Abstract
We present the results of a multicenter clinical trial using Epstein-Barr virus (EBV)–specific cytotoxic T lymphocytes (CTLs) generated from EBV-seropositive blood donors to treat patients with EBV-positive posttransplantation lymphoproliferative disease (PTLD) on the basis of the best HLA match and specific in vitro cytotoxicity. Thirty-three PTLD patients who had failed on conventional therapy were enrolled. No adverse effects of CTL infusions were observed and the response rate (complete or partial) in 33 patients was 64% at 5 weeks and 52% at 6 months. Fourteen patients achieved a complete remission, 3 showed a partial response, and 16 had no response at 6 months (5 died before completing treatment). At 5 weeks, there was a significant trend toward better responses with higher numbers of CD4+ cells in infused CTL lines (P = .001) that were maintained at 6 months (P = .001). Patients receiving CTLs with closer HLA matching responded better at 6 months (P = .048). Female patients responded better than male patients, but the differences were not statistically significant. Our results show that allogeneic CTLs are a safe and rapid therapy for PTLD, bypassing the need to grow CTLs for individual patients. The response rate in this poor prognosis patient group is encouraging.
Introduction
Posttransplantation lymphoproliferative disease (PTLD) is a common complication of solid organ transplantation that carries a high morbidity and mortality.1 The majority of tumors are of B-cell origin and are associated with Epstein-Barr virus (EBV). EBV is a herpes virus that induces proliferation and transformation of B lymphocytes in vitro and is associated with several human B-cell tumors including Burkitt lymphoma and Hodgkin disease.2,3 Most people acquire EBV silently during early childhood, but if primary infection is delayed until after puberty then it is associated with infectious mononucleosis (IM) in around 25% of cases.4 More than 90% of adults carry EBV as a persistent infection of B lymphocytes. Cytotoxic T lymphocytes (CTLs) that recognize both latent and lytic viral antigens are important in controlling EBV reactivation.5 EBV-specific CTLs are readily detectable in peripheral blood of healthy seropositive carriers, but in transplant recipients, who require immunosuppressive drugs, the activity of virus-specific CTLs is suppressed, thus predisposing the patients to PTLD. In addition primary EBV infection following transplantation carries a high risk of PTLD, and since most recipients who are EBV seronegative before transplantation are children, there is an increased incidence of the PTLD in the pediatric transplant population.6,7
PTLD is most common within the first year following transplantation during high-dose immunosuppression, but may occur at any stage, even after several decades.8 The clinical presentation of PTLD is variable and it is often difficult to diagnose. Lesions may be single or multiple, nodal or extra nodal—common sites being the gut, brain and transplanted organ. PTLD may present as an IM-like syndrome particularly following primary EBV infection. The tumor encompasses a spectrum of histologic types ranging from hyperplasia to monomorphic lymphoma.8 Early lesions may be polyclonal with progression to monoclonality. In EBV-associated PTLD, tumor cells contain viral DNA and express the small EB-encoded RNAs (EBERS) and a variety of viral proteins. Most tumors show full latent viral gene expression (EB nuclear antigens [EBNAs] 1, 2, 3a, 3b, 3c, and leader protein [LP]; latent membrane proteins [LMPs] 1, 2), with a few cells expressing lytic cycle genes. Expression of the EBNA 3 proteins, which are immunodominant for CTL responses, indicates that most PTLD tumors grow opportunistically in the absence of effective T-cell control of the persistent virus infection. However viral antigen expression is variable within and between tumors, some showing more restricted forms of latency either without evidence of EBNA 2 and 3 proteins, or a Burkitt-like EBNA 1 only phenotype.9
First-line treatment for PTLD is immunosuppression dose reduction, which is successful in inducing tumor regression in a proportion of cases.10 However this treatment may be limited by the onset of graft rejection due to reduced immunosuppression, and other forms of treatment such as chemotherapy, radiotherapy, and rituximab (anti-CD20 monoclonal antibody) are often required.11,12 Despite these treatments, the overall mortality from PTLD in solid organ transplantation is around 50%.13 Another approach to PTLD treatment is to enhance the EBV-specific CTL response that is suppressed in transplant recipients. Cytotoxic T-cell therapy to prevent and/or treat PTLD was first used successfully in allogeneic hematopoietic stem cell transplant recipients where stem cell donor's peripheral blood was used as a source of EBV-specific CTLs for infusion.14–16 However, since donor blood is not generally available for solid organ recipients with PTLD, we established a frozen bank of 100 EBV-specific CTLs generated from the peripheral blood of Scottish blood donors.17 We previously conducted a pilot study in which 8 patients with progressive PTLD were treated with these CTLs selected on the basis of best HLA matches between the CTL donor and PTLD patient. Three patients attained complete remission.18 In this present study, we used these partially HLA-matched allogeneic CTLs in a phase 2 multicenter clinical trial to treat 33 patients (31 solid organ and 2 stem cell transplant recipients) with biopsy proven EBV-positive PTLD that had failed conventional treatment. The overall response rate was 64% at 5 weeks and 52% at 6 months.
Patients, materials, and methods
Study design
This phase 2 multicenter clinical trial aimed to test the safety and efficacy of banked allogeneic EBV-specific CTLs used on a best HLA-match basis to treat PTLD. Inclusion criteria were as follows: biopsy-proven EBV-positive PTLD and a tumor where response could be measurable. Exclusion criteria were as follows: pregnancy and Karnofsky scale of 10 or lower. Standard therapy involved weekly intravenous infusions of CTLs (2 × 106 CTLs per kg body weight) for 4 weeks—doses established previously in a pilot study.18 Patients from 19 transplantation centers were recruited to the trial (listed in “Acknowledgments”) with informed written consent from patients or guardians obtained in accordance with the Declaration of Helsinki. Preliminary data from the first 7 patients included in this trial were published elsewhere.18 The trial was approved by the Multicentre Research Ethics Committee for Scotland and by local research ethics committees for each center. This trial was also registered with European Clinical Trial Database, ClinicalTrials.gov, and the United Kingdom National Cancer Research Network.
