lethal GVHD mediated by donor CD4+ and CD8+ T cells and reverses skin fibrosis
To examine the effects that donor CD8+ T cells have on the therapy for cutaneous GVHD by anti-CD137, we introduced total B10.D2 spleen/lymph node cells into irradiated Balb/c mice. Even though donor CD8+ T cells alone could not induce cutaneous GVHD, 1 × 107 total donor spleen/lymph node cells resulted in severe lethal GVHD, making it impossible to test the therapeutic effect of anti-CD137 in this clinical setting (data not shown). Therefore, we induced cutaneous GVHD with a lower number of donor T cells (6 × 106 spleen/lymph node cells) and treated recipient mice with anti-CD137 or control Ig 30 days after disease induction. We found that anti-CD137 significantly increased survival rate compared with control Ig (Figure 5A). Importantly, skin fibrosis and inflammation were ameliorated in the surviving mice treated with anti-CD137 in the category of clinical score, percentage of mice with ulcers, gross observation, and histopathology (Figure 5B-E). These results indicate that anti-CD137 can inhibit lethal GVHD induced by donor CD4+ and CD8+ T cells.
Anti-CD137 blocks lethal GVHD induced by donor CD4+ and CD8+ T cells. On day 0, Balb/c recipient mice were sublethally irradiated and reconstituted with 5 × 106 BM cells and 6 × 106 total spleen/lymph node cells from B10.D2 donor mice. Anti-CD137 (200 μg per mouse) was administered 30 days after disease induction, and changes of mean clinical scores and survival rate were counted thereafter (n = 7 for control Ig group; n = 12 for anti-CD137 group). (A) Survival rate. (B) Changes of mean clinical scores. (C) Comparison of the percentage of mice with ulcers between day 30 and day 59 after disease induction. (D) Gross observation of the skin lesions in the posterior neck area at day 59 after disease induction. (E) Histopathology of the skin at day 59 after disease induction (H&E staining). Images were acquired using an Olympus BX51TF light microcope, 4×/0.10 oil objective, and an Olympus C-3000 zoom camera and processed using Image-Pro Plus, version 4. Original magnification, × 40.
Anti-CD137 blocks lethal GVHD induced by donor CD4+ and CD8+ T cells. On day 0, Balb/c recipient mice were sublethally irradiated and reconstituted with 5 × 106 BM cells and 6 × 106 total spleen/lymph node cells from B10.D2 donor mice. Anti-CD137 (200 μg per mouse) was administered 30 days after disease induction, and changes of mean clinical scores and survival rate were counted thereafter (n = 7 for control Ig group; n = 12 for anti-CD137 group). (A) Survival rate. (B) Changes of mean clinical scores. (C) Comparison of the percentage of mice with ulcers between day 30 and day 59 after disease induction. (D) Gross observation of the skin lesions in the posterior neck area at day 59 after disease induction. (E) Histopathology of the skin at day 59 after disease induction (H&E staining). Images were acquired using an Olympus BX51TF light microcope, 4×/0.10 oil objective, and an Olympus C-3000 zoom camera and processed using Image-Pro Plus, version 4. Original magnification, × 40.
Discussion
Despite improvements in the practice of allogeneic hematopoietic stem cell transplantation, the prevalence of cGVHD has increased for various reasons.2 One of the difficulties in combating cGVHD is a lack of understanding about the pathophysiology of this T-cell–mediated syndrome. Accordingly, few therapeutic approaches have targeted pathogenic T cells specific for host mH antigens. Previous animal studies have shown that short-term administration of an agonistic mAb to CD137 blocks autoimmune disease progression, including SLE-like cGVHD.24-30 In this study, we demonstrated that a single injection of anti-CD137 was effective in reversing already established cutaneous GVHD with human cGVHD features. When we injected anti-CD137 into mice exhibiting full-blown cutaneous GVHD with alopecia and skin remodeling, anti-CD137 reduced increased collagen deposition and completely resolved skin inflammation. Surprisingly, a majority of mice recovered from cutaneous GVHD showed normal hair growth at the sites of skin lesions, and remained healthy. Therefore, our result might constitute a basis for effective immunotherapy of cutaneous GVHD patients with anti-CD137.
Interestingly, in vivo engagement of CD137 during the induction phase of cGVHD induced severe aGVHD in mice that are genetically susceptible to cGVHD (Figure 1). Since in MHC-matched and multiple mH-mismatched, donor-recipient pairings, target antigens determine GVHD phenotype,14 this observation suggests a possibility that in vivo stimulation of CD137 can alleviate the anergy of CD4+ T cells (presumably Th1 cells) to nonimmunodominant mH antigens derived from the intestine. However, we believe that this is not the case, considering that anti-CD137 can down-regulate the activity of both Th1 and Th2 CD4+ T cells (J.K. and B.K., unpublished observations, September 21, 2006), as reported in many studies.24,25,30-34 Rather, a more plausible explanation for our observations is that anti-CD137 may amplify a “cytokine storm” by enforcing the production of cytokines by various types of cells primed by irradiation,38 and this amplified cytokine storm deteriorates the wasting disease mediated by donor CD4+ T cells (J.K. and B.K., unpublished observations, July 15, 2006). Ongoing research to clarify these issues is currently under way in our laboratory. On the other hand, there is a report demonstrating that alleviating CD8+ T-cell anergy can result in the cGVHD→aGVHD shift.39 In vivo stimulation of CD137 with agonistic anti-CD137 is well known to be effective in breaking CD8+ T-cell anergy40 and expanding subimmunodominant antigens.41 Consistent with this, treatment with anti-CD137, simultaneously with transfer of donor CD8+ T cells and T-cell–depleted BM, activates CD8+ T cells that otherwise remain anergized to mH antigens derived from the skin and other target organs, resulting in the development of a hybrid form of aGVHD and cGVHD in the recipient (J.K. and B.K, unpublished observations, December 17, 2005). In this regard, it is worthwhile noticing that, although donor CD8+ T cells alone may fall into anergy in recipient mice in our cGVHD, they may be activated with the help of donor CD4+ T cells as well as by engagement with costimulatory molecules and induce lethal cGVHD. In the curing model of cutaneous GVHD, however, anti-CD137 is equally effective in reversing cutaneous GVHD induced by donor CD4+ T cells or donor CD4+ and CD8+ T cells. Therefore, it will be needed to define the mechanism(s) underlying the therapeutic effect of anti-CD137 on cutaneous GVHD caused by CD4+ and CD8+ T cells.
