Abstract
Gene replacement therapy is complicated by the risk of an immune response against the therapeutic transgene product, which in part is determined by the route of vector administration. Our previous studies demonstrated induction of immune tolerance to coagulation factor IX (FIX) by hepatic adeno-associated viral (AAV) gene transfer. Using a regulatory T-cell (Treg)–deficient model (Rag-2−/− mice transgenic for ovalbumin-specific T-cell receptor DO11.10), we provide first definitive evidence for induction of transgene product-specific CD4+CD25+ Tregs by in vivo gene transfer. Hepatic gene transfer–induced Tregs express FoxP3, GITR, and CTLA4, and suppress CD4+CD25− T cells. Tregs are detected as early as 2 weeks after gene transfer, and increase in frequency in thymus and secondary lymphoid organs during the following 2 months. Similarly, adoptive lymphocyte transfers from mice tolerized to human FIX by hepatic AAV gene transfer indicate induction of CD4+CD25+GITR+ that suppresses antibody formation to FIX. Moreover, in vivo depletion of CD4+CD25+ Tregs leads to antibody formation to the FIX transgene product after hepatic gene transfer, which strongly suggests that these regulatory cells are required for tolerance induction. Our study reveals a crucial role of CD4+CD25+ Tregs in preventing immune responses to the transgene product in gene transfer.
Introduction
Genetic disease can potentially be cured by the introduction of a functional copy of the defective gene. Stable gene transfer results in a continuous supply of the functional gene product, and proof-of-principle for this concept has been achieved in humans with severe immune deficiencies and in animal models of several genetic diseases.1,2 However, gene replacement therapy is complicated by the risk of an immune response against the therapeutic transgene product. A particularly serious concern in treatments that rely on systemic delivery of the transgene product is the potential for formation of an antibody response to the therapeutic protein. A humoral immune response could not only neutralize the gene therapy but also interfere with conventional protein therapy.3,4 Animal models of genetic disease have been used to study the risk of immune responses in gene therapy for lysosomal storage disorders and systemic protein deficiencies such as the X-linked bleeding disorder hemophilia
The goal of in vivo gene transfer for systemic protein delivery is to direct transgene expression to a particular tissue, thereby turning the target cells into factories that produce the therapeutic protein and secrete it into circulation. However, because of the genetic defect in the recipient of gene transfer, the functional protein may represent a neoantigen that can be subject to an adaptive immune response. Activation signals derived from the gene transfer process (ie, from the procedure or the vector) may trigger a local immune response that can lead to T- and B-cell activation in draining lymph nodes and subsequently in other lymphoid organs such as the spleen.5,6 Nonetheless, sustained correction of lysosomal storage disease in mice and of canine and murine hemophilia B (deficiency in coagulation factor IX, FIX) has been achieved in juvenile and adult animals by in vivo hepatic gene transfer using adeno-associated viral (AAV) vectors.7–10 Moreover, it has been demonstrated that the lack of immune responses in these experiments was the result of induction of immune tolerance by the hepatic route.10,11 Experimental animals continued to express a human FIX (hFIX) transgene product without antibody formation even after challenge by administration of hFIX in adjuvant.11 Lymphocytes from tolerized mice failed to proliferate and to secrete cytokines in response to in vitro restimulation with hFIX, indicating a lack of T helper cell responses.11
While our laboratory has obtained evidence for induction of CD4+ T-cell anergy and deletion using ovalbumin (ova) as a model antigen in hepatic AAV-mediated gene transfer, studies with hFIX also provided evidence for induction of CD4+ regulatory T cells (Tregs).11,12 These cells were found to suppress antibody and cytotoxic T-cell responses to hFIX, thereby allowing for secondary gene transfer with a more immunogenic adenoviral vector.13 The subset of CD4+ cells responsible for suppression had not yet been identified. Analysis of splenocytes showed an increase in CD4+CD25+GITR+ and in FoxP3 message after challenge of tolerized mice by systemic administration of adenoviral vector expressing hFIX.13 These data suggested a potential role for CD4+CD25+ Tregs. Interestingly, in the ova model system (mice transgenic for a T-cell receptor, TCR, for ova), the clonal population of ova-specific CD4+ T cells contained an increased percentage of CD4+CD25+ Tregs at 2 months after gene transfer, by which time deletion had resulted in a reduction of the total number of TCR transgenic CD4+ T cells.12 Removal of CD25+ cells partially restored responsiveness of CD4+CD25− cells to ova in vitro.12 However, ova-specific CD4+CD25+ Tregs in this system had been present prior to gene transfer likely due to incomplete allelic exclusion of endogenous TCRs and stimulation with self-antigens.14 Therefore, it remained unclear whether hepatic transgene expression could induce CD4+CD25+ Tregs. In general, little is known about the role of Tregs in gene transfer.
Several subsets of regulatory T cells are known to be inducible by specific routes of antigen administration. In recent years, it has become evident that there are also naturally occurring Tregs, which are required to prevent autoimmunity.15 These cells typically comprise 5% to 10% of CD4+ T cells and constitutively express CD25, the α-chain of IL-2R, and CD4+CD25+ Tregs suppress CD4+CD25− T cells in a cell contact–dependent manner in vitro.16 Additionally, CD4+CD25+ Tregs express cytotoxic T-lymphocyte–associated antigen-4 (CTLA4), glucocorticoid-induced tumor necrosis factor receptor family–related gene (GITR) and transcription factor forkhead box P3 (FoxP3). FoxP3 is a master control gene for the development and function of naturally occurring Tregs, and is currently the most specific molecular marker for this T-cell subpopulation.17–19 Recently, increasing evidence has been obtained that CD4+CD25+ Tregs can also be induced, and that these cells may not be derived exclusively from thymic development, since it is possible to convert CD4+CD25− cells to CD4+CD25+ Tregs.20
Here, we demonstrate that in vivo hepatic gene transfer induces CD4+CD25+ Tregs that suppress antibody formation to the transgene product. Our results strongly suggest that these regulatory cells are required for tolerance induction, and we provide definite evidence for induction of transgene product–specific CD4+CD25+GITR+FoxP3+ Tregs. We conclude that augmenting CD4+CD25+ Treg function will be crucial in preventing immune responses to the transgene product in gene transfer.
