Abstract
Shwachman-Diamond syndrome (SDS; OMIM 260400), an inherited bone marrow failure syndrome, is caused by mutations in both alleles of the SBDS gene, which encodes a protein of unknown function. Here we report heterozygosity for the 258 + 2 T>C SBDS gene mutation previously identified in SDS patients in 4 of 91 patients with apparently acquired aplastic anemia (AA) but not in 276 ethnically matched controls (Fisher exact test, P < .004). Affected patients were young and had a poor outcome; they had reduced SBDS expression but no evidence of the pancreatic exocrine failure or skeletal abnormalities typical of SDS. Length of telomeres in granulocytes of SBDS heterozygous patients was short for their age, and in SDS patients with both SBDS alleles affected further analyzed, granulocytes' telomeres were even shorter, correlating in length with SBDS expression. Higher heterogeneity in telomere length also was observed in SDS patients. Telomerase activity of SBDS-deficient patients' lymphocytes was comparable with controls, and no physical interaction between SBDS protein and telomerase complex components (TERT or TERC) was established. We propose that heterozygosity for the 258 + 2 T>C SBDS mutation predisposes to AA by accelerating telomere shortening of leukocytes via a telomerase-independent mechanism.
Introduction
Mutations to SBDS underlie Shwachman-Diamond syndrome (SDS), an inherited syndrome featuring bone marrow failure, pancreatic exocrine insufficiency, skeletal defects, and an elevated risk for developing hematologic cancers.1 The functional consequences of SBDS mutation have not yet been characterized, although short telomeres of leukocytes have been observed in affected patients.2 Structural and functional analyses of SBDS Archae and yeast orthologues suggest involvement in RNA processing, and the protein's nucleolar localization in mammalian cells further supports this role.3,4 Mutations in affected patients result from gene conversion between SBDS and an SBDSP pseudogene, the 2 most common being insertion of a premature stop codon and splice site disruption with resultant translation truncation.5
SDS represents one inherited bone marrow failure syndrome; others include dyskeratosis congenita (DKC) and Fanconi anemia.1,6 These syndromes typically present in early childhood with a constellation of symptoms and signs and are associated with poor outcomes. In contrast, acquired bone marrow failure, or acquired aplastic anemia (AA), can occur during any decade of life and usually responds to treatment with either intensive immunosuppressive therapy or HLA-matched sibling hematopoietic stem cell transplantation, both of which are successful in the majority of cases.7
Common to both acquired AA and constitutional marrow failure syndromes is association with short-for-age telomeres from malfunction of telomere repair components. Telomeres protect chromosome ends from erosion and thus cells from senescence; their elongation can be observed in highly proliferative tissues.8 Both telomerase, a reverse-transcription enzyme complex, and ALT (alternative lengthening of telomeres), an incompletely defined pathway involving sister-chromatid exchange, are known telomere-lengthening mechanisms.8,9 Accelerated telomere shortening of leukocytes is found in approximately one third of patients with acquired AA10 and is virtually universal in DKC and SDS.2,11 Some of the cases of acquired AA with telomere shortening may be explained by mutations in TERT or TERC, which code for, respectively, telomerase reverse transcriptase and the RNA template of the telomerase complex.12–14 Autosomal recessive DKC is also caused by TERC mutations, and X-linked DKC results from mutation to dyskerin, a protein that associates with the telomerase complex and participates in rRNA processing in addition to modulating telomerase activity.15,16
As the number of patients with acquired AA featuring telomere shortening exceeds the number of patients with telomerase complex mutations, we hypothesized that other genetic determinants also contribute to telomere erosion in AA. Given the link between SDS and telomere attrition, in the present study we investigated whether mutations in the SBDS gene also occurred in acquired AA, and we explored the role of SBDS protein in telomere maintenance.