It is difficult to draw a definite conclusion on the involvement of Th1/Th2 CD4+ T cells in the pathogenesis of cutaneous GVHD. Regardless of the involvement of CD4+ T-cell subsets in the development of cutaneous GVHD, however, anti-CD137 is effective in reversing active cutaneous GVHD even in the absence of donor CD8+ T cells. We routinely detected a significantly increased population of apoptotic cells in mice with cutaneous GVHD 4 to 6 days after anti-CD137 treatment (Figure 4C). As blocking of the Fas pathway recovers rate of apoptosis and levels of cytokine production induced by anti-CD137 to the extent comparable with control Ig-treated mice, it appears that apoptosis of donor CD4+ T cells is a prerequisite for the therapeutic effect of anti-CD137. (Interestingly, CD137-Fc protein also can induce apoptosis of human lymphocytes presumably via the ligand for CD137 independently of the Fas pathway.42 ) Prior studies have shown that agonist mAbs to costimulatory molecules use different death pathways for the induction of AICD of donor CD4+ T cells in aGVHD models; whereas anti-CD28 deletes alloantigen-specific donor T cells in IFN-γ–dependent ways,43 anti-GITR induces apoptosis of alloreactive CD4+ T cells that is dependent of the Fas pathway.44 The common death pathway shared by CD137 and GITR may be one of many common features in their molecular structure and functions.39,44-46
Despite the impressive preventive/therapeutic effect of anti-CD137 on spontaneous or experimentally induced diseases, the underlying mechanisms are rather complicated. Deletion of pathogenic CD4+ T cells and/or B cells provides an adequate explanation for anti-CD137–mediated immunotherapeutic effect in some diseases.24,25,30 As shown in this study, pathogenic T cells may be deleted by AICD. Massive AICD of CD4+ T cells is easily detectable in vivo in mice that received anti-CD137 together with strong allostimulation (eg, cGVHD)30 or in mice in which TCR transgenic CD4+ T cells are transferred when they are immunized with an antigen and simultaneously treated by anti-CD137.24 Massive depletion of B cells occurs in SLE28 and SLE-like cGVHD30 by anti-CD137 and is associated with the abrogation of autoantibody production and, thus, diminution of disease severity. In this case, IFN-γ seems to play a critical role in nonspecifically deleting B cells.24,26 However, since autoantibody production by B cells requires help from CD4+ T cells, we propose that the primary reason for anti-CD137–mediated immunotherapy of autoimmune diseases lies in deletion of CD4+ T cells rather than nonspecific deletion of B cells. In allergic asthma, Polte et al33 demonstrated that CD137 stimulation induces anergy of CD4+ T cells. However, this observation may not necessarily be contradictory to our results. It is well known that deletion of antigen-specific T cells can lead to peripheral tolerance to the antigen. If anti-CD137 causes mice to lose a majority of antigen-specific CD4+ T cells (in this case, Th2 cells), CD4+ T cells isolated from those mice would poorly respond to antigen after adoptive transfer into immunodeficient mice or after in vitro restimulation with antigen. The most convincing evidence supporting the induction of CD4+ T-cell anergy by anti-CD137, which Polte et al showed, is that the unresponsive CD4+ T cells, derived from anti-CD137–treated mice, proliferated in response to antigen when IL-2 was added to the culture medium in the in vitro restimulation experiment. This phenomenon could occur in experimental conditions in which CD4+ T cells (which escape from AICD) with a low-affinity TCR for antigen might be activated by a high concentration of epitope peptide in the presence of IL-2, as shown by the authors. This issue may be fully elucidated using monoclonal autoreactive CD4+ T cells, which we are trying to prepare in our laboratory.
In conclusion, the findings reported here clearly showed that anti-CD137 is effective in reversing advanced cutaneous GVHD. In vivo engagement of costimulatory molecules may provide effective therapies against disorder caused by inappropriate T-cell activation.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This work was supported by grants from the Korea Research Foundation (C00088) and the Korea Health 21 Research and Development Project of the Korean Ministry of Health and Welfare (A040004).
Authorship
Contribution: B.K. designed research; J.K., H.J.K., J.K., and K.P. performed research; H.Y. contributed vital reagents; J.K., H.-J.C., S.H.N., H.R.C., and B.K. analyzed data; B.K. wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Byungsuk Kwon, Department of Biological Science, University of Ulsan, San29, Mukeo-dong, Nam-ku, Ulsan 680–749, Republic of Korea; e-mail: bkwon@mail.ulsan.ac.kr.