Cytotoxic T-cell bank and in vitro testing
The establishment and management of the CTL bank have been reported elsewhere.17 Briefly, peripheral blood mononuclear cells (PBMCs) were obtained from HLA-typed, EBV-seropositive blood donors at Scottish National Blood Transfusion Services with informed consent. Lymphoblastoid cell lines (LCLs) were established by in vitro EBV infection and used as stimulators to grow EBV-specific CTLs by published methods.17 CTLs were tested in standard chromium release assays for cytotoxicity against autologous phytohemagglutinin (PHA)–stimulated blasts, autologous and mismatched LCLs, and K562 cell line to detect NK-cell activity. When deemed EBV specific, CTLs were screened for bacterial, viral, and fungal contaminants; stained and analyzed by fluorescence-activated cell sorting (FACS) (for TCRαβ, CD3, CD4, CD8, CD19, CD16/7, CD56, CD57, and a wide range of T-cell activation and differentiation markers); and then frozen in vials until required.17
Patient/CTL matching procedure
HLA A and B antigens were typed by a one-stage microcytotoxicity test using the First and Second Lambda monoclonal class I 72-well trays (One Lambda, VH Bio, Newcastle, United Kingdom) and HLA DR by low-resolution polymerase chain reaction (PCR).17 The HLA profile (A, B, DR) of the potential CTL recipients was used to identify the closest matched banked CTLs and 2 to 3 CTLs were thawed and tested for cytotoxicity in chromium release assays against autologous LCLs and PHA blasts, K562, patient PHA blasts, and, where available, patient LCLs. The CTLs showing the highest specific killing against patient LCLs with low killing of patient PHA blasts and K562 were selected for infusion.18
Immunohistochemistry
The diagnosis of PTLD and its classification were determined by routine histologic examination. EBER staining was carried out by in situ hybridization using a commercial kit (Dako, Cambridge, United Kingdom), and EBNA 1 and 2, and LMP 1 expression were detected by routine staining using commercially available antibodies (EBNA 2 no. M7004, LMP-1 no. M0897 from Dako; EBNA 1 no. Ab8329 from AbCam, Cambridge, United Kingdom) and detection system (Dako EnVision kit) after antigen retrieval (pressure cooking 3 minutes at pressure in Tris/EDTA). Tumor cell clonality was assessed by in situ hybridization for κ and λ mRNA (Dako).
Patient monitoring
Tumor response (complete, partial, or no response) was recorded 5 weeks and 6 months after CTL therapy as determined by clinical evaluation, imaging (x-ray, computed tomography [CT], magnetic resonance imaging [MRI] scan as appropriate) or endoscopy, and was decided by the physicians involved in patient care. Clinical monitoring was carried out weekly for 4 weeks and then at regular intervals for 6 months. A complete response (CR) was defined as complete disappearance of all measurable tumor masses and any other manifestations of disease.19 A partial response (PR) was achieved if the overall tumor mass decreased 50% or more in size where tumors were measurable clinically and/or by imaging and the patient's clinical condition remained stable or improved. No response (NR) was defined as tumor masses that remained the same size or increased in size and/or the patient's clinical condition deteriorated.
Quantitative EBV load by real-time PCR
EBV DNA levels were monitored by real-time PCR of EBV polymerase gene using Corbett Rotor-gene 3000 machine (Corbett Research, Cambridge, United Kingdom) in PBMCs taken before and after each CTL infusion and thereafter at clinical monitoring sessions.20 Briefly, DNA was extracted using QIAamp DNA Mini Kit as per the manufacturer's instructions (Qiagen, Crawley, United Kingdom) and stored at −70°C. DNA (5 microliters [μL])/1 μg) was then amplified in a 25-μL reaction volume containing primers and Taq polymerase (Promega, Southampton, United Kingdom). EBV-positive Raji cells were used to obtain a standard curve from a dilution series starting with 100 μg DNA/tube and then 10-fold serial dilutions to give a final concentration of 10 pg DNA/tube. Viral DNA copy number in test samples was estimated by extrapolating from the Raji standard curve. β-Globin was always included in each PCR run as a house keeping gene. EBV load in test samples was expressed as the relative copy number normalized against the amount of amplified β-globin DNA.
T-cell receptor (TCR) spectratyping
Functionally rearranged TCR β chain variable (BV) gene subfamilies were amplified across the complementarity determining region 3 (CDR3) encoding regions using 23 subfamily-specific primers and a FAM-conjugated β chain constant region–specific primer.21,22 RNA was extracted from 5 × 106 PBMCs or CTLs using the Rneasy mini kit and Qiashredder system (Qiagen) as per the manufacturer's instructions and was converted to complementary DNA (cDNA) using random hexamers and the Thermoscript RT system from Invitrogen (Paisley, United Kingdom). PCR amplifications were performed on 1 μL cDNA in a total volume of 20 μL containing variable and constant primers and 0.5 U of Amplitaq Gold (Applied Biosystems, Foster City, CA) polymerase. PCR product (1 μL) was diluted in 10 μL nuclease-free water and then further diluted 1 in 10 with Hi-Di formamide (containing Genescan 500LIZ size standard) before electrophoresis in an ABI 3730 (Dye set 5) automated sequencer. ABI Genemapper software (version 3.7) was used to analyze data (Applied Biosystems).
Alloantibody screen
Pre- and post-CTL infusion sera were tested for antibodies directed against mismatched HLA antigens on the infused CTLs by microlymphocytotoxicity assay. Briefly, serum samples were screened against a selected panel of peripheral blood lymphocytes from 50 HLA-typed volunteers, for the detection of class I antibodies and 30 patients with B-cell chronic lymphocytic leukemia for the detection of class II antibodies. Antibody screening was performed using the standard microlymphocytotoxicity assay, initial incubation time (45 minutes) with extension (120 minutes) after complement (Cedar Lane, Hornby, ON). Target cell viability was assessed after staining with acridine orange and ethidium bromide. Antibody specificities were identified by computer-assisted analysis (using 2 × 2 contingency tables) of panel reactivity profiles.
Statistical analysis
Statistical analysis was carried out on an intent-to-treat basis. The overall survival rate was calculated by nonparametric Kaplan-Meier method. Fisher exact test was used to identify those patient characteristics that were significantly associated with treatment response (complete or partial). For quantitative characteristics (eg, time between transplantation and PTLD development and level of EBV-specific CTL killing) a chi-squared trend test was conducted to examine whether the proportion of patients responding to treatment either increased or decreased across the levels of the characteristic. Multivariate analysis of complete and partial responders was not possible because of the limited number of patients in each group. All analyses were conducted using Stata 9.0 (Strata Corp, College Station, TX).
Results
PTLD patients
Thirty-three patients were recruited to the trial. Five patients (2 males and 3 females) died from causes unrelated to the CTL therapy before completing the course of treatment (patients 29-33; Table 1). These patients were severely ill (widespread primary disease, bacterial/fungal septicemia, multiorgan failure, severe graft rejections) prior to CTL therapy but were nevertheless included in the intent-to-treat analyses.