Patients, materials, and methods
Patients and controls
Peripheral blood samples were obtained from 91 unrelated patients with the diagnosis of acquired aplastic anemia (females, 40; males, 51; age range, 5 to 79 years; median age, 37 years) treated at a single institution (the Hematology Branch, National Heart, Lung, and Blood Institute, National Institutes of Health). The diagnosis of aplastic anemia was based on the criteria of the International Agranulocytosis and Aplastic Anemia Study.17 Of 91 patients, 58% were self-reported as Caucasian, 15% as African descendents, 19% as Hispanics, and 8% as Asians. Patients came from the United States and from several Latin-American and Asian countries; patients or their guardians provided written informed consent for genetic testing, according to protocols approved by the institutional review board of the NHLBI. Informed consent was obtained in accordance with the Declaration of Helsinki. Samples from 274 healthy persons were analyzed as controls: 117 (43%) were white (94 from Human Variation Panel HD100CAU, Coriell Cell Repositories [http://locus.umdnj.edu/nigms/cells/humdiv.html], and 23 from SNP500Cancer [http://snp500cancer.nci.nih.gov]), 111 (41%) black (88 from Human Variation Panel HD100AA and 23 from SNP500Cancer), 23 (8%) Hispanic (from SNP500Cancer), and 23 (8%) Asian (from SNP500Cancer).18 Peripheral blood samples from SDS patients were collected in 2 different institutions (Great Ormond Street Hospital, London, United Kingdom, and The Hospital for Sick Children, The University of Toronto, Toronto, ON) according to respective local institutional review boards. Diagnosis was made according to standard criteria.19
Mutation analysis
DNA was extracted from peripheral blood leukocytes, and polymerase chain reaction (PCR) amplification of the SBDS gene's 5 exons and proximal intronic regions was performed as previously described.20 Primers specifically amplify SBDS but not SBDSP. Genomic DNA (10 ng) was amplified using TaKaRa LA Taq (TaKaRa Bio, Shiga, Japan) in a PTC-220 thermocycler (MJ Research, Cambridge, MA) according to the following parameters: 95°C for 2 minutes; 95°C for 30 seconds, 55°C for 50 seconds, and 72°C for 4 minutes, 35 cycles; and 72°C for 5 minutes. PCR products were tested for amplification in an agarose gel, and purified using the QIAquick PCR purification kit (Qiagen, Baltimore, MD). Purified products were sequenced using specific primers (Table S1, available on the Blood website; see the Supplemental Materials link at the top of the online article) and Big Dye Terminator kit v3.1 (Applied Biosystems, Foster City, CA), cleaned of extra dye with the DyEx extraction kit (Qiagen), dried, resuspended in formamide, and analyzed in an ABI3700 automated genetic-sequencer analyzer (Applied Biosystems). Sequencing results were analyzed using Sequencher 4.5 software (Gene Codes, Ann Arbor, MI). All sequences were determined in both directions.
Telomere length and telomerase activity
The average length of telomere repeats at chromosome ends in individual peripheral blood leukocytes was measured by flow fluorescence in situ hybridization, as previously reported.21 Telomere length and heterogeneity were also measured by Southern blot using the TeloTAGGG kit (Roche, Mannheim, Germany). Telomere smears were divided into 30 boxes, and length analysis performed according to manufacturer's instructions using ImageQuant software (Amersham Biosciences, Piscataway, NJ).
For assaying telomerase activity, peripheral blood cells were enriched for stimulated T cells by culturing in RPMI 1640 with l-glutamine and 10% heat-inactivated fetal bovine serum (FBS) in the presence of phytohemagglutinin (5 μg/mL) and interleukin-2 (40 IU/mL) for 4 days at 37°C with 5% CO2. Samples were processed in quadruplicate using the TRAPeze XL Telomerase Detection method according to manufacturer's instructions (Chemicon International, Temecula, CA).
SBDS antibody
A polyclonal antibody against SBDS was generated in rabbits using the terminal 18 amino acids of the SBDS protein (Ac-SLEVLNLKDVEEGDEKFE-amide) by BioSource (Hopkinton, MA). Before our antibody was ready for use, an aliquot of polyclonal antibody was kindly provided by Dr A. Shimamura (Dana-Farber Cancer Institute, Boston, MA).
Plasmid constructs and TERC synthesis
pcDNA3 vector (Invitrogen, Frederick, MD) was modified to include an N-terminally positioned FLAG epitope preceded by a Kozak initiation sequence. TERT and SBDS cDNAs were acquired from ATCC (Manassas, VA) and cloned into the modified pcDNA3 vector using restriction sites EcoR1 and Xba1. SBDS-84X, which encodes the first 84 amino acids of SBDS, was generated by PCR using an EcoR1-SBDS wild-type 5′ primer and the Xba1 3′ primer 5′-CCCACTCAGTGTTTGTAAATGTTTCCTAATCTAGAGCG-3′ and cloned into modified pcDNA3 as done for SBDS. All constructs were verified by DNA sequencing. Wild-type TERC cDNA was amplified from genomic DNA, transcribed using the MAXIscript kit (Ambion, Austin, TX), and agarose gel purified. TERC RNA was biotinylated with EZ-Link Psoralen-PEO-Biotin (Pierce, Rockford, IL).
Cell culture and expression
HEK 293T and HeLa cells were cultured in DMEM and IMDM, respectively, supplemented with 10% FBS, l-glutamine, and antibiotics. Transfection was performed at 60% to 80% confluency using FuGene 6 (Roche, Indianapolis, IN) for 20 hours.