Materials and methods
Viral vectors
AAV vectors harboring the ova or green fluorescence protein (GFP) transgene under the control of the human elongation factor-1α (EF1α) enhancer/promoter (AAV-EF1α-ova or AAV-EF1α-GFP) were as previously described.12 Both expression cassettes contain the first intron of the human EF1α gene and a human growth hormone polyadenylation signal. Vector AAV-ApoE/hAAT-hFIX contains the hepatocyte-specific expression cassette for hFIX as published.11,21 This cassette includes the ApoE enhancer, hepatocyte control region, hAAT promoter, hFIX cDNA (including a 1.4-kb portion of intron 1 of the F9 gene), and bovine growth hormone polyA signal. All expression cassettes are flanked by AAV-2 inverted terminal repeats. AAV vector (serotype 2) was produced by triple transfection of HEK-293 cells, purified by CsCl-gradient centrifugation, and quantitated by slot blot hybridization as described.22
Animal studies
Male C57BL/6 (4-6 weeks of age) and male DO11.10 TCR transgenic mice (BALB/c background, 4-6 weeks of age) were purchased from Jackson Laboratories (Bar Harbor, ME). DO11.10-tg Rag-2 knockout (DO11.10Rag-2−/−) mice were purchased from Taconic (Hudson, NY). Animals were housed in The Children's Hospital of Philadelphia's and University of Florida's Animal Laboratory Facilities with 12-hour light and dark cycles along with unlimited access to food and water. AAV vector (50 μL per injection) was delivered into the portal vein by splenic capsule injection with a Hamilton syringe (Reno, NV) following a ventral midline incision as described.11,23 Immunizations were done by subcutaneous injection on the back using 5 μg human FIX protein formulated in complete Freund adjuvant (CFA; Life Technologies, Rockville, MA). Blood samples were collected from the mice via the retro-orbital plexus using heparinized capillary tubes. Depletion of CD25+ cells was performed every 2 weeks for 6 weeks (starting at the day of vector administration) by intraperitoneal injections of rat antimurine CD25 monoclonal antibody (affinity purified, preservative-free; BioLegend, San Diego, CA) at a dose of 30 μg/mouse (∼ 1.5 mg/kg). Control mice received nonspecific rat IgG.
Assay for antibody formation to hFIX
Immunocapture assay for determination of anti-hFIX titers was performed as published.24,25 Briefly, microtiter plates were coated with plasma-derived human FIX protein (1 μg/mL, mononine; Armour Pharmaceutical, Kankakee, IL), and mouse plasma samples were applied at a 1:20 dilution. In parallel, wells were coated with 2-fold serial dilutions of purified mouse immunoglobulin IgG (Sigma-Aldrich, St Louis, MO) starting at 2000 ng/mL for generation of a standard curve. Anti-hFIX was detected with horseradish peroxidase–conjugated antibody (in 1:2000 dilution) specific for mouse IgG (Roche Molecular Biochemicals, Indianapolis, IN). Antibody levels were measured by OD reading at 450 nm following incubation with o-phenylen diamine substrate.
Flow cytometry
Spleens, thymus, inguinal nodes, and portal nodes were harvested from mice and processed to produce single-cell suspensions as previously described.25 Viable splenocytes and lymphocytes were counted using a hemacytometer and trypan blue. Cells were then placed in culture or used for analysis by flow cytometry. Fixation and antibody staining of cell surface markers were carried out using standard protocols. DO11.10 TCR specific to ova 323-339 peptide was detected by using the monoclonal KJ1-26 antibody conjugated to the FITC fluorochrome (CALTAG, Burlingame, CA).26 Other monoclonal antibodies used were FITC-conjugated anti-CD4, PE-conjugated anti-CTLA4 (BD Bioscience, San Jose, CA), allophycocyanin-conjugated anti-CD25 (CALTAG, Burlingame, CA), and PE-conjugated anti-GITR (R&D Systems, Minneapolis, MN). Stain for CTLA4 was intracellular. Nuclear stain for transcription factor FoxP3 was performed with the eBiosciences (San Diego, CA) kit. Controls for all stains included isotype controls and unstained cells. Flow cytometry was performed on a FACSCalibur (Becton Dickinson, Mountain View, CA), and data were analyzed with CellQuest software (BD Bioscience).
Adoptive T-cell transfer
C57BL/6 mice (naive or 2 months after hepatic gene transfer with AAV vector) were killed and splenocytes isolated. CD4+ T cells were purified from pooled splenocytes by magnetic cell sorting (MACS) using a column for positive selection with antimurine CD4 (Miltenyi Biotech, Auburn, CA) according to the manufacturer's instructions. Similarly, CD4+CD25+ and CD4+CD25− cells were isolated by magnetic cell sorting using the Miltenyi Biotech CD4CD25 Isolation Kit. Here, CD4+ cells are negatively selected followed by CD25+ positive selection following the manufacturer's instructions (Miltenyi Biotech). Alternatively, CD4+GITR+ cells were separated by a positive selection with biotin-conjugated anti-GITR following enrichment of CD4+ cells. Analysis by flow cytometry showed more than 85% purity of CD4+ T cells with less than 1% CD8+ T cells and less than 7% B cells. CD4+CD25+ cells were more than 80% pure (< 15% CD4+CD25−). CD4+GITR+ cells were more than 75% pure. CD4-depleted cells (5 × 107/recipient mouse), CD4+ cells (1 × 107/recipient mouse), CD4+CD25− cells (1 × 107/recipient mouse), CD4+CD25+ cells (1 × 106/recipient mouse), CD4+GITR− cells (1 × 107/recipient mouse), or CD4+GITR+ cells (1 × 106/recipient mouse) were adoptively transferred to naive wild-type C57BL/6 mice via tail vein injection. Recipient mice were subcutaneously injected with 5 μg hFIX in CFA 24 hours after adoptive transfer. Anti-hFIX IgG titers in plasma were measured 3 weeks after immunization.11,27
Reverse-transcription–polymerase chain reaction (RT-PCR) and real-time PCR
Total cellular RNA was extracted from 5 × 105 MACS-purified cells using RNeasy Mini Kit (QIAGEN, Valencia, CA). The total amount of RNA was reverse transcribed using Superscript II reverse-transcriptase and oligo(dT)12-18 primer (Invitrogen, Carlsbad, CA) in a final volume of 20 μL. The relative quantity of FoxP3 message in each sample was initially estimated by semiquantitative PCR for FoxP3 and a housekeeping gene (HPRT).19 Reaction mixture (25 μL) contained 1.5 mM MgCl2, 0.2 mM dNTP, 0.5 μM forward and reverse primer, and approximately 2.5 units of PuReTaq DNA polymerase (Amersham Biosciences, Piscataway, NJ). PCRs were performed on a MyCycler Thermal Cycler (Bio-Rad Laboratories, Hercules, CA). For HPRT amplifications, PCRs consisted of a 2-minute denaturation step at 94°C followed by 30 cycles of 1 minute at 94°C, 1 minute at 60°C, and 1 minute at 72°C. For FoxP3, reactions were conducted as described for HPRT except that annealing temperature was 57°C and the number of cycles 32. The primer sequences were as follows: FoxP3, 5′-CAG CTG CCT ACA GTG CCC CTA G-3′ and 5′-CAT TTG CCA GCA GTG GGT AG-3′ (S6); HPRT, 5′-GT TGG ATA CAG GCC AGA CTT TGT TG-3′ and 5′-GAA GGG TAG GCT GGC CTA TAG GCT-3′.
FoxP3 mRNA levels were also quantified by real-time PCR using the ABI/PRISM 7700 sequence detection system (Applied Biosystems, Foster City, CA).
Analyses were performed using primers, an internal fluorescent TaqMan probe specific to FoxP3 or HPRT, and the QuantiTect Probe PCR Kit (QIAGEN). PCRs contained 0.4 μM primers and 0.2 μM TaqMan probe, and consisted of a 10-minute at 95°C denaturation step followed by 40 cycles of 15 seconds at 95°C and 60 seconds at 60°C. The primer and TaqMan probe sequences were as follows: FoxP3 primers, 5′-CCC AGG AAA GAC AGC AAC CTT-3′ and 5′-TTC TCA CAA CCA GGC CAC TTG-3′; FoxP3 probe, 5′-FAM-ATC CTA CCC ACT GCT GGC AAA TGG AGT C-3′; HPRT primers, 5′-TGA AGA GCT ACT GTA ATG ATC AGT CAA C-3′ and 5′-AGC AAG CTT GCA ACC TTA ACC A-3′; and HPRT probe, 5′-VIC-TGC TTT CCC TGG TTA AGC AGT ACA GCC C-3′. To eliminate amplifications from contaminating genomic DNA, these primers were designed to span an intron/exon boundary and thus to anneal specifically to cDNA.19 Normalized values for FoxP3 mRNA expression in each sample were calculated as the relative quantity of FoxP3 divided by the relative quantity of HPRT (× 100). All samples were run in duplicate.
In vitro coculture of CD4+ subpopulations and cytokine release assay
CD4+CD25+ from AAV-EF1α-ova–treated DO11.10-tg RAG-2−/− and CD4+CD25− cells from naive DO11.10-tg were isolated via the MACS following manufacturer's instructions (Miltenyi Biotech). Antigen-presenting cells (APCs) were prepared from naive DO11.10-tg mice using the MACS CD90 separation system. CD4+CD25+ cells were cocultured with CD4+CD25− at different ratios and stimulated with 100 μg/mL ova in the presence of APCs in 96-well plates. Cells were cultured in 2-MLC (mixed leukocyte culture media including 2% fetal bovine serum) media at 37°C in 10% CO2 as published.12 Supernatants were collected after 3 days and tested for the level of interleukin-2 (IL-2). IL-2 enzyme-linked immunosorbent assay (ELISA) used specific antibodies from Pharmingen (San Diego, CA) and was carried out according to manufacturer's instructions.
Statistical analysis
Results are expressed as mean ± SEM. Comparisons among groups were made by unpaired Student t test. Differences were considered significant at P less than or equal to .05.
Results
In vivo gene transfer induces transgene product–specific regulatory CD4+CD25+ Tregs in DO11.10-tg RAG-2−/− mice
Several studies have hinted at the involvement of CD4+CD25+ Tregs in tolerance induction by in vivo gene transfer, yet no conclusive evidence that gene transfer can induce this type of regulatory cell has been presented.12,13,28 To obtain direct evidence that gene transfer can induce CD4+CD25+ Tregs, we performed hepatic gene transfer studies in DO11.10-tg RAG-2−/− BALB/c mice, which are transgenic for TCR DO11.10 (encoded by rearranged Vα13 and Vβ8.2 genes) but lack endogenous TCRs.14 The transgenic TCR is specific for a chicken ova peptide, amino acids 323 to 339, presented by the MHC class II molecule I-Ad, and is expressed in more than 99% of CD4+ T cells in these animals. DO11.10-tg RAG-2−/− mice lack CD4+CD25+ Tregs, presumably because of a lack of TCRs that recognize self-antigens. Mice received hepatic gene transfer with AAV-EF1α-ova or AAV-EF1α-GFP vector or did not receive gene transfer. Eight weeks later, lymphocytes were isolated from spleen, thymus, portal nodes, and inguinal nodes for analysis by flow cytometry. As expected, 1% or less of CD4+ cells of GFP-transduced and untransduced controls stained for CD25 (Figure 1). However, ova-transduced mice showed a significant increase in percent of CD25+ cells among the CD4+TCR+ population in multiple lymphoid organs (3.5- to 17-fold of controls, Figure 1).
Percent CD25+ T cells of CD4+ DO11.10 TCR+ cells in DO11.10-tg RAG-2−/− BALB/c mice as determined by flow cytometry. (A) Scatter graphs show staining of CD25 and DO11.10 TCR for gated CD4+ cells. This example represents inguinal lymph node cells from control mice and animals that received hepatic gene transfer with 1 × 1012 vg AAV-EF1α-ova vector. Mice were killed 8 weeks after gene transfer. Antibody stainings were FITC-conjugated KJ1-26 (for DO11.10 TCR) and PE-conjugated CD25. (B) Summary of results for different lymphoid organs including fold difference between groups. Mice had received no gene transfer or AAV-EF1α-GFP or AAV-EF1α-ova. Results from spleens and thymus were average (± SD), while lymph nodes cells were pooled prior to flow cytometry (n = 5/cohort). *P < .01 compared with control groups.
Percent CD25+ T cells of CD4+ DO11.10 TCR+ cells in DO11.10-tg RAG-2−/− BALB/c mice as determined by flow cytometry. (A) Scatter graphs show staining of CD25 and DO11.10 TCR for gated CD4+ cells. This example represents inguinal lymph node cells from control mice and animals that received hepatic gene transfer with 1 × 1012 vg AAV-EF1α-ova vector. Mice were killed 8 weeks after gene transfer. Antibody stainings were FITC-conjugated KJ1-26 (for DO11.10 TCR) and PE-conjugated CD25. (B) Summary of results for different lymphoid organs including fold difference between groups. Mice had received no gene transfer or AAV-EF1α-GFP or AAV-EF1α-ova. Results from spleens and thymus were average (± SD), while lymph nodes cells were pooled prior to flow cytometry (n = 5/cohort). *P < .01 compared with control groups.
Gene transfer–induced CD4+CD25+ Tregs express FoxP3, GITR, and CTLA-4
To further characterize induced CD4+CD25+ cells from DO11.10-tg RAG-2−/− mice, we determined expression levels of FoxP3 in various subpopulations of splenocytes derived from DO11.10-tg Rag+/+ (RAG+, these mice contain naturally occurring CD4+CD25+ Tregs, which were used as positive control) or DO11.10-tg RAG-2−/− (RAG−) mice. CD4+CD25+ cells from AAV-ova treated DO11.10-tg RAG-2−/−, in contrast to CD4+CD25− cells or to CD4+ cells from DO11.10-tg RAG-2−/− that had not received gene transfer, expressed high levels of FoxP3 as determined by real-time PCR (> 2-log induction, Figure 2A,B). When splenocytes were analyzed for FoxP3 expression by flow cytometry, 2.6% to 3.4% of CD4+ cells from AAV-ova transduced DO11.10-tg RAG-2−/− mice were CD25+FoxP3+ (8 weeks after gene transfer) compared with 0.3% in control mice that had not received gene transfer (Figure 2C and data not shown, n = 2 per group). Furthermore, a functional assay of CD4+CD25+ cells was performed. CD4+CD25+ cells from AAV-ova transduced DO11.10-tg RAG-2−/− and CD4+CD25− cells isolated from DO11.10-tg mice were cocultured in vitro followed by determination of IL-2 production. CD4+CD25+ cells suppressed IL-2 expression of CD4+CD25− cells following in vitro stimulation with ova in a cell dose–dependent manner (Figure 2D). Similar results were obtained for IFN-γ secretion (data not shown). Note CD4+CD25+ cells by themselves did not produce IL-2 in response to stimulation, and that low doses of these cells were sufficient to demonstrate a suppressive effect on CD4+CD25− cells (Figure 2D).
Characterization of regulatory CD4+CD25+ cells isolated from AAV-EF1α-ova–transduced DO11.10-tg RAG-2−/− mice. Expression of FoxP3 in a subpopulation of CD4+ splenocytes derived from DO11.10-tg BALB/c (RAG+) or DO11.10-tg RAG-2−/− (RAG−) mice. (A) Splenocytes were sorted into CD4+ cells and further separated into CD25+ or CD25− cells. cDNA from each population was subjected to PCR using FoxP3- or HPRT (hypoxanthine–guanine phosphoribosyl–transferase)–specific primers. (B) Quantification of relative FoxP3 mRNA levels in indicated CD4+ T-cell subsets. cDNA samples were subjected to real-time quantitative PCR analyses using primers and an internal fluorescent probe specific for FoxP3 or HPRT. The relative quantity of FoxP3 in each sample was normalized to the relative quantity of HPRT. Shown are average results for 3 independent experiments (± SD). (C) Flow cytometry for FoxP3 expression. Shown are examples for percent CD25+FoxP3+ of CD4+ splenocytes in individual mice (8 weeks after gene transfer with AAV-ova or naive control). (D) Cell dose–dependent suppression of IL-2 expression in DO11.10-tg CD4+CD25− cells by CD4+CD25+ isolated from AAV-EF1α-ova–transduced DO11.10-tg RAG-2−/− mice upon in vitro coculture (in the presence of APCs) and stimulation with ova. Data represent average (± SD) from n = 4 cultures based on cells pooled from several mice.
Characterization of regulatory CD4+CD25+ cells isolated from AAV-EF1α-ova–transduced DO11.10-tg RAG-2−/− mice. Expression of FoxP3 in a subpopulation of CD4+ splenocytes derived from DO11.10-tg BALB/c (RAG+) or DO11.10-tg RAG-2−/− (RAG−) mice. (A) Splenocytes were sorted into CD4+ cells and further separated into CD25+ or CD25− cells. cDNA from each population was subjected to PCR using FoxP3- or HPRT (hypoxanthine–guanine phosphoribosyl–transferase)–specific primers. (B) Quantification of relative FoxP3 mRNA levels in indicated CD4+ T-cell subsets. cDNA samples were subjected to real-time quantitative PCR analyses using primers and an internal fluorescent probe specific for FoxP3 or HPRT. The relative quantity of FoxP3 in each sample was normalized to the relative quantity of HPRT. Shown are average results for 3 independent experiments (± SD). (C) Flow cytometry for FoxP3 expression. Shown are examples for percent CD25+FoxP3+ of CD4+ splenocytes in individual mice (8 weeks after gene transfer with AAV-ova or naive control). (D) Cell dose–dependent suppression of IL-2 expression in DO11.10-tg CD4+CD25− cells by CD4+CD25+ isolated from AAV-EF1α-ova–transduced DO11.10-tg RAG-2−/− mice upon in vitro coculture (in the presence of APCs) and stimulation with ova. Data represent average (± SD) from n = 4 cultures based on cells pooled from several mice.
In order to further investigate the phenotype of these regulatory cells induced by hepatic gene transfer, we analyzed CD4+CD25+ cells from various lymphoid organs of DO11.10-tg RAG-2−/− by flow cytometry 60 days after vector administration (Figure 3A). Of total CD4+CD25+ cells, more than 95% were GITR+ and more than 65% were CTLA4+ in spleen, thymus, portal nodes, and inguinal nodes (Figure 3B-C). In summary, hepatic transgene expression induced a population of Tregs that is CD4+CD25+FoxP3+GITR+CTLA4+ T cells and therefore comparable with naturally occurring CD4+CD25+ Tregs (Figures 2–3).
Analyses of cell populations from lymphoid organs of DO11.10-tg RAG-2−/− mice by flow cytometry 60 days after AAV-EF1α-ova gene transfer. (A) Quantitation of CD25+ of TCR+CD4+, CD25+GITR+ and CD25+CTLA4+ of CD4+ cells from spleen in naive or vector-treated mice. Percentages of dual-positive cells (top-right quadrant) are indicated. Note that AAV-EF1α-ova–transduced mice showed substantial increase in CD25+, CD25+GITR+, and CD25+CTLA4+ of total CD4+ cells compared with control animals. Antibody stain was FITC-conjugated KJ1-26 or CD4, PE-conjugated GITR or CTLA4, and APC-conjugated CD25. Representative examples of FACS density plots are shown for individual samples of splenocytes from DO11.10-tg RAG-2−/− mice. Also graphed are percent GITR+ (B) and CTLA4+ (C) of CD4+CD25+ cells derived from spleen, thymus, portal nodes, and inguinal nodes 60 days after AAV-EF1α-ova gene delivery (B,C).
Analyses of cell populations from lymphoid organs of DO11.10-tg RAG-2−/− mice by flow cytometry 60 days after AAV-EF1α-ova gene transfer. (A) Quantitation of CD25+ of TCR+CD4+, CD25+GITR+ and CD25+CTLA4+ of CD4+ cells from spleen in naive or vector-treated mice. Percentages of dual-positive cells (top-right quadrant) are indicated. Note that AAV-EF1α-ova–transduced mice showed substantial increase in CD25+, CD25+GITR+, and CD25+CTLA4+ of total CD4+ cells compared with control animals. Antibody stain was FITC-conjugated KJ1-26 or CD4, PE-conjugated GITR or CTLA4, and APC-conjugated CD25. Representative examples of FACS density plots are shown for individual samples of splenocytes from DO11.10-tg RAG-2−/− mice. Also graphed are percent GITR+ (B) and CTLA4+ (C) of CD4+CD25+ cells derived from spleen, thymus, portal nodes, and inguinal nodes 60 days after AAV-EF1α-ova gene delivery (B,C).
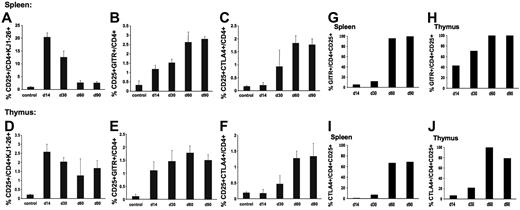
Finally, we analyzed lymphocyte populations in thymus and spleen in DO11.10-tg RAG-2−/− mice as a function of time after administration of AAV-ova vector. As shown in Figure 4, CD4+CD25+GITR+ were detected above background as early as 14 days after vector administration (Figure 4B,E), and CD4+CD25+CTLA4+ were increased after 30 days (Figure 4C,F). The regulatory T-cell population steadily increased up to day 60 and remained unchanged by day 90. During the first month after gene transfer, 10% to 20% of CD4+ splenocytes were CD25+, but only approximately 10% of these cells were GITR+, suggesting that the majority of CD4+CD25+ splenocytes at these early time points were activated T cells rather than regulatory cells (Figure 4A,G). However, data from later time points indicate that CD4+CD25+ splenocytes almost exclusively represented Tregs by day 60 (95%-100% were GITR+ and 70%-100% CTLA4+, Figure 4G-J).
Analyses of lymphocyte populations in DO11.10-tg RAG-2−/− mice as a function of time after administration of AAV-EF1α-ova. Examined tissues were spleens and thymus. Shown is the summary of percent CD25+ (A,D), CD25+GITR+ (B,E), and CD25+CTLA4+ (C,F) of total CD4+ cells derived from spleen or thymus. Note that more than 99% of CD4+ cells in these mice express ova-specific TCR DO11.10 and are therefore KJ1-26+. Antibody staining was carried out using FITC-conjugated KJ1-26 or CD4, PE-conjugated GITR or CTLA4, APC-conjugated CD25. Results are average ± SD for n = 3 mice. Also shown is a summary of percent GITR+ (G,H), and CTLA4+ (I,J) of CD4+CD25+ cells from spleen (G,I) or thymus (H,J) as a function of time after vector administration.
Analyses of lymphocyte populations in DO11.10-tg RAG-2−/− mice as a function of time after administration of AAV-EF1α-ova. Examined tissues were spleens and thymus. Shown is the summary of percent CD25+ (A,D), CD25+GITR+ (B,E), and CD25+CTLA4+ (C,F) of total CD4+ cells derived from spleen or thymus. Note that more than 99% of CD4+ cells in these mice express ova-specific TCR DO11.10 and are therefore KJ1-26+. Antibody staining was carried out using FITC-conjugated KJ1-26 or CD4, PE-conjugated GITR or CTLA4, APC-conjugated CD25. Results are average ± SD for n = 3 mice. Also shown is a summary of percent GITR+ (G,H), and CTLA4+ (I,J) of CD4+CD25+ cells from spleen (G,I) or thymus (H,J) as a function of time after vector administration.
Next, we wanted to test for induction of CD4+CD25+ Tregs in transfer of a therapeutic hFIX gene.
Induction of CD4+CD25+GITR+ cells that suppress antibody formation to hFIX
In previous studies on liver-directed gene transfer with AAV-hFIX vector, we have shown evidence that tolerance induction involves regulatory CD4+ lymphocytes.11,13 To test whether suppression of antibody formation to hFIX is mediated by induced CD4+CD25+ Tregs, we performed adoptive transfer studies. In order to be able to draw conclusions about the relative contribution of different subsets of splenocytes, magnetically sorted cells were transferred in numbers that reflect their relative proportions in donor spleens as determined by flow cytometry (data not shown). Consistent with our previous findings, adoptively transferred total splenocytes or CD4+ cells (but not CD4− cells) from mice that had received hepatic AAV-hFIX gene transfer suppressed antibody formation against hFIX in immunized syngeneic recipients (Figure 5A). Transfer of CD4+CD25+ cells resulted in a level of suppression of antibody formation that was identical to that obtained with total CD4+cells (∼ 70% suppression of the antibody response), while CD4+CD25− cells were only marginally effective (Figure 5A, ∼ 40% suppression). Nonspecific CD4+CD25+ cells (isolated from naive mice) gave highly variable results, but on average failed to suppress antibody formation (Figure 5A).
In order to further establish that suppression is mediated by CD4+CD25+ Tregs, the following additional experiments were performed. It is known that CD4+CD25+ Tregs constitutively express GITR, a receptor that upon binding to its ligand on dendritic cells can mediate loss of the suppressive phenotype (this signaling pathway is thought to allow dendritic cells to override Treg function).29,30 We adoptively transferred CD4+GITR+ or CD4+GITR− cells from C57BL/6 mice that received hepatic gene transfer to naive C57BL/6 (Figure 5B). CD4+GITR+ cells from AAV-hFIX–treated animals suppressed the production of anti-hFIX after immunization and were similarly effective as CD4+CD25+ cells in the previous experiment. Nonspecific CD4+GITR+ cells (isolated from naive mice) were significantly less effective in suppression. Compared with control CD4+GITR− cells, CD4+GITR− splenocytes from AAV-hFIX–transduced mice also showed suppression in this experiment (albeit inefficient compared with CD4+GITR+ cells). However, flow cytometry showed substantial contamination of CD4+GITR− cells with CD4+GITR+ cells due to a poor depletion by magnetic cell sorting (data not shown). It is therefore possible that contaminating CD4+GITR+ cells mediated the suppression. From the totality of the results of the adoptive transfer experiments presented in Figure 5, we can surmise that hepatic gene transfer has induced CD4+CD25+GITR+ Tregs that suppressed antibody formation to hFIX. Other subsets of splenocytes or nonspecific Tregs failed to suppress or were comparatively inefficient in suppression of antibodies.
Plasma levels of IgG1 anti-hFIX 3 weeks after immunologic challenge by subcutaneous administration of 5 μg hFIX formulated in CFA in C57BL/6 mice that had received adoptive transfer of splenocytes from naive (white bars, controls) or vector-treated (hatched bars, AAV-FIX) C57BL/6 mice. Adoptive transfer was by tail vein injection 24 hours before challenge. Vector-treated mice had received hepatic gene transfer with 1011 vg/animal AAV-ApoE/hAAT-hFIX vector 6 weeks before the experiment. (A) Total splenocytes (5 × 107, bars 1-2), CD4+ T-cell–depleted splenocytes (5 × 107, bar 3), CD25+-depleted CD4+ T cells (9 × 106, bars 6, 8), MACS-purified CD4+ T cells (107, bars 4-5), or CD4+CD25+ cells (1 × 106, bars 7, 9) were transferred. Each bar is average antibody titer for 6 animals (± SD). *P < .05 compared with control splenocytes. **P < .01 compared with control CD4+ cells. ***P < .01 compared with CD4+CD25− cells or to control CD4+ cells. (B) CD4+GITR+ (bars 12-13) or CD4+GITR− splenocytes (bars 10-11) were transferred. Each bar is average antibody titer for 4 animals (± SD). *P < .05 compared with CD4+GITR− cells or to control CD4+GITR+ cells.
Plasma levels of IgG1 anti-hFIX 3 weeks after immunologic challenge by subcutaneous administration of 5 μg hFIX formulated in CFA in C57BL/6 mice that had received adoptive transfer of splenocytes from naive (white bars, controls) or vector-treated (hatched bars, AAV-FIX) C57BL/6 mice. Adoptive transfer was by tail vein injection 24 hours before challenge. Vector-treated mice had received hepatic gene transfer with 1011 vg/animal AAV-ApoE/hAAT-hFIX vector 6 weeks before the experiment. (A) Total splenocytes (5 × 107, bars 1-2), CD4+ T-cell–depleted splenocytes (5 × 107, bar 3), CD25+-depleted CD4+ T cells (9 × 106, bars 6, 8), MACS-purified CD4+ T cells (107, bars 4-5), or CD4+CD25+ cells (1 × 106, bars 7, 9) were transferred. Each bar is average antibody titer for 6 animals (± SD). *P < .05 compared with control splenocytes. **P < .01 compared with control CD4+ cells. ***P < .01 compared with CD4+CD25− cells or to control CD4+ cells. (B) CD4+GITR+ (bars 12-13) or CD4+GITR− splenocytes (bars 10-11) were transferred. Each bar is average antibody titer for 4 animals (± SD). *P < .05 compared with CD4+GITR− cells or to control CD4+GITR+ cells.
Because the transcription factor FoxP3 is currently the most specific molecular marker for CD4+CD25+ Tregs,19,29,31 we sought to examine and compare FoxP3 expression levels in CD4+ T cells after hepatic gene transfer of AAV-hFIX vector. Total RNA was isolated from CD4+ and CD4− splenocytes from naive C57BL/6 mice or from mice that were expressing hepatocyte-derived hFIX because of prior AAV-hFIX gene transfer (performed 6 weeks earlier). Given the fact that the frequency of antigen-specific T cells is low (approximately 1 in 105 T cells in a naive mouse has been estimated)32 and that regulatory T cell are effective in low numbers, it may not be surprising that we found no difference in the relative level of FoxP3 expression in tolerized mice compared with animals that had not received gene transfer (Figure 6). However, if mice were challenged by immunization with hFIX in CFA, naive controls showed a subsequent slight decrease in FoxP3 message in CD4+ cells, whereas tolerized mice had a 1.5- to 2.5-fold increase in FoxP3 message (Figure 6; no FoxP3 expression was detected in CD4− T cells from naive or vector-treated animals). Therefore, the failure of tolerized mice to form antibodies to hFIX correlates with an increased Treg response to the challenge.
Quantification of relative FoxP3 mRNA levels in CD4+ or CD4− T cells derived from naive control or AAV-ApoE/hAAT-hFIX gene-transferred C57BL/6 mice 60 days before the experiment. Some of control or gene-transduced animals were challenged by subcutaneous administration of 5 μg hFIX formulated in CFA 5 days prior to PCR analysis. cDNA samples were subjected to real-time quantitative PCR analyses using primers and an internal fluorescent probe specific for FoxP3 or HPRT. The relative quantity of FoxP3 in each sample was normalized to the relative quantity of HPRT. FoxP3 transcript levels in naive CD4+ cells are reported as 100%. Results were shown as average ± SD (n = 3 per group). *P < .05 compared with other experimental groups.
Quantification of relative FoxP3 mRNA levels in CD4+ or CD4− T cells derived from naive control or AAV-ApoE/hAAT-hFIX gene-transferred C57BL/6 mice 60 days before the experiment. Some of control or gene-transduced animals were challenged by subcutaneous administration of 5 μg hFIX formulated in CFA 5 days prior to PCR analysis. cDNA samples were subjected to real-time quantitative PCR analyses using primers and an internal fluorescent probe specific for FoxP3 or HPRT. The relative quantity of FoxP3 in each sample was normalized to the relative quantity of HPRT. FoxP3 transcript levels in naive CD4+ cells are reported as 100%. Results were shown as average ± SD (n = 3 per group). *P < .05 compared with other experimental groups.
In vivo depletion of CD4+CD25+ T cells leads to antibody formation to hFIX
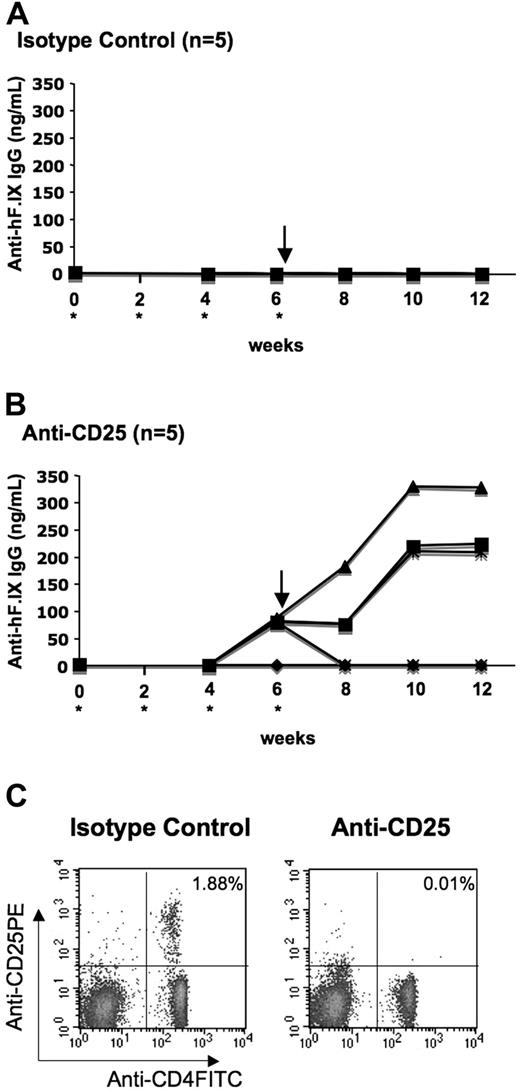
Others have shown that administration of anti-CD25 monoclonal antibody (PC61) results in rapid and efficient depletion of CD4+CD25+ cells as determined by secondary staining with a mAb directed against a different CD25 epitope.33,34 In order to test for the requirement of CD4+CD25+ Tregs for tolerance to the transgene product, we injected C57BL/6 mice either with rat antimouse CD25 or with isotype control rat IgG1 every 2 weeks for 6 weeks starting on the day of liver-directed gene transfer of AAV-hFIX (Figure 7A,B). Depletion of CD4+CD25+ cells in anti-CD25–treated mice and lack of depletion in control mice were confirmed by flow cytometry on peripheral blood cells (Figure 7C). Note that 4 of 5 mice that had received anti-CD25 treatment developed antibodies to hFIX by week 6, while control animals did not (0/5). Moreover, after subsequent challenge with hFIX/CFA, titers of anti-hFIX in 3 of 5 mice from the anti-CD25–treated group increased further (Figure 7B). These antibodies were IgG1 subclass. In contrast, control animals did not show any antibody response even after challenge (Figure 7A). These data demonstrate a requirement for CD4+CD25+ Tregs in tolerance induction to hFIX by hepatic gene transfer.
Depletion of CD4+CD25+ cells in C57BL/6 mice after gene transfer with AAV-ApoE/hAAT-hFIX vector. Shown are plasma levels of IgG anti-hFIX as a function of time after vector administration. Animals were injected intraperitoneally with either rat IgG1 (A, isotype control) or rat antimouse CD25 monoclonal antibody PC61 (B) every 2 weeks (up to week 6) starting on the day of liver-directed gene transfer (asterisk). Mice were challenged with 5 μg hFIX formulated in CFA at week 6. (C) Analyses of peripheral blood lymphocytes by flow cytometry showed depletion of CD4+CD25+ cells after repetitive anti-CD25 injection in C57BL/6 mice. Antibody staining was carried out using FITC-conjugated anti-CD4 and anti–PE-conjugated CD25.
Depletion of CD4+CD25+ cells in C57BL/6 mice after gene transfer with AAV-ApoE/hAAT-hFIX vector. Shown are plasma levels of IgG anti-hFIX as a function of time after vector administration. Animals were injected intraperitoneally with either rat IgG1 (A, isotype control) or rat antimouse CD25 monoclonal antibody PC61 (B) every 2 weeks (up to week 6) starting on the day of liver-directed gene transfer (asterisk). Mice were challenged with 5 μg hFIX formulated in CFA at week 6. (C) Analyses of peripheral blood lymphocytes by flow cytometry showed depletion of CD4+CD25+ cells after repetitive anti-CD25 injection in C57BL/6 mice. Antibody staining was carried out using FITC-conjugated anti-CD4 and anti–PE-conjugated CD25.
Discussion
Several laboratories have demonstrated induction of immune tolerance to systemic proteins by hepatocyte-restricted transgene expression following in vivo gene transfer.7,10,11,35–37 How exactly the hepatic environment permits establishment of tolerance, whereas other target tissues often direct local immune responses to gene transfer, is unclear. Nonetheless, progress has been made toward a model of how liver-derived transgene expression leads to absence of T- and B-lymphocyte responses. Experimental animals maintain tolerance after subsequent challenge by administration of the protein antigen in adjuvant or by secondary gene transfer with an immunogenic adenoviral vector.10,11,13 Our laboratory provided evidence for induction of anergy and deletion of transgene product–specific CD4+ T cells during the first 2 months after gene transfer.12 However, we also found that tolerance induction to hFIX was associated with induction of regulatory CD4+ T cells capable of suppressing antibody and cytotoxic T lymphocyte (CTL)/lymphocytic inflammatory responses to hFIX.11,13 Additional analyses of splenocytes and in vitro assays on CD4+ subsets suggested that regulation might be an integral part of tolerance.12,13
In this new study, we demonstrate that suppression of antibodies to hFIX by hepatic gene transfer–induced CD4+ Tregs is primarily mediated by CD4+CD25+ Tregs (Figure 5, adoptive transfer experiments). Moreover, we provide first definitive evidence for induction of CD4+CD25+ Tregs, which are phenotypically comparable with naturally occurring Tregs. These cells are induced in vivo by stimulation with the transgene product, express FoxP3, GITR, and CTLA4, and suppress CD4+CD25− cells in a standard in vitro assay. The level of induction of FoxP3 transcript was similar to data published by others using transgenic mouse models instead of viral gene transfer.14 Our data suggest generation of CD4+CD25+ Tregs in the thymus. Mice show the highest output of T cells from the thymus at 6 weeks of age, but continue to produce thymic emigrants in a relatively age-independent fashion.38 Additional experiments will be required to address whether thymic development is strictly required for induction of CD4+CD25+ Tregs as opposed to conversion of CD4+CD25− T cells to Tregs by antigen presentation in secondary lymphoid organs or nonlymphoid organs. Such studies may have implications for treatment of humans, who typically show reduced thymic output with increased age.
Experimental results from antibody-mediated depletion of CD25+ cells early after gene transfer strongly suggest that CD4+CD25+ Tregs are required for tolerance induction. Others had similar findings for tolerance induction to FVIII by ex vivo gene transfer to B cells.39 In our study, animals treated with control immunoglobulin remained tolerant without evidence for antibody formation to hFIX, whereas most of the CD25-depleted mice formed antibodies. Anti-CD25 is used clinically to deplete activated T cells (which transiently express CD25), in order to prevent rejection of organ transplants.40,41 Consequently, we did not exclusively deplete Tregs, but also suppressed effector T-cell responses. Antibody formation to hFIX is T helper cell–dependent in mice (CD4-deficient mice consistently fail to form anti-hFIX, published25 and unpublished data, R.W.H., April 2005). Therefore, while anti-hFIX titers in CD25-depleted animals were generally low, these experiments almost certainly underestimate the strength of the immune response in absence of Tregs. Interestingly, thus far, we have failed to break tolerance in a similar experiment, when we depleted CD25+ cells 2 months after gene transfer (data not shown). At early time points (ie, the induction phase of tolerance) the role of Tregs may be even more critical than at later time points (ie, during the maintenance phase of tolerance) because effector T cells may become activated while other tolerance mechanisms such as T-cell anergy and deletion have not yet been effectively established.12 Nevertheless, our published data on suppression of inflammatory responses to the liver following secondary gene transfer with adenoviral vector (expressing immunogenic viral gene products in addition to hFIX) suggest that there is a role for Tregs for maintenance of tolerance as well.13 C57BL/6 mice can mount robust hFIX-specific T- and B-cell responses, indicating that tolerance to murine FIX does not extend to the human protein.25 On the other hand, we cannot rule out that homology to endogenous FIX and perhaps even to other related proteins such as factors VII or X contributes to tolerance induction by activation of naturally occurring CD4+CD25+ Tregs that recognize shared epitopes (in addition to the induced transgene product–specific Tregs).
Based on the current study and our earlier paper by Dobrzynski et al, we propose the following model for establishment of CD4+ T-cell tolerance to the transgene product.12 Unresponsiveness of transgene product–specific CD4+ T cells increases during the first 2 months after hepatic gene transfer. Transgene product–specific CD4+CD25+ Tregs are induced by 2 weeks and steadily increase in frequency until reaching a plateau by 2 months. Likely linked to the Treg response, the transgene product–specific CD4+ T-cell population becomes gradually more anergic in a time course similar to that of Treg induction. Deletion (as part of a central tolerance mechanism) leads to a net reduction in the total number of transgene product–specific CD4+ T cells between 1 and 2 months. After 2 months, no significant further changes in the T-cell population are observed unless an additional challenge with antigen occurs.
In the adoptive transfer studies, suppression of antibody formation to hFIX was evident without a need for preconditioning of recipient mice, suggesting a strong suppressive effect. At the same time, adoptively transferred Tregs failed to completely suppress antibody formation. While this may reflect a technical limitation of the experiment, the combination of our new results (depletion experiment) and previous data (failure to induce tolerance in Fas-deficient mice) suggests that Treg induction and T-cell deletion are both required for robust tolerance that is not broken by a stringent challenge such as administration of antigen in CFA.11 Since Fas-knockout mice are deficient in activation-induced cell death rather than in thymic deletion of T cells, it is likely that an additional peripheral deletion mechanism is involved in tolerance induction, which requires further studies.
Our results also show that immune regulation during tolerance induction by hepatic gene transfer is distinct from mucosal (nasal or oral) tolerance, which is typically characterized by induction of Th2 and Th3 cells secreting large amounts of the suppressive cytokines IL-10 and TGF-β upon in vitro restimulation with the antigen.27 Splenocytes from hepatic tolerized mice fail to secret significant amounts of these cytokines, suggesting also lack of Tr1 cell induction. Others have shown that feeding of ovalbumin to immune-competent DO11.10-tg BALB/c mice leads to an accumulation of ova-specific CD4+FasL+ intrahepatic T cells, which secrete high levels of IL-4 and have regulatory function by inducing apoptotic cell death of CD4+ T cells.42 We also analyzed intrahepatic lymphocytes in DO11.10-tg Rag-2+/+ BALB/c mice at different time points after hepatic AAV-ova gene transfer and found no evidence for an increase in ova-specific CD4+FasL+ cells, but again found an increase in CD4+CD25+ cells (data not shown). Despite these differences between mucosal and hepatic tolerance, it appears that CD4+CD25+ Tregs also play a role in mucosal tolerance.27,43
Antigen-specific CD4+CD25+ Tregs are capable of suppressing CTL and antibody responses to the transgene product (this study and data published by others).44 Our new data demonstrate induction of CD4+CD25+ Tregs by hepatic gene transfer, which is required for tolerance induction and suppression of antibody formation to the transgene product. These results indicate a critical role of CD4+CD25+ Tregs in immune tolerance to the transgene product following in vivo gene transfer, which has wide-reaching implications for systemic protein delivery by in vivo gene transfer. Gene therapy protocols should result in optimal activation of CD4+CD25+ Tregs, and immune modulation strategies aimed at preventing responses to vectors or transgene products should be designed to preserve CD4+CD25+ Treg function.
An Inside Blood analysis of this article appears at the front of this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This work was supported by NIH grants R01 AI/HL51390-01 (R.W.H.) and P01 HL078810 (C.T. and R.W.H.).
We thank Dr S. Waddington for critical suggestions to this study and M. Cooper for technical assistance.
National Institutes of Health
Authorship
Contribution: O.C. performed most experiments, analyzed data, and wrote parts of the paper; E.D. performed experiments leading to Figure 1; L.W. performed part of experiments shown in Figure 2; S.N. performed part of experiments resulting in Figure 7B; B.M. optimized assays leading to Figure 2D; C.P.T. designed experiments and analyzed data; R.W.H. directed the study, supervised experiments, and wrote parts of the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Roland W. Herzog, University of Florida Cancer and Genetics Research Center, 1376 Mowry Road, Room 203, Gainesville, FL 32610; e-mail: rherzog@ufl.edu.