Abstract
Scurfy mice develop CD4 T-cell–mediated lymphoproliferative disease leading to death within 4 weeks of age. The scurfy mutation causes loss of function of the foxp3 gene (foxp3sf), which is essential for development and maintenance of naturally occurring regulatory CD4 T cells (nTregs). In humans, mutations of the foxp3 gene cause immune dysregulation, polyendocrinopathy, enteropathy, and X-linked syndrome (IPEX). In most patients with IPEX and also in scurfy mice, T cells show hyperreactivity and levels of Th1- and Th2-associated cytokines are substantially elevated. We report that removal of CD28 expression rescued scurfy mice from early death. Longer-term surviving CD28-deficient scurfy mice still had lymphoproliferative disorder, but their CD4 T cells showed decreased interferon-γ and no sign of interleukin-4 or interleukin-10 hyperproduction. Furthermore, injection of CTLA4-Ig to block CD28-B7 interactions substantially improved the survival of scurfy mice by blocking effector T-cell differentiation. These data support the hypothesis that CD28-B7 interactions play a critical role in the etiology of lethal autoimmune disease in scurfy mice by stimulating the differentiation of antigen-activated naive T cells into effector T cells.
Introduction
The scurfy mutation causes loss of function of the foxp3 gene, which is essential for development and maintenance of naturally occurring regulatory CD4+CD25+ T cells (nTregs).1 Disease in scurfy mice is characterized by lymphocytic infiltration of lymph nodes, spleen, liver, and skin.2 CD4+ T cells from these mice were hyperresponsive to T-cell receptor (TCR) stimulation and produced highly elevated levels of cytokines, including interleukin (IL)-2, -4, -5, -10, -13, interferon (IFN)-γ, granulocyte-macrophage colony-stimulating factor, and tumor necrosis factor-α.3,4 These hyperreactive T cells were relatively resistant to immunosuppressive drugs.4,5 When adoptively transferred into nu/nu recipients, CD4 T cells from scurfy mice induced a rapid wasting disease indicating that these cells play a central role in scurfy disease development.6
CD28 plays a pivotal role in antigen–peptide-induced proliferation of naive T cells,7 whereas CD28 costimulation increases the level of IL-2 mRNA by transcriptional and posttranscriptional mechanisms,8–10 up-regulates expression of the antiapoptotic protein Bcl-XL that promotes cell survival,11–13 and elevates antigen receptor proximal events by strengthening immunologic synapse.14,15 CD28 dependency decreases under conditions of high antigenic load or in the presence of high avidity peptide agonists.16 In contrast to naive T cells, proliferation and cytokine production by recently activated T cells and memory T cells are less dependent on CD28 costimulation.17 CD28/B7 costimulation plays a critical role also in the control of Th1/Th2 balance.7 Differentiation of naive CD4+T cells toward Th2, but not Th1 phenotype, is dependent on CD28/B7 costimulation both in vitro and in vivo.18,19
CTLA-4 is an inhibitory cell surface receptor of the CD28 family and down-regulates T cell responses. Although CD28 is constitutively expressed on resting and activated T cells, CTLA-4 is not expressed by resting T cells but is induced on activation.20 CD28 and CTLA-4 compete for binding to CD80 (B7.1) and CD86 (B7.2). Studies suggest that CD86 preferentially interacts with CD28, in contrast to CD80, which binds to CTLA-4 more strongly than to CD28.21 Thus, a fusion protein consisting of the extracellular domain of CTLA-4 and the Fc part of IgG, CTLA4Ig, through its binding to CD80 and CD86 inhibits CD28-mediated positive signals and blocks T-cell responses to prevent autoimmune diseases and transplant rejections (reviewed in 22 ). Disruption of the CTLA-4 gene leads to uncontrolled T cell lymphoproliferation in mice leading to death by 3 to 4 weeks after birth. Lymphoproliferation in CTLA-4-deficient mice is mediated by CD28 signaling, because mice lacking all 3 molecules, CTLA-4, B7.1, and B7.2 (ctla-4−/−, b7.1−/−, 7.2−/−), or both CTLA-4 and CD28 (ctla-4−/−cd28−/−) showed no hyperproliferation.23,24 It should be noted that nTregs constitutively express higher amounts of CTLA-4 than do naive conventional T cells, and previous studies have implicated a role for CTLA-4 in the suppressive mechanism used by nTregs.25,26
CD28 also plays a critical role in autoimmunity. Studies using antigen-induced experimental autoimmune encephalomyelitis, a model of human multiple sclerosis, showed that disruption of CD28/B7 interactions at the time of immunization was associated with a reduction in the severity of the autoimmune disease.27–29 Blockade of CD28/B7 interactions also reduced disease severity in mouse models of myocarditis,30 arthritis,31 thyroiditis,32 and myasthenia gravis.33 In the New Zealand black/New Zealand white mouse strain, which develops an antibody-dependent lupus-like autoimmune syndrome, treatment with CTLA4Ig or a combination of anti-B7–1 and anti-B7–2 antibodies prevented the development and progression of the disease.34,35
We examined the role of CD28 in the pathogenesis of autoimmune disease development in scurfy mice. The data demonstrate that CD28 plays a pivotal role in the early death of mice with the scurfy mutation. However, lymphoproliferative disorders of CD28-deficient scurfy mice remained as severe as those of CD28-sufficient scurfy mice, showing that aberrant T-cell proliferation caused by loss of nTregs is not sufficient to cause the early death of scurfy mice.
Materials and methods
Mice
Female scurfy mice, CD28 knockout mice (backcrossed to C57BL/6 for either 8 or 12 generations, respectively), and C57BL/6 mice were purchased from Jackson Laboratory (Bar Harbor, ME). Female foxp3sf/+ mice were crossed with cd28−/− males to obtain foxp3sf/Ycd28+/− and foxp3sf/+cd28+/− progeny. Female foxp3sf/+cd28+/− mice were then backcrossed with cd28−/− males to obtain foxp3sf/Ycd28−/− males. From this mating, we screened 4 litters and obtained 3 cd28−/−scurfy males, which were mated with cd28−/−foxp3sf/+ females to generate cd28−/−scurfy males and females. Mice carrying the scurfy mutation or CD28 gene disruption were identified by polymerase chain reaction using primers and conditions as suggested by Jackson laboratory. All procedures have been reviewed and approved by the Medical College of Georgia Institutional Animal Care and Use Committee (IACUC).
Antibodies and flow cytometry
Fluorochrome- and biotin-coupled monoclonal antibodies directed against CD4 (RMA 4–5), CD8 (53–6.7), CD62L (MEL-14), CD25 (7D4), CD3 (17A2), and DX5 were purchased from BD Biosciences (San Jose, CA). Anti-CD3 (2c11) and CD28 (37–51) were from eBiosciences (San Diego, CA). Stained cells were analyzed on a FACS Calibur (BD Biosciences).
T-cell proliferation and cytokine assay
Defined T-cell populations were sorted by Mo-Flo (Dako Colorado, Fort Collins, CO) or FACSaria (BD Biosciences) cell sorter. Lymph node T cells were purified by panning or a Mo-Flo cell sorter and were cultured with plate-bound anti-CD3 and soluble anti-CD28 antibody (0.5 mg/mL) in RPMI 1640 supplemented with 5% fetal calf serum (FCS), penicillin/streptomycin, 2 mM l-glutamine, and 50 μM 2-mercaptoethanol in 96-well plates. After 48 hours culture, supernatant (50 μL) was collected for cytokine analysis and plates were then pulsed with 0.5 μCi/well of [3H]-thymidine to measure proliferation. Cytokine production was analyzed by enzyme-linked immunosorbent assay as described.36
Histology
Tissues were fixed in 10% buffered formalin and embedded in paraffin. Hematoxylin and eosin–stained sections (5 μm) were mounted with Cytoseal60 (Richard Allan Scientific, Kalamazoo, MI) and analyzed by light microscopy. Images were acquired at room temperature using an Olympus Provis (Model AX70TRF; Tokyo, Japan) microscope equipped with Spot Camera (model 1.3.0; Diagnostic Instruments, Sterling Heights, MI) and Spot software.
CTLA4-IgG2a treatment
One hundred micrograms CTLA4-IgG2a in 50μL phosphate-buffered saline (PBS) was injected intraperitoneally on days 7, 11, 15, and 19 postpartum.
Results
Loss of CD28 expression rescues scurfy mice from early death
To examine the role of CD28 in the pathogenesis of scurfy mice, we bred female (foxp3sf/+) mice with cd28 gene knockout (cd28−/−) mice. Male F1 mice with a cd28+/−foxp3sf genotype (n = 9) survived longer than parental scurfy mice (n = 9) (mean survival time 63 days and 24 days, respectively). From F1 female cd28+/−foxp3sf/+ mice, we derived male cd28−/−foxp3sf mice (n = 10) 50% of which survived longer than 200 days and were fertile (Figure 1A). These data demonstrate that CD28 is a component of the lethal autoimmune disease caused by the scurfy mutation.
State of T cells from CD28 deficient scurfy mice. (A) Lifespan of scurfy (sf), CD28 heterozygous scurfy [sf/CD28(+/−)], and CD28-deficient scurfy [sf/CD28(−/−)] mice (P < .001). (B) Total number of live cells present in inguinal lymph nodes (left panel) and subpopulations of lymph node cells (right panel). CD4+ cells: □, CD8+ cells: ▒, B220+ cells: ■, (mean ± SD, n = 3). (C) CD4/CD8 phenotypes of lymph node cells (top row) and thymocytes (bottom row). Cells from 25-day-old C57BL/6 (WT), CD28-deficient scurfy [CD28(−/−) scurfy], and cd28+/+scurfy (scurfy) mice were stained for CD4 and CD8 and analyzed by flow cytometry. Numbers