Western blot analysis
Peripheral blood lymphocytes were isolated by density gradient centrifugation, washed in cold PBS, and lysed with M-PER lysis buffer (Pierce) on ice for 30 minutes. Lysates were cleared by centrifugation at 12 000g at 4°C for 20 minutes, and protein concentration was measured using the DC Protein Assay (BioRad, Hercules, CA). Western blotting was performed according to standard protocols using HRP-conjugated anti-FLAG M2 antibody (Sigma, St Louis, MO) 1:1000, anti-SBDS 1:1000, or anti-B-Actin (AbCam, Cambridge, United Kingdom) 1:10 000.
Coimmunoprecipitation
HeLa and 293T cells were rinsed in cold PBS and lysed on ice by scraping into cold M-PER lysis buffer followed by three 5-second sonication pulses. Lysates were cleared by centrifugation and incubated at 4°C with 10 μL anti-FLAG M2 preblocked agarose beads (Sigma) for 4 to 6 hours. Beads were preblocked for 1 hour at room temperature in 100 ng/μL BSA, 100 ng/μL casein, 250 ng/μL tRNA, and 100 ng/μL glycogen. Following immunoprecipitation, beads were washed 4 times with M-PER supplemented with 0.1% Triton-X 100 and 4 additional times with PBS + 0.1% Triton-X 100.
Mammalian 2-hybrid analysis
The Mammalian 2-Hybrid Assay Kit (Stratagene, La Jolla, CA) was used according to the manufacturer's instructions. SBDS was cloned into pCMV-BD fused 3′ to GAL 4 BD using EcoR1 and Xba1 restriction sites; TERT was similarly cloned into pCMV-AD fused 3′ to NF-kB. Properly orientated SBDS and TERT clones were selected by Pst1 and Xho1 restriction enzyme digestion, respectively. Plasmids were cotransfected with GAL4-binding pFR-Luc plasmid in HeLa and HEK 293T cells using FuGene 6 (Roche), and SBDS expression was confirmed by Western analysis. If brought into close proximity, NF-κB activates GAL4 to up-regulate transcription of the cotransfected luciferase reporter gene.
Rabbit reticulocyte lysate system and TERC immunoprecipitation
FLAG-tagged SBDS, SBDS-84X, and TERT were expressed with the TnT Quick Coupled Transcription/Translation System (Promega, Madison, WI) for 2 hours at 37°C in the presence of 100 ng biotinylated TERC. Lysates were diluted in 20 volumes M-PER and subjected to FLAG-immunoprecipitation as above in the presence of 100 U/mL RNAse inhibitor. RNA was extracted from beads using TRIzol reagent (Invitrogen), separated on a 6% polyacrylamide-7M urea-TBE gel, transferred to a positively charged nylon membrane, and developed using the BrightStar biodetect kit (Ambion).
Statistical analysis
Differences in the frequencies of genetic variations between samples from patients with aplastic anemia and controls were evaluated by means of Fisher exact test. Differences in age between patients with aplastic anemia with and without SBDS mutations were investigated using the Mann Whitney U test.
Results
Mutation analysis
We screened 91 consecutive patients with acquired AA and 276 ethnically matched controls for mutations in SBDS. Four patients were heterozygous for the 258 + 2 T>C mutation at intron 2 (Table 1), which was absent in controls (Fisher exact test, P < .004). This mutation—also found in SDS patients—is thought to disrupt the donor splice site in intron 2, engaging a cryptic upstream splice site at positions 251 to 252 and leading to protein truncation at codon 84 by frameshift.5 Two nonpathogenic polymorphisms were identified at similar allele frequencies in patients and controls (Table 2).
The 4 AA patients heterozygous for SBDS 258 + 2 T>C mutation were young (median age, 15.5 years; range, 5 to 19 years) in comparison with other aplastic anemia patients analyzed (median age, 29 years; Mann-Whitney U test, P = .02), and had no clinical history of pancreatic exocrine failure or skeletal abnormalities. Serum lipase, amylase, and fecal fat obtainable from 2 individuals were normal (data not shown). Patients also tested negative for chromosomal hypersensitivity to DNA cross-linking agents. The medical history, family history, and the physical examination of the patients were not suggestive of an inherited syndrome. Parental genetic screening, possible in 2 cases, demonstrated that the mothers harbored the mutation, confirming its germ-line origin. Both (as well as a third mother not tested [Table 1]) had long histories of subclinical mild anemia, although no quantitative abnormalities in leukocytes or platelets were observed. Patient C also was found to be heterozygous for telomerase reverse transcriptase (TERT) nonsynonymous gene variant A1062T, which we recently observed to correlate with short telomeres and to modulate telomerase activity (R.T.C., J. Regal, P.M.L., unpublished data, April 2007).
SBDS expression
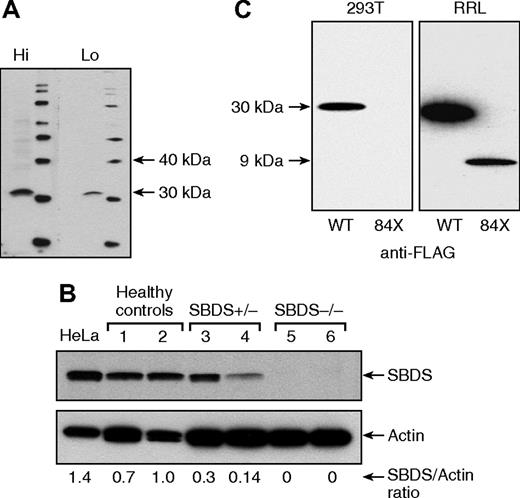
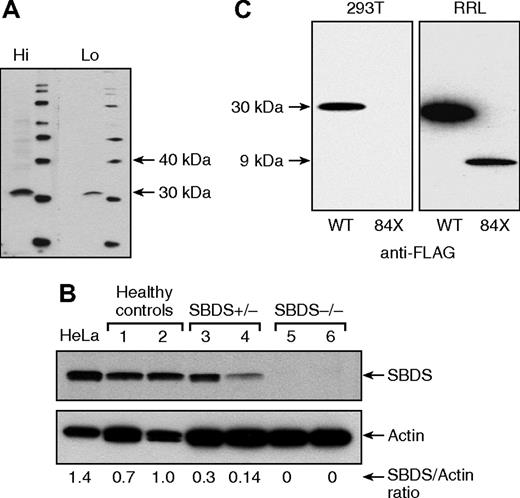
A polyclonal antibody against SBDS was raised (Figure 1A), and by Western blotting, we observed that SBDS expression was evident in primary lymphocytes of healthy controls, partially decreased in SBDS heterozygous AA patients, and was undetectable in SDS patients, in which both alleles are affected (Figure 1B). To clarify the expression and function of the 258 + 2 T>C mutation truncated product, a FLAG-tagged construct of SBDS N-terminal 84 amino acids (FLAG-SBDS-84X) was transfected into HEK 293T cells and tested in the cell-free rabbit reticulocyte lysate (RRL) system. Whereas FLAG-SBDS-84X was clearly detected in RRL system, indicating the sensitivity of Western blot to probe the truncated protein, no stable FLAG-SBDS-84X expression was observed in HEK 293T cells (Figure 1C), indicating that the 258 + 2 T>C SBDS mutation results in a nonexpressed product and impairs SBDS function(s), probably by a mechanism of haploinsufficiency.
SBDS protein expression in peripheral blood lymphocytes. (A) A polyclonal antibody against the terminal 18 amino acids of the SBDS was generated in rabbits with adequate sensitivity and specificity at high (Hi, 1:500) and low (Lo, 1:2,000) dilutions, recognizing a protein with ∼31 kDa. (B) By Western blot, SBDS expression was evident in HeLa cells and peripheral blood lymphocytes from healthy controls, partially diminished in SBDS heterozygous aplastic anemia patients, and undetectable in patients with SDS. (C) FLAG-tagged SBDS (FLAG-SBDS-WT) and FLAG-tagged construct of SBDS N-terminal 84 amino acids (FLAG-SBDS-84X) were transfected into HEK 293T, but only the full-length protein was expressed (left panel), whereas in the cell-free rabbit reticulocyte expression system (RRL), both products were expressed.
SBDS protein expression in peripheral blood lymphocytes. (A) A polyclonal antibody against the terminal 18 amino acids of the SBDS was generated in rabbits with adequate sensitivity and specificity at high (Hi, 1:500) and low (Lo, 1:2,000) dilutions, recognizing a protein with ∼31 kDa. (B) By Western blot, SBDS expression was evident in HeLa cells and peripheral blood lymphocytes from healthy controls, partially diminished in SBDS heterozygous aplastic anemia patients, and undetectable in patients with SDS. (C) FLAG-tagged SBDS (FLAG-SBDS-WT) and FLAG-tagged construct of SBDS N-terminal 84 amino acids (FLAG-SBDS-84X) were transfected into HEK 293T, but only the full-length protein was expressed (left panel), whereas in the cell-free rabbit reticulocyte expression system (RRL), both products were expressed.
Telomere length and telomerase activity in SBDS-deficient primary cells
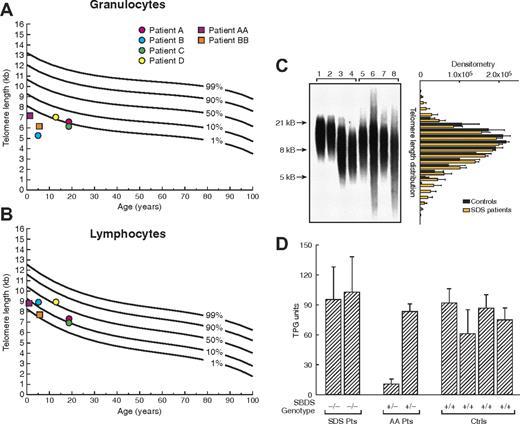
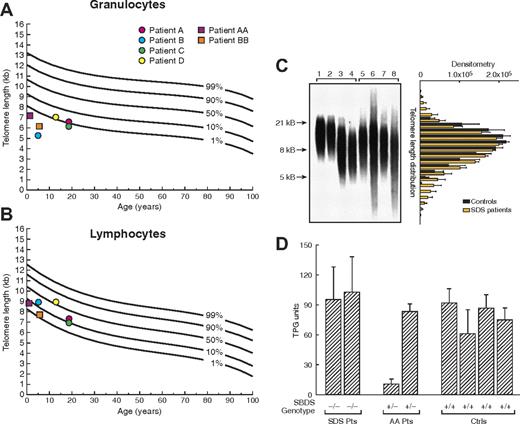
Using flow-fluorescent in situ hybridization (FISH), we examined the length of telomeres of leukocyte subsets in SBDS heterozygous patients with acquired AA and patients with SDS. All 4 SBDS heterozygous patients from our series had short telomeres of granulocytes (Figure 2A). In contrast, lymphocytes' telomeres were normal in length (Figure 2B). We further evaluated SDS patients with both SBDS alleles mutated, whose telomeres were even shorter in all leukocytes in comparison with patients heterozygous for the SBDS 258 + 2 T>C mutation: in granulocytes, telomere length was below the first percentile of expected-for-age curve and in lymphocytes between the first and tenth percentiles (Figure 2), indicating a correlation between telomere length and SBDS expression. Except for patient C, who also carries a TERT polymorphism likely to impair telomerase activity, the consistency of telomere shortening in SBDS heterozygous patients, coupled with shorter telomeres in SDS patients, implicates SBDS in telomere length maintenance. We further explored the length of telomeres in SBDS-deficient patients by Southern blot analysis, which allows quantification of length heterogeneity. Measured as the coefficient of variation (CV), heterogeneity in relative telomeric fragment length was significantly increased in patients with SBDS mutations compared with healthy controls (Figure 2C).
Telomere length in peripheral blood leukocytes. (A,B) Telomere length in peripheral blood granulocytes and lymphocytes from patients with acquired aplastic anemia (AA) carrying 258 + 2 T>C SBDS gene mutation (●) and patients with Shwachman-Diamond syndrome (SDS; ■). Telomere lengths were measured by flow fluorescence in situ hybridization analysis. Lines represent the 1st, 10th, 50th, 90th, and 99th percentiles of telomere length in age-matched healthy controls' granulocytes and lymphocytes, based on a reference group of 400 persons. (C) Telomere length of leukocytes also was measured by Southern blot allowing quantification of length heterogeneity by calculating the coefficient of variation (CV). Length heterogeneity was significantly increased in SDS patients compared with healthy controls. Lanes 1 and 2, umbilical cord blood of healthy subjects; lanes 3 and 4, age-matched healthy subjects; lanes 5 to 8, patients with SDS. Bar graphic on the right represents the analysis of variation of optical density (mean ± SEM) in telomere length for controls (1-4) and SDS patients (5-8), indicating the higher variability in telomere length in SDS patients. (D) Cultured lymphocytes from patients with SDS (−/−), with acquired AA heterozygous for SBDS gene 258 + 2 T>C mutation (+/−), and healthy controls (+/+) were assayed for telomerase activity using the fluorescent telomeric repeat-amplification protocol (TRAP). Telomerase activity was measured in quadruplicate and indicated in total product generated (TPG) units. Error bars represent standard deviation.
Telomere length in peripheral blood leukocytes. (A,B) Telomere length in peripheral blood granulocytes and lymphocytes from patients with acquired aplastic anemia (AA) carrying 258 + 2 T>C SBDS gene mutation (●) and patients with Shwachman-Diamond syndrome (SDS; ■). Telomere lengths were measured by flow fluorescence in situ hybridization analysis. Lines represent the 1st, 10th, 50th, 90th, and 99th percentiles of telomere length in age-matched healthy controls' granulocytes and lymphocytes, based on a reference group of 400 persons. (C) Telomere length of leukocytes also was measured by Southern blot allowing quantification of length heterogeneity by calculating the coefficient of variation (CV). Length heterogeneity was significantly increased in SDS patients compared with healthy controls. Lanes 1 and 2, umbilical cord blood of healthy subjects; lanes 3 and 4, age-matched healthy subjects; lanes 5 to 8, patients with SDS. Bar graphic on the right represents the analysis of variation of optical density (mean ± SEM) in telomere length for controls (1-4) and SDS patients (5-8), indicating the higher variability in telomere length in SDS patients. (D) Cultured lymphocytes from patients with SDS (−/−), with acquired AA heterozygous for SBDS gene 258 + 2 T>C mutation (+/−), and healthy controls (+/+) were assayed for telomerase activity using the fluorescent telomeric repeat-amplification protocol (TRAP). Telomerase activity was measured in quadruplicate and indicated in total product generated (TPG) units. Error bars represent standard deviation.
To determine telomerase activity in SBDS mutant patients, primary cultured T cells were assayed. Though intersubject variability was high, telomerase activity in SDS and SBDS+/− AA patients was not different from that in healthy controls (Figure 2D). Low telomerase activity of stimulated leukocytes in one heterozygous patient may be explained by other unknown genetic or environmental factors influencing telomerase activity in a given individual. However, the telomerase function among SDS patients (with minimal SBDS expression), the other heterozygous patient, and controls was comparable.
Association between SBDS and telomerase complex components
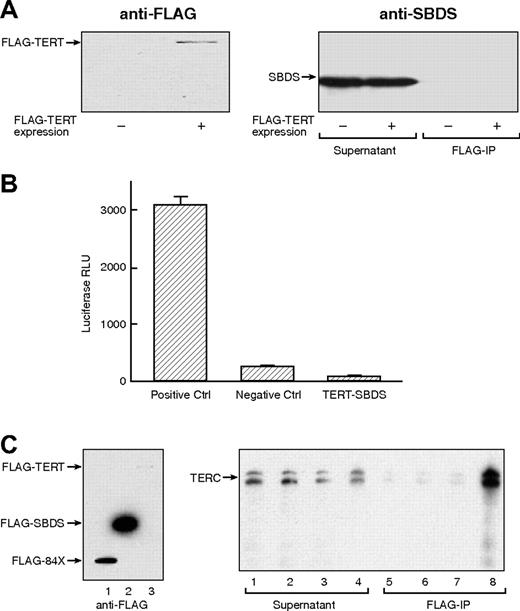
We explored a possible physical association between SBDS and components of the telomerase complex. Both entities are localized to nucleoli, and the putative RNA processing activity of SBDS is compatible with the rRNA pseudouridylation activity of telomerase dyskerin. FLAG-tagged TERT was expressed in HEK 293T cells, which endogenously express high levels of SBDS. Following immunoprecipitation of FLAG-TERT (Figure 3A left panel), no SBDS was detected in precipitates probed with anti-SBDS antibody (Figure 3A right panel). Lack of TERT-SBDS interaction was additionally demonstrated using a mammalian 2-hybrid assay, which can detect weaker or more transient protein-protein interactions (Figure 3B). TERC RNA was incubated with cell-free RRL expressing FLAG-TERT, FLAG-SBDS, and negative controls. Following FLAG immunoprecipitation, RNA was extracted from the precipitates and probed for TERC. As predicted, FLAG-TERT efficiently precipitated TERC; FLAG-SBDS, however, demonstrated no affinity for TERC (Figure 3C).
Lack of physical interaction between SBDS and telomerase complex components. (A) FLAG-tagged TERT construct was transfected into HeLa and HEK 293T cells. Left panel illustrates HeLa cell lysates transfected (+) or not transfected (−) with FLAG-TERT probed for FLAG-TERT with an anti-FLAG monoclonal antibody, indicating effective TERT expression. Immunoprecipitation with anti-FLAG antibody was performed and precipitates probed for SBDS (right panel). (B) TERT-SBDS interaction was further explored using the 2 hybrid system. Luciferase activity indicates close proximity of NF-κB and yeast GAL4. (C) Association between SBDS and TERC RNA. TERC RNA was synthesized in vitro and incubated with rabbit reticulocyte lysate (RRL) expressing FLAG-TERT, FLAG-SBDS, FLAG-SBDS-84X, or empty vector (left panel; lane 1, RRL expressing FLAG-SBDS-84X; lane 2, RRL expressing FLAG-SBDS; and lane 3, RRL expressing FLAG-TERT). Following FLAG immunoprecipitation, RNA was extracted from the precipitates and examined for TERC. Right panel illustrates Northern blot for TERC: lane 1, non-transfected RRL supernatant; lane 2, FLAG-SBDS-84X-transfected RRL supernatant; lane 3, FLAG-SBDS-transfected RRL supernatant; lane 4, FLAG-TERT-transfected RRL supernatant; lane 5, non-transfected RRL precipitate; lane 6, FLAG-SBDS-84X-transfected RRL precipitate; lane 7, FLAG-SBDS-transfected RRL precipitate; lane 8, FLAG-TERT-transfected RRL precipitate. Despite the low expression FLAG-TERT by the RRL, it efficiently precipitated TERC. In contrast, large amounts of FLAG-SBDS and FLAG-SBDS-84X failed to precipitate TERC to any appreciable degree.
Lack of physical interaction between SBDS and telomerase complex components. (A) FLAG-tagged TERT construct was transfected into HeLa and HEK 293T cells. Left panel illustrates HeLa cell lysates transfected (+) or not transfected (−) with FLAG-TERT probed for FLAG-TERT with an anti-FLAG monoclonal antibody, indicating effective TERT expression. Immunoprecipitation with anti-FLAG antibody was performed and precipitates probed for SBDS (right panel). (B) TERT-SBDS interaction was further explored using the 2 hybrid system. Luciferase activity indicates close proximity of NF-κB and yeast GAL4. (C) Association between SBDS and TERC RNA. TERC RNA was synthesized in vitro and incubated with rabbit reticulocyte lysate (RRL) expressing FLAG-TERT, FLAG-SBDS, FLAG-SBDS-84X, or empty vector (left panel; lane 1, RRL expressing FLAG-SBDS-84X; lane 2, RRL expressing FLAG-SBDS; and lane 3, RRL expressing FLAG-TERT). Following FLAG immunoprecipitation, RNA was extracted from the precipitates and examined for TERC. Right panel illustrates Northern blot for TERC: lane 1, non-transfected RRL supernatant; lane 2, FLAG-SBDS-84X-transfected RRL supernatant; lane 3, FLAG-SBDS-transfected RRL supernatant; lane 4, FLAG-TERT-transfected RRL supernatant; lane 5, non-transfected RRL precipitate; lane 6, FLAG-SBDS-84X-transfected RRL precipitate; lane 7, FLAG-SBDS-transfected RRL precipitate; lane 8, FLAG-TERT-transfected RRL precipitate. Despite the low expression FLAG-TERT by the RRL, it efficiently precipitated TERC. In contrast, large amounts of FLAG-SBDS and FLAG-SBDS-84X failed to precipitate TERC to any appreciable degree.
Discussion
In the present study, we found that heterozygosis for 258 + 2 T>C SBDS mutation was associated with acquired AA and telomere shortening of leukocytes. That SBDS contributes to telomere length maintenance is indicated by telomere shortening in leukocytes of SBDS-deficient patients and the inverse correlation between SBDS expression and telomere length in patients' primary leukocytes.
We identified among acquired AA patients an SBDS mutation, 258 + 2 T>C, previously observed in SDS patients in homozygous or compound heterozygosis.5 Leukocytes of SBDS heterozygous AA patients had partial SBDS expression, whereas SDS patients had no detectable SBDS. Shwachman-Diamond syndrome phenotype—bone marrow failure, pancreatic exocrine insufficiency, skeletal abnormalities—is likely due to significantly reduced SBDS expression. Parents of SDS patients do not manifest features of the disease, establishing its recessive inheritance pattern. In murine models, complete disruption of Sbds expression results in embryonic lethality at day 6.5.22 Partial SBDS expression had been previously noted in SDS patients possessing homozygous 258 + 2 T>C mutations,4 suggesting that minimal amounts of protein are required for cell survival and that bypassing of the cryptic splice site may occur. No patient has been found homozygous for the most common truncation mutation, 183-184TA>CT, which creates a stop codon at amino acid position 62 and likely generates no functional protein. We observed that truncated SBDS-84X is expressed by cell-free RRL but not by cell-culture, indicating that 258 + 2 T>C mutation protein product is not adequately expressed and that disease results from haploinsufficiency. Taking these results together, we speculate that individuals heterozygous for SBDS mutations, though not usually symptomatic, are at risk for manifestations of SBDS deficiency that are milder than in SDS patients, including less severely shortened telomeres, weaker penetrance, and more variable age of onset. Additionally, disease is limited to the highly proliferative hematopoietic compartment.
In primary leukocytes from SBDS-deficient patients and healthy volunteers, telomere length correlated with SBDS expression. In patients with acquired AA heterozygous for SBDS mutation, granulocytes' telomeres were short and lymphocytes' telomeres were in the normal range. In patients with SDS, granulocytes' telomeres were very short and lymphocytes' telomeres were short, implicating SBDS in the maintenance of telomere length (Figure 2). The exception is patient C, who also carries a TERT 1062A>T gene variant that is likely to directly modulate telomerase activity. We can speculate that the coincidence of rare gene mutations in different genes associated with short telomeres underscores the potential of gene-gene interaction potentiating their effects on telomere shortening and disease phenotype—patient C rapidly evolved to myelodysplasia with monosomy 7, an uncommon complication of aplastic anemia for age 5 years.23 Our findings on telomere shortening associating with SBDS deficiency are in agreement with a recent report indicating that, in 32Dcl3 myeloid cells, down-regulation of SBDS expression by small interference RNA causes abnormal telomere shortening.24 As telomerase activity was preserved in lymphocytes of these patients, SBDS likely does not directly modulate telomerase. Nevertheless, the assay used in this study, the telomere repeat amplification protocol (TRAP), reports telomerase activity in cell lysates reconstituted with functional TERT and TERC alone; the effects of telomerase modulators might not be recognized by this method. Indeed, leukocytes of DKC patients were previously found to have normal telomerase activity using the same method.11 Thus, that SBDS modulates telomerase activity cannot be excluded. As SBDS is predicted to play a role in RNA processing and contains putative RNA-binding sites,3,25 binding to telomerase RNA component TERC was plausible; however, using different methods, we failed to demonstrate any physical association between SBDS and telomerase complex components, namely TERT and TERC (Figure 3). Although in vivo chaperone-mediated SBDS-TERC association cannot be ruled out, our studies indicate that SBDS does not modulate telomerase activity through direct interaction. Together, these results suggest that SBDS influences telomere length via a telomerase-independent pathway.
We observed an increased heterogeneity in telomere length of leukocytes of patients with SBDS mutations, which showed very short and very long telomeres. This could be explained by the differential impact of telomere shortening in distinct leukocyte subsets, as granulocytes seem to be more vulnerable to telomere attrition than do lymphocytes. However, while high telomere length heterogeneity is more pronounced in SDS patients compared with SBDS heterozygous AA patients, variability of telomere shortening among subsets of leukocytes is less evident in SDS patients, making this explanation unlikely. High telomere length heterogeneity is a hallmark of ALT activation,9 suggesting that this pathway might be activated in SBDS-deficient patients. Although ALT activation has been clearly demonstrated only in aberrant situations, there is indirect evidence that ALT might be induced in normal hematopoietic cells26 and immortalized lymphocytes.27 Another feature of ALT cells is the association of promyelocytic leukemia (PML) protein bodies to telomeres,9 but in our patients' lymphocytes we were unable to detect any ALT-associated PML bodies or a physical association between SBDS and PML (data not shown). However, ALT-associated PML bodies are not found in all ALT cells28 and some telomerase-negative human cells in which telomeres are maintained in the absence of ALT-associated PML bodies.29
The clinical relevance of SBDS mutation in the course of marrow failure in our patients with acquired AA was suggested by their poor outcomes. AA in children generally responds to immunosuppression: approximately 70% recover and more than 90% survive.7 Of our 4 patients, 3 never responded to immunosuppression and the fourth relapsed after a short partial response. One additionally later developed myelodysplasia with monosomy 7; one third of SDS patients eventually develop chromosome 7 abnormalities during the course of their disease,1 and monosomy 7 also is the most common cytogenetic abnormality in patients with acquired AA.23 Two patients have died. Although the number of patients is small, these results indicate that screening patients with acquired AA, especially young patients, may have clinical importance to determine therapeutic options.
In conclusion, whereas mutations in both SBDS gene alleles cause SDS, we found that heterozygosis for 258 + 2 T>C SBDS mutation was a risk factor for acquired AA. SBDS deficiency appears to predispose to marrow failure by causing telomere shortening of leukocytes via a telomerase-independent pathway. Shwachman-Diamond syndrome joins DKC as a constitutional marrow failure syndrome sharing its genetic basis with acquired AA. Though diverse in its genetic origins, telomere shortening of leukocytes seems to be a common pathway leading to aplastic anemia in both acquired and constitutional bone marrow failure syndromes.
Acknowledgments
This work was supported by the National Institutes of Health (NIH) Intramural Research Program.
The authors are grateful to Olga Nuñez for assisting in patient care, Irma Vulto for her excellent technical assistance, and Orian Shirihai for valuable critical review.
National Institutes of Health
Authorship
Contribution: R.T.C., S.A.G., K.L.W., and S.K. performed experiments; P.M.L. performed flow-FISH analysis; P.J.A. and Y.D. provided SDS patients' samples; S.J.C. provided healthy control samples; R.T.C., S.A.G., and N.S.Y. analyzed data and wrote the paper; Y.D., P.J.A., S.J.C., and P.M.L. analyzed data and helped write the paper. R.T.C. and S.A.G. contributed equally to this work.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Rodrigo T. Calado, Hematology Branch, NHLBI/NIH, 10 Center Dr, Bldg 10/CRC, Rm 3E-5140, Bethesda, MD 20892-1202; e-mail: calador@nhlbi.nih.gov.