Patients' characteristics and outcome of CTL infusions
| Pt no. . | Sex . | Tx/time to PTLD . | Age at CTL, y . | Pre-Tx EBV* . | PTLD sites . | PTLD histology† . | Pre-CTL EBV DNA‡ . | Previous Rx for PTLD . | Outcome, 5 wk . | Outcome, 6 mo . |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | F | Liver/3 mo | 1 | Pos | Tonsil, stomach, bowel | Monomorphic | 106 | RIS, ACV | CR | CR |
| 2 | F | Liver/6.5 y | 76 | NK | Bowel | Hodgkin | ND | RIS, surgery | CR | CR |
| 3 | M | Heart/5.5 y | 51 | NK | Orbit, pectoral | Monomorphic | 454 | RIS | CR | CR |
| 4 | F | Stem cell/1.5 mo | 30 | Pos | LN (multisites), blood | Polymorphic | ND | RIS, rituximab | CR | CR |
| 5 | M | Bone marrow/4 mo | 27 | NK | LN | Polymorphic | ND | RIS, Chemo, radio | CR | CR |
| 6 | F | Kidney/9 y | 68 | NK | LN, bone marrow | Burkitt | 24 000 | RIS, chemo | CR | CR |
| 7 | F | Kidney/19 y | 67 | NK | LN (multisites) | Hodgkin | ND | RIS | CR | CR |
| 8 | F | Liver/9 mo | 13 | Neg | Bowel, LN | Hyperplastic | ND | RIS, VCV | CR | CR |
| 9 | F | Liver/3 mo | 50 | NK | LN (multisites) | Hodgkin | ND | RIS, radio | CR | CR |
| 10 | M | Liver, small bowel/21 mo | 3 | Neg | Sigmoid colon, duodenum | Hyperplastic | 1200 | RIS | CR | CR |
| 11 | F | Liver/4.2 y | 5 | Neg | LN | Hyperplastic | 339 | RIS | CR | CR |
| 12 | M | Liver, small bowel/9 mo | 2 | Neg | Small bowel, LN | Hodgkin | 1052 | RIS, GCV | CR | CR |
| 13 | F | Kidney/23 mo | 19 | Neg | Brain (multiple) | Polymorphic | ND | RIS, rituximab, chemo, radio | PR | CR |
| 14 | F | Kidney/23 mo | 35 | Neg | Brain (multiple) | Monomorphic | ND | RIS | PR | CR |
| 15 | M | Kidney/3.5 y | 41 | Neg | Gingivae, adrenals | Monomorphic | 4000 | RIS | PR | PR |
| 16 | M | Kidney/18.5 y | 64 | NK | Kidney, bladder, LN | Monomorphic | 100 000 | RIS, chemo, radio | PR | PR |
| 17 | M | Liver/4.3 y | 60 | NK | Liver, abdomen LN | Polymorphic | 640 | RIS | PR | PR |
| 18 | M | Kidney/5.3 y | 11 | Pos | Tonsil, LN (multisites) | Polymorphic | 1.2 × 106 | RIS | PR | NR |
| 19 | M | Heart/20 y | 33 | NK | LN (multisites) | Hyperplastic | ND | RIS | PR | NR |
| 20 | M | Liver/9 y | 14 | Neg | LN (multisites) | Hodgkin | ND | RIS | PR | NR |
| 21 | F | Heart, lungs/3 y | 51 | Pos | Brain (mulitple) | Monomorphic | ND | RIS, rituximab, chemo, radio | PR | NR; died |
| 22 | M | Kidney/8 y | 61 | Pos | Large pelvic mass (kidney, bladder) | Monomorphic | 24 000 | RIS, rituximab, chemo, radio | NR | NR; died |
| 23 | M | Liver/4.7 y | 49 | NK | Blood, bone marrow | Polymorphic | ND | RIS | NR | NR |
| 24 | M | Kidney/10 y | 48 | NK | Eye, spine, bladder, LN | Monomorphic | 7900 | RIS, rituximab | NR | NR |
| 25 | M | Kidney/8.8 y | 51 | NK | LN (multisites) | Monomorphic | 50 752 | RIS | NR | NR |
| 26 | M | Kidney/13 y | 19 | NK | Brain (multiple) | Polymorphic | 3200 | RIS | NR | NR |
| 27 | M | Lung/3 mo | 8 | NK | LN (multisites) | Polymorphic | 704 | RIS, rituximab, Anti-IL6, GCV | NR | NR |
| 28 | M | Liver/8.5 y | 61 | NK | LN (multisites) | Monomorphic | 1827 | RIS | NR | NR |
| 29 | M | Kidney/6 y | 53 | NK | Lungs, LN | Polymorphic | ND | RIS, rituximab, chemo | NR; died d8 | NR; died |
| 30 | F | Lung/3 mo | 58 | Pos | Lung, LN | Monomorphic | ND | RIS, rituximab, chemo | NR–Died d11 | NR; died |
| 31 | F | Kidney/6 mo | 12 | Neg | Kidney, spleen, liver, bone marrow, pleura | Hyperplastic | 2127 | RIS, rituximab | NR; died d10 | NR; died |
| 32 | M | Liver, small bowel/21 mo | 3 | Neg | Jejunam, duodenum, sigmoid colon, rectum | Hyperplastic | ND | RIS | NR; died d25 | NR; died |
| 33 | F | Liver/4 mo | 69 | Pos | Liver allograft | Hyperplastic | ND | RIS | NR; died d10 | NR; died |
| Pt no. . | Sex . | Tx/time to PTLD . | Age at CTL, y . | Pre-Tx EBV* . | PTLD sites . | PTLD histology† . | Pre-CTL EBV DNA‡ . | Previous Rx for PTLD . | Outcome, 5 wk . | Outcome, 6 mo . |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | F | Liver/3 mo | 1 | Pos | Tonsil, stomach, bowel | Monomorphic | 106 | RIS, ACV | CR | CR |
| 2 | F | Liver/6.5 y | 76 | NK | Bowel | Hodgkin | ND | RIS, surgery | CR | CR |
| 3 | M | Heart/5.5 y | 51 | NK | Orbit, pectoral | Monomorphic | 454 | RIS | CR | CR |
| 4 | F | Stem cell/1.5 mo | 30 | Pos | LN (multisites), blood | Polymorphic | ND | RIS, rituximab | CR | CR |
| 5 | M | Bone marrow/4 mo | 27 | NK | LN | Polymorphic | ND | RIS, Chemo, radio | CR | CR |
| 6 | F | Kidney/9 y | 68 | NK | LN, bone marrow | Burkitt | 24 000 | RIS, chemo | CR | CR |
| 7 | F | Kidney/19 y | 67 | NK | LN (multisites) | Hodgkin | ND | RIS | CR | CR |
| 8 | F | Liver/9 mo | 13 | Neg | Bowel, LN | Hyperplastic | ND | RIS, VCV | CR | CR |
| 9 | F | Liver/3 mo | 50 | NK | LN (multisites) | Hodgkin | ND | RIS, radio | CR | CR |
| 10 | M | Liver, small bowel/21 mo | 3 | Neg | Sigmoid colon, duodenum | Hyperplastic | 1200 | RIS | CR | CR |
| 11 | F | Liver/4.2 y | 5 | Neg | LN | Hyperplastic | 339 | RIS | CR | CR |
| 12 | M | Liver, small bowel/9 mo | 2 | Neg | Small bowel, LN | Hodgkin | 1052 | RIS, GCV | CR | CR |
| 13 | F | Kidney/23 mo | 19 | Neg | Brain (multiple) | Polymorphic | ND | RIS, rituximab, chemo, radio | PR | CR |
| 14 | F | Kidney/23 mo | 35 | Neg | Brain (multiple) | Monomorphic | ND | RIS | PR | CR |
| 15 | M | Kidney/3.5 y | 41 | Neg | Gingivae, adrenals | Monomorphic | 4000 | RIS | PR | PR |
| 16 | M | Kidney/18.5 y | 64 | NK | Kidney, bladder, LN | Monomorphic | 100 000 | RIS, chemo, radio | PR | PR |
| 17 | M | Liver/4.3 y | 60 | NK | Liver, abdomen LN | Polymorphic | 640 | RIS | PR | PR |
| 18 | M | Kidney/5.3 y | 11 | Pos | Tonsil, LN (multisites) | Polymorphic | 1.2 × 106 | RIS | PR | NR |
| 19 | M | Heart/20 y | 33 | NK | LN (multisites) | Hyperplastic | ND | RIS | PR | NR |
| 20 | M | Liver/9 y | 14 | Neg | LN (multisites) | Hodgkin | ND | RIS | PR | NR |
| 21 | F | Heart, lungs/3 y | 51 | Pos | Brain (mulitple) | Monomorphic | ND | RIS, rituximab, chemo, radio | PR | NR; died |
| 22 | M | Kidney/8 y | 61 | Pos | Large pelvic mass (kidney, bladder) | Monomorphic | 24 000 | RIS, rituximab, chemo, radio | NR | NR; died |
| 23 | M | Liver/4.7 y | 49 | NK | Blood, bone marrow | Polymorphic | ND | RIS | NR | NR |
| 24 | M | Kidney/10 y | 48 | NK | Eye, spine, bladder, LN | Monomorphic | 7900 | RIS, rituximab | NR | NR |
| 25 | M | Kidney/8.8 y | 51 | NK | LN (multisites) | Monomorphic | 50 752 | RIS | NR | NR |
| 26 | M | Kidney/13 y | 19 | NK | Brain (multiple) | Polymorphic | 3200 | RIS | NR | NR |
| 27 | M | Lung/3 mo | 8 | NK | LN (multisites) | Polymorphic | 704 | RIS, rituximab, Anti-IL6, GCV | NR | NR |
| 28 | M | Liver/8.5 y | 61 | NK | LN (multisites) | Monomorphic | 1827 | RIS | NR | NR |
| 29 | M | Kidney/6 y | 53 | NK | Lungs, LN | Polymorphic | ND | RIS, rituximab, chemo | NR; died d8 | NR; died |
| 30 | F | Lung/3 mo | 58 | Pos | Lung, LN | Monomorphic | ND | RIS, rituximab, chemo | NR–Died d11 | NR; died |
| 31 | F | Kidney/6 mo | 12 | Neg | Kidney, spleen, liver, bone marrow, pleura | Hyperplastic | 2127 | RIS, rituximab | NR; died d10 | NR; died |
| 32 | M | Liver, small bowel/21 mo | 3 | Neg | Jejunam, duodenum, sigmoid colon, rectum | Hyperplastic | ND | RIS | NR; died d25 | NR; died |
| 33 | F | Liver/4 mo | 69 | Pos | Liver allograft | Hyperplastic | ND | RIS | NR; died d10 | NR; died |
Pt indicates patient; Tx, transplantation; y, years; Rx, treatment; F, female; wk, weeks; mo, months; Pos, positive; RIS, reduction of immunosuppression; ACV, aciclovir; CR, complete response; NK, not known; ND, not detected; M, male; LN, lymph nodes; chemo, chemotherapy; radio, radiotherapy; Neg, negative; VCV, valaciclovir; GCV, ganciclovir; PR, partial response; and NR, no response.
Serum anti-VCA IgG.
All PTLD tumor biopsies were positive for EBERs by in situ hybridization.
Copies of EBV DNA per 106 PBMCs.
Of the 33 PTLD patients in the trial, 19 were male and 14 female; the age range was 1 to 76 years (Tables 1, 2). Transplant types were as follows: stem cell, 2; heart, 2; kidney, 13; liver, 10; liver and small bowel, 3; lung, 2; heart and lung, 1. Details of time from transplantation to PTLD development, histologic types and sites of PTLD, and pretransplantation EBV serostatus (where available) are shown in Tables 1 and 2. All patients had immunosuppression dose reduction before receiving CTL therapy (stepwise dose reduction or immediate maximum dose reduction or complete withdrawal of immunosuppression according to individual transplantation centers' protocols). Twelve patients had additional rituximab and/or antivirals, and 8 had chemotherapy and/or radiotherapy (Table 1). With the exception of 3 patients (patients 13, 21, and 30) receiving concurrent rituximab and 3 patients (patients 15, 18, and 23) with continued immunosuppression dose reduction, all other patients had stopped all forms of therapy 2 to 8 weeks before starting CTL and were considered for CTLs due to their progressive or nonresponsive disease and in some cases, impending graft rejection. Their immunosuppression was re-escalated before CTL infusions. Tumor biopsies from all patients were positive for EBERs by in situ hybridization.
Analyses of outcome in association with various parameters
| . | No. of patients . | No. of responders (%) . | |
|---|---|---|---|
| 5 wk . | 6 mo . | ||
| Sex | |||
| Female | 14 | 11 (79) | 10 (71) |
| Male | 19 | 10 (53) | 7 (37) |
| P | — | .16 | .08 |
| Age at CTL infusion | |||
| Younger than 16 y | 10 | 7 (70) | 5 (50) |
| 16 to 49 y | 9 | 6 (67) | 5 (56) |
| 50 y or older | 14 | 8 (57) | 7 (50) |
| P trend | — | .51 | .98 |
| Time to PTLD from transplantation | |||
| Fewer than 2 y | 14 | 9 (64) | 9 (64) |
| 2 y or longer | 19 | 12 (63) | 8 (42) |
| P | — | .999 | .30 |
| Single vs multiple tumor sites | |||
| Single site | 4 | 3 (75) | 3 (75) |
| Multiple sites | 29 | 18 (62) | 14 (48) |
| P | — | .999 | .60 |
| Sites involved | |||
| Nodal | 11 | 8 (73) | 5 (45) |
| Extranodal | 15 | 9 (60) | 8 (53) |
| Both | 7 | 4 (57) | 4 (57) |
| P | — | .72 | .999 |
| Histologic type of PTLD | |||
| Hyperplastic | 5 | 4 (80) | 3 (60) |
| Hodgkin lymphoma | 5 | 5 (100) | 4 (80) |
| Polymorphic | 9 | 5 (56) | 4 (44) |
| Monomorphic | 14 | 7 (50) | 6 (43) |
| P | — | .23 | .61 |
| Clonality of tumor* | |||
| Polyclonal | 7 | 4 (57) | 2 (29) |
| Monoclonal† | 10 | 8 (80) | 8 (80) |
| Negative | 2 | 2 (100) | 2 (100) |
| P | — | .78 | .80 |
| Percentage of CD4 cells in infused CTL lines | |||
| Less than 1% | 12 | 3 (25) | 2 (17) |
| 1% to 4.9% | 9 | 7 (78) | 5 (56) |
| 5% or more | 12 | 11 (92) | 10 (83) |
| P trend | — | .001 | .001 |
| Number of HLA matches at HLA A, B, and DR loci | |||
| 2/6 | 3 | 2 (67) | 1 (33) |
| 3/6 | 13 | 7 (54) | 5 (38) |
| 4/6 | 7 | 3 (43) | 3 (43) |
| 5/6 | 10 | 9 (90) | 8 (80) |
| P trend | — | .17 | .048 |
| . | No. of patients . | No. of responders (%) . | |
|---|---|---|---|
| 5 wk . | 6 mo . | ||
| Sex | |||
| Female | 14 | 11 (79) | 10 (71) |
| Male | 19 | 10 (53) | 7 (37) |
| P | — | .16 | .08 |
| Age at CTL infusion | |||
| Younger than 16 y | 10 | 7 (70) | 5 (50) |
| 16 to 49 y | 9 | 6 (67) | 5 (56) |
| 50 y or older | 14 | 8 (57) | 7 (50) |
| P trend | — | .51 | .98 |
| Time to PTLD from transplantation | |||
| Fewer than 2 y | 14 | 9 (64) | 9 (64) |
| 2 y or longer | 19 | 12 (63) | 8 (42) |
| P | — | .999 | .30 |
| Single vs multiple tumor sites | |||
| Single site | 4 | 3 (75) | 3 (75) |
| Multiple sites | 29 | 18 (62) | 14 (48) |
| P | — | .999 | .60 |
| Sites involved | |||
| Nodal | 11 | 8 (73) | 5 (45) |
| Extranodal | 15 | 9 (60) | 8 (53) |
| Both | 7 | 4 (57) | 4 (57) |
| P | — | .72 | .999 |
| Histologic type of PTLD | |||
| Hyperplastic | 5 | 4 (80) | 3 (60) |
| Hodgkin lymphoma | 5 | 5 (100) | 4 (80) |
| Polymorphic | 9 | 5 (56) | 4 (44) |
| Monomorphic | 14 | 7 (50) | 6 (43) |
| P | — | .23 | .61 |
| Clonality of tumor* | |||
| Polyclonal | 7 | 4 (57) | 2 (29) |
| Monoclonal† | 10 | 8 (80) | 8 (80) |
| Negative | 2 | 2 (100) | 2 (100) |
| P | — | .78 | .80 |
| Percentage of CD4 cells in infused CTL lines | |||
| Less than 1% | 12 | 3 (25) | 2 (17) |
| 1% to 4.9% | 9 | 7 (78) | 5 (56) |
| 5% or more | 12 | 11 (92) | 10 (83) |
| P trend | — | .001 | .001 |
| Number of HLA matches at HLA A, B, and DR loci | |||
| 2/6 | 3 | 2 (67) | 1 (33) |
| 3/6 | 13 | 7 (54) | 5 (38) |
| 4/6 | 7 | 3 (43) | 3 (43) |
| 5/6 | 10 | 9 (90) | 8 (80) |
| P trend | — | .17 | .048 |
Clonality of tumor not known for 14 patients.
Includes 5 Hodgkin lymphomas.
The 33 PTLD trial patients were enrolled from 19 transplantation centers; 19 were treated as inpatients and 14 received CTL infusions as outpatients. Twenty-three patients received 4 CTL infusions, but individual patients received 1 (patients 12, 30, and 33), 2 (patients 17, 29, and 31), 3 (patient 32), 6 (patient 22), and 8 (patients 14 and 21) infusions.18 The infusions were well tolerated with no evidence of acute infusion reactions. No adverse effect on the transplanted organ or evidence of CTL-versus-host disease was observed.
Clinical response to CTL infusions
At 5 weeks after the start of CTL infusions, 21 patients (64%) showed a response (complete or partial) to therapy and 12 patients did not respond (Tables 1–2). Twelve patients achieved a complete response (CR) defined as complete PTLD tumor regression and improvement in graft functions and clinical status, and 9 patients showed a partial response (PR) defined as clinical improvement and 50% reduction in overall tumor mass where tumors were measurable clinically and/or by imaging. The 5 patients who died before completion of CTL treatment were classified as nonresponders.
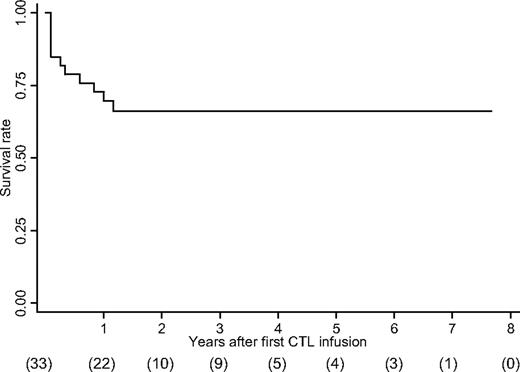
The overall survival rate is shown in Figure 1. At 6 months, 26 patients (79% survival) were still alive (Tables 1, 2). One patient who showed a partial response to CTL therapy at 5 weeks died 3 months later from severe cytomegalovirus infection (patient 21, she was in CR at the time of death), and one patient from the NR group died due to an extensive tumor burden (patient 22). Three patients who initially had shown a partial response relapsed by 6 months.
The Kaplan-Meier overall survival rate. The values in brackets indicate the number of patients followed up at each time point after the first CTL infusion.
The Kaplan-Meier overall survival rate. The values in brackets indicate the number of patients followed up at each time point after the first CTL infusion.
Two patients with partial responses at 5 weeks achieved complete response at 6 months bringing the number of patients showing CR to CTL therapy to 14. One CR patient has since relapsed and 13 remain disease free, 1 to 7.5 years after completing CTL therapy.
Two of the 3 patients (patients 15 and 16) with PR at 6 months are still alive (18 and 20 months after infusions, respectively). Patient 15 had a further course of reduction of immunosuppression. The third PR patient (patient 17) had a relapse and died 7 months later.
Thus, overall a response (complete or partial) to CTL therapy was recorded in 21 (64%) of 33 patients at 5 weeks and 17 (52%) of 33 patients at 6 months. Because the number of participants is relatively small, partial and complete responders were aggregated for comparison with the nonresponder group in further analyses.
Response in female compared with male patients
The response was better among female than male patients at both 5 weeks (79% versus 53%, respectively) and 6 months (71% versus 37%, respectively), but these differences were not statistically significant (Table 2). However, when analyses were restricted to 28 patients who successfully completed treatment, the results became significant: at both 5 weeks and 6 months all (11 of 11 and 10 of 10, respectively) female patients showed a response, whereas at the same time points, 10 of 17 and 7 of 16 male patients responded (P = .02 at 5 weeks and P = .004 at 6 months). There was no association between the sex of CTL donors or CD4 T-cell counts in CTLs and sex-related response (data not shown). The age of the patients did not affect outcome.
EBV serostatus, time of onset of PTLD, and outcome
Pretransplantation EBV serology was available from 17 of 33 patients (Table 1). Ten patients were seronegative before transplantation and developed PTLD following primary EBV infection. Seven (70%) of these 10 previously EBV-seronegative patients showed a response (6 CR and 1 PR) to CTL at 6 months compared with 2 (29%) of 7 patients who were already EBV seropositive before transplantation.
The interval between transplantation and PTLD development varied from 6 weeks to 19 years (median time, 3.5 years). The response at 5 weeks to CTL therapy did not differ significantly between early and late onset of disease (Table 2): 9 (64%) of 14 early (PTLD < 2 years after transplantation) compared with 12 (63%) of 19 late (PTLD > 2 years after transplantation) PTLD responded (P = .999). At 6 months, all 9 of 14 patients with early onset disease showed a sustained response, whereas only 42% (8/19) of patients with late onset PTLD maintained the initial response (P = .30).
Degree of HLA matching
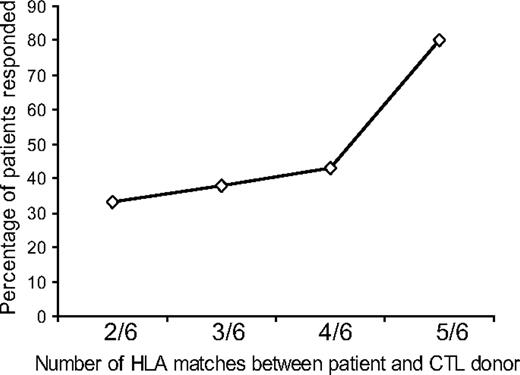
CTL lines used in the trial were selected for individual patients on the basis of the number of HLA matches (A, B, and DR), with preference given to HLA class I antigen (A and B) matching as EBV antigens expressed in relation to HLA class I molecules are known to be the main targets for CTLs.5 The number of HLA matches at HLA A, B, and DR loci varied from 2 of 6 to 5 of 6, and there was an association between the number of matches and patient outcome, with those with a higher number of matches responding better at 6 months than those with fewer matches at 6 months; this reached a statistical significance (P = .048; Figure 2; Table 2). The response rate was not related to matching or mismatching of any particular HLA antigen locus (A, B, or DR; data not shown).
Relation between the degree of HLA matching between the patient and CTL donor and clinical outcome. The number of HLA antigen matches between patients and CTL donors showed a trend toward a sustained clinical response at 6 months with greater matching that reached a statistical significance (P = .048).
Relation between the degree of HLA matching between the patient and CTL donor and clinical outcome. The number of HLA antigen matches between patients and CTL donors showed a trend toward a sustained clinical response at 6 months with greater matching that reached a statistical significance (P = .048).
Better outcome with higher percentage of CD4 cells in CTLs
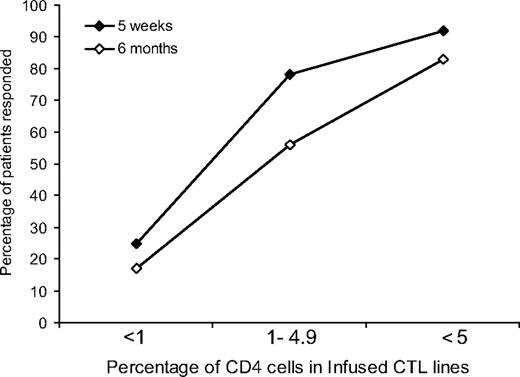
All CTLs used for in vivo infusions were polyclonal and contained both CD4+ and CD8+ cell populations, with the percentage of CD3+, CD4+ cells ranging from less than 1% to 60%. At 5 weeks after the first infusion, there was a statistically significant trend (P = .001) toward better responses in those receiving CTLs with a higher percentage of CD4 cells. Responses for patients receiving CTLs with less than 1%, 1% to 4.9%, and 5% or more CD4 cells were as follows: 3 (25%) of 12, 7 (78%) of 9, and 11 (92%) of 12, respectively (Figure 3). This significant trend was maintained at 6 months with 2 (18%) of 12, 5 (56%) of 9, and 10 (83%) of 12 patients responding, respectively (P = .001) (Figure 3).
Relation between clinical outcome and the percentage of CD4+ cells in infused CTL lines. A higher percentage of PTLD patients responded to CTL therapy when the infused CTL lines contained a higher percentage of CD4+ cells. This trend was statistically significant both at 5 weeks (♦) and at 6 months (◇) (P = .001 and P = .001, respectively).
Relation between clinical outcome and the percentage of CD4+ cells in infused CTL lines. A higher percentage of PTLD patients responded to CTL therapy when the infused CTL lines contained a higher percentage of CD4+ cells. This trend was statistically significant both at 5 weeks (♦) and at 6 months (◇) (P = .001 and P = .001, respectively).
CTL cytotoxicity and outcome
The level of EBV-specific CTL killing in vitro of the autologous LCLs measured prior to CTL infusion ranged from 19% to 74%, and CTL killing of the patient LCLs (determined either prior to or following the CTL infusions depending on when the LCLs became available for testing) ranged from less than 1% to 60%. No association was seen between level of in vitro killing of autologous or patient's LCLs by CTLs and tumor response (data not shown).
PTLD morphology and outcome
Prior to CTL therapy, 29 patients had tumors at multiple anatomic sites, whereas 4 were single-site tumors (Table 2). Three (patients 2, 5, 11) of the 4 single-site tumors showed a complete response to CTL therapy, and this response was maintained at 6 months. In comparison 18 (62%) of 29 tumors involving multiple sites showed a response at 5 weeks, and this was reduced to 14 (48%) of 29 at 6 months. Five patients had PTLD involving the central nervous system; 4 (patients 13, 14, 21, 26) with multiple lesions in brain parenchyma, of which 2 achieved a complete response at 6 months, 1 had no response, and 1 showed a partial response at 5 weeks but later died of cytomegalovirus infection (autopsy showed complete regression of the tumor). The other patient (patient 24) with widespread PTLD involving eye, spine, and lymph nodes did not respond to CTLs.
There was no significant association between response to CTL therapy and histologic type, clonality, or viral gene expression of the PTLD tumors; however, it was notable that 4 (80%) of the 5 hyperplastic tumors and all 5 Hodgkin-type PTLDs responded to CTLs compared with 56% and 50% response rates for polymorphic and monomorphic tumors, respectively (Table 2). The only Burkitt-like tumor showed a complete response that was sustained at 6 months (Table 1).
EBV viral load in peripheral blood
EBV DNA was detected in PBMCs of 17 of 33 PTLD patients tested before the first CTL infusion, the viral load ranging from 339 to 1.22 million copies per million PBMCs. Pre-CTL infusion levels of EBV DNA for all 33 patients are shown in Table 1. Four (patients 22, 24, 27, and 31) of 9 patients who had received prior anti-CD20 monoclonal antibody rituximab had detectable EBV DNA in their PBMCs before CTL infusion.
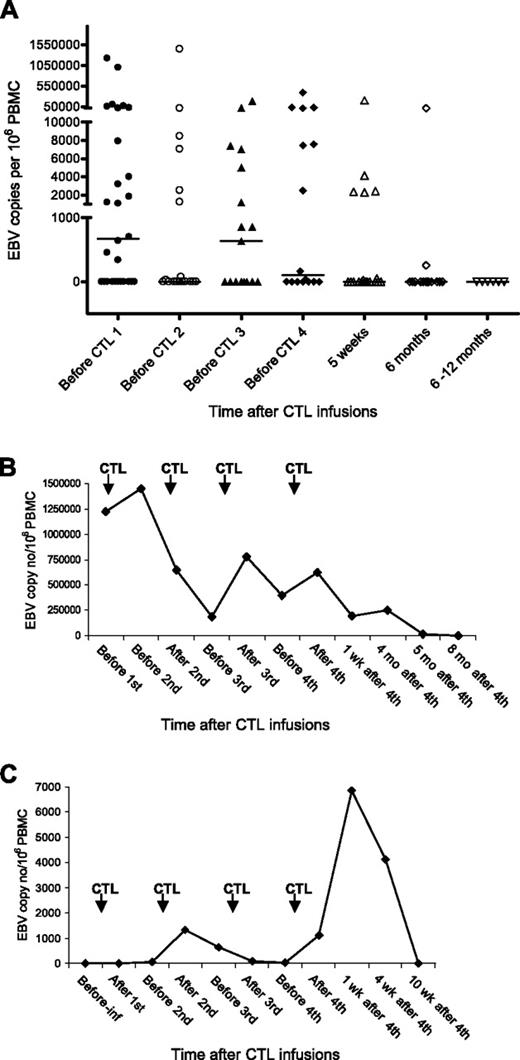
Ten of the 17 patients with preinfusion EBV DNA later showed a complete/partial response to CTL therapy and 7 patients had no response. Viral load fluctuated during the CTL infusions and became undetectable between 1 week to 8 months after the last CTL infusion in all patients (Figure 4A). There was no association between the rate of decrease in viral load and the response to therapy (Figure 4B represents patient 18, PR at 5 weeks, NR at 6 months). Some patients (including 9 who had no detectable DNA before CTL infusion) showed a transient increase in EBV load in PBMCs immediately after CTL infusions (Figure 4C; patient 2 CR).
EBV load in a PTLD patient following CTL infusions. DNA was extracted from PBMCs and amplified using real-time PCR. The EBV copy number was calculated using a standard curve obtained from dilutions of EBV-positive Raji cell lines. (A) EBV DNA levels in PBMCs of all patients. The black bars show the median value at each time point. The level was taken as 0 where EBV DNA was undetectable. (B) EBV DNA levels in PBMCs of patient 18. (C) EBV DNA levels in PBMCs of patient 2. Pre-inf indicates before CTL infusion; wks, weeks; and mo, months.
EBV load in a PTLD patient following CTL infusions. DNA was extracted from PBMCs and amplified using real-time PCR. The EBV copy number was calculated using a standard curve obtained from dilutions of EBV-positive Raji cell lines. (A) EBV DNA levels in PBMCs of all patients. The black bars show the median value at each time point. The level was taken as 0 where EBV DNA was undetectable. (B) EBV DNA levels in PBMCs of patient 18. (C) EBV DNA levels in PBMCs of patient 2. Pre-inf indicates before CTL infusion; wks, weeks; and mo, months.
Monitoring of infused CTLs
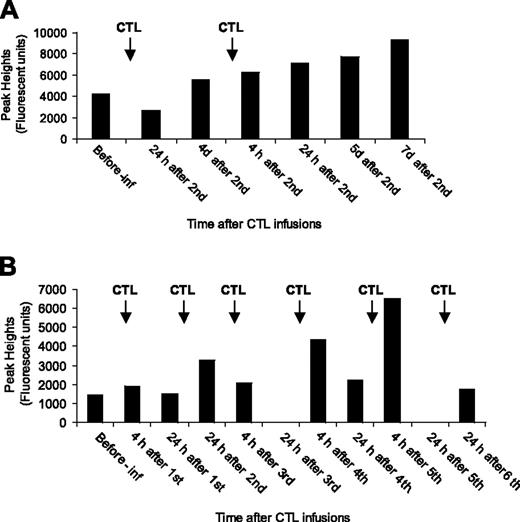
T-cell receptor (TCR) spectratyping was performed on preinfusion and serial postinfusion PBMCs from 5 patients and on the infused CTLs. The timing of samples tested ranged from 4 hours to 7 days after infusion. The number of monoclonal TCR subfamilies identified in the infused CTLs ranged from 2 to 8 (average number, 4) with no specific pattern of family usage nor any particular subfamily being dominant. Where possible the monoclonal subfamilies identified in the CTLs were traced in the patient PBMC samples. For successful tracing, the CTL monoclonal subfamily signal had to be strong with the same base pair peak in the pretreatment recipients' PBMC producing a weak signal. Tracing was successful in 3 of the 5 patients (1 complete responder, 1 partial responder, and 1 nonresponder), with a total of 7 subfamilies traced over the treatment period (2, 4, and 6 infusions). Two different patterns of CTL subfamily trace were observed; a gradual increase in the peak signal with each infusion (2 of 7 subfamilies) and a maximum signal detected at 4 or 24 hours after each infusion (5 of 7 subfamilies; Figure 5A, B, PR and NR, respectively). In one patient, an elevated CTL subfamily could be detected 7 days after the second infusion, but no further samples were available to test.
Monitoring of infused CTLs. PBMCs from patients' peripheral blood were analyzed by TCR spectratyping method to detect infused CTLs. (A) Trace analysis of BV subfamily 3 (204 base pair peak) in patient 17's PBMCs before and after infusion. (B) Trace analysis of BV subfamily 4 (341 base pair peak) in patient 22's PBMCs before and after infusion. Pre-inf indicates before CTL infusion; h, hours; and d, days.
Monitoring of infused CTLs. PBMCs from patients' peripheral blood were analyzed by TCR spectratyping method to detect infused CTLs. (A) Trace analysis of BV subfamily 3 (204 base pair peak) in patient 17's PBMCs before and after infusion. (B) Trace analysis of BV subfamily 4 (341 base pair peak) in patient 22's PBMCs before and after infusion. Pre-inf indicates before CTL infusion; h, hours; and d, days.
Monitoring of alloantibody response
Testing of pretransplantation and posttransplantation plasma samples from all trial participants revealed that only one patient had developed an antibody response against a mismatched donor HLA antigen (A2; data not shown).
Discussion
We report the first phase 2 clinical trial using partially HLA-matched allogeneic CTLs for PTLD therapy. Using EBV-specific CTLs grown from peripheral blood of healthy blood donors to treat PTLD on a best HLA-match basis, we show a 52% (17/33) response rate at 6 months. CTL infusions proved simple to administer on an outpatient basis, and no treatment-related acute or long-term toxicity was observed. Thus, since all patients in the trial had failed to respond to conventional PTLD treatments, our response rate of 52% with allogeneic CTLs is encouraging and represents a new, safe, and alternative approach to treating PTLD.
Previous studies using donor-derived EBV-specific CTLs to prevent or treat PTLD in stem cell transplant recipients have reported promising results, but this strategy cannot be used for PTLD in solid organ recipients as donor blood is generally not available.14,15 For solid organ recipients, autologous CTLs have been used with some success, but the time and expense required to generate individual CTLs for each patient restricts its wider application.23–25 To bypass these restrictions, we developed a CTL bank in Edinburgh that provides a source of EBV-specific CTLs for rapid treatment of PTLD on a best HLA-matched basis.
The primary outcome measure in this trial was tumor response at 6 months as indicated by clinical evaluation in addition to radiologic or MRI scanning where appropriate. Twenty-six of the 33 patients reached the 6-month time point with a response rate (partial or complete) of 52% (17/33). There was a suggestion that response to treatment may be better among female than male patients, but a larger trial is needed to confirm this. If such a difference exists, then the reasons are not clear.
In common with previous studies using conventional PTLD treatment, our results show some indication of a better, sustained response to CTL therapy in early onset disease.26 Thus, we found that PTLD tumors arising within 2 years of transplantation showed a better response rate at 6 months than those arising at a later time point (64% versus 42%), although the differences were not statistically significant. It is notable that 3 of 4 tumors involving a single anatomic site showed a complete response.12,26 At the time of enrollment into our trial, most patients had progressive disease despite having received conventional therapy. In particular, immunosuppression dose reduction was first-line treatment for all patients (Table 1), and for this reason we cannot exclude the possibility that some of the responses we observed were due to the delayed effect of this treatment rather than CTLs. However, we have previously reported complete tumor regression after allogeneic CTL therapy in a child with an EBV-associated brain lymphoma related to a primary immunodeficiency, a situation in which the level of immunosuppression could not be manipulated.27
The CTLs used in this study were selected for infusion on a best HLA-match basis, with the number of matches at HLA A, B, and DR loci varying from 2 to 5. We recorded a significant increase (P = .048) in response rate with increased number of HLA matches between CTL donor and recipient, again indicative of the tumor responses being CTL mediated (Figure 2). The CTL lines in the Edinburgh bank were derived from healthy donors and, in order to maintain multiple epitope specificities, were not cloned prior to in vivo use. Most lines consist of CD8 T cells with a minor population of CD4 cells.17 The outcome of CTL therapy in this trial correlated with the level of CD4 cells within CTLs, with a highly significant improvement in outcome with higher numbers of CD4 cells (Figure 3). This is not an entirely unexpected result as CD4 cells provide help to CD8 cytotoxic T cells, and although most CD8 cells in the CTLs had an effector phenotype (CD45RO+, HLA-DR+, CD69+, CD150+), CD4 cells may have provided additional signals that enhanced their survival in vivo.
Although the timing of the fall in EBV load in peripheral blood that we regularly observed during and following CTL therapy suggests that the CTLs were active in vivo, it is important to note that the rate of fall did not correlate with response to tumor (Figure 4). Thus events in the peripheral blood do not always reflect those in the tissues or tumor sites, and viral load data should be interpreted with caution. Interestingly, in some cases, a transient rise in plasma EBV load occurred immediately after CTL infusions, a phenomenon recorded by others during treatment of PTLD with autologous CTLs and thought to indicate specific lysis of tumor cells.25
Heslop et al have previously shown that stem cell transplant donor-derived CTLs survive long term in recipients and can control reactivated persistent EBV infection up to 18 months after infusion.28 But considering the partial HLA matching and allogeneic nature of the CTLs used in this trial, we expected them to be short-lived in vivo since the mismatched HLA molecules would be targeted by the host immune system. However only one patient developed detectable antialloantibodies during the study, and this individual achieved a complete response. This finding can perhaps be explained by the immunosuppressive therapy that all patients were receiving inhibiting primary immune responses. Using clonotyping to monitor individual T-cell clones within infused CTLs, we demonstrated survival of these cells in peripheral blood for up to 7 days (Figure 5), and in one patient (patient 13) where tetramers were available to detect individual EBV peptide–specific clones, infused CTLs were detected at 194 days. In this case, the CTLs used contained a high level (34%) of CD4+ T cells, again suggesting that these cells may have prolonged in vivo survival (M.K.G. and D.H.C., data not shown).
Compared with our 52% response rate at 6 months, a recent phase 2 clinical trial using rituximab to treat PTLD reported a response rate of 44% at day 80. Rituximab is available for immediate use and is easy to administer. This study enrolled patients whose only previous treatment was reduction of immunosuppression, and CNS tumors were excluded, whereas, in our trial 50% of patients had received and failed on a variety of therapies in addition to reduction of immunosuppression (Tables 1, 2) and patients with CNS PTLD (n = 5) were included.12 Chemotherapy is commonly preferred for aggressive tumors and gives a comparable response rate to that reported here, however severe toxicity and infections are recurring problems.11,29 Thus there is an urgent need for large multicenter trials comparing and combining these treatment options in order to establish the best treatment practice for patients at all stages of the disease.
In summary, we have shown that partially HLA-matched allogeneic T-cell therapy is a safe and effective option for PTLD and that a CTL bank overcomes the restrictions implicit in autologous CTL therapy. During the trial, frozen CTLs were sent from Edinburgh to France, Sweden, and Australia where they were used to treat PTLD. Thus a single CTL bank could be a valuable international resource.
An Inside Blood analysis of this article appears in the front of this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The work was funded by The Cancer Research UK (grant nos. SP 2278/0202 and C307/A3869). The authors would like to thank the lead clinicians of the transplantation centers for referring patients to this trial: Dr Amrolia, Great Ormond Street Hospital, London; Dr Cavenagh, Barts and the London National Health Service Trust; Dr Culligan, Aberdeen Royal Infirmary; Dr Fitzsimons, Gartnavel General Hospital, Glasgow; Drs Gustafsson, Jernberg and Barkholt, Karolinska University Hospital, Stockholm, Sweden; Dr Hunter, Leicester Royal Infirmary; Dr Mackie, Western General Hospital, Edinburgh; Dr Marcus, Addenbrooke's Hospital, Cambridge; Dr Mead, Southampton General Hospital; Drs Minard and Mahlaoui at Hopital Necker Enfants-Malades, Paris, France; Prof Mufti and Dr Devereux, King's College Hospital, London; Dr O'Connor, The Royal Belfast Hospital For Sick Children; Dr Pettitt, Royal Liverpool University Hospital; Dr Picton, Manchester Royal Infirmary; Dr Poynton, University Hospital of Wales, Cardiff; and Drs Wright and Snowden, Royal Hallamshire Hospital, Sheffield. The authors also thank the coordinators and staff at all transplantation units for collecting and sending samples to Edinburgh. Hazel Gavin and Elaine Edgar provided secretarial help. Dr Jill Douglas provided technical help.
Authorship
Contribution: D.H.C. was the principal investigator; D.H.C., T.H., M.T., P.L.A., and A.J.S. were grant holders; T.H. and D.H.C. designed the trial, supervised and trained the team members, liaised with clinicians, provided clinical advice, interpreted and analyzed data, and drafted the paper; G.M.W. was the trial coordinator, liaised with transplantation centers, and maintained databases; G.M.W., M.M.J., G.U., P.W., and D.B. established and maintained CTL bank, analyzed patients' samples, and performed quality control/sterility checks; M.M.J. also carried out EBV PCR; K.M. performed TCR spectratyping; C.D.H. performed statistical analyses; C.B. provided histologic diagnosis on tumor samples; M.T., P.L.A., and A.J.S. advised on trial design; M.T. coordinated the blood donors; P.L.A. provided clinical advice and phenotyping of cells; D.K., A.M., and M.K.G. enrolled 3 or more PTLD patients to the trial; all the authors read and commented on the final version of the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Tanzina Haque, Centre for Virology, Royal Free Hospital and University College Medical School, Rowland Hill Street, London NW3 2PF; e-mail: t.haque@medsch.ucl.ac.uk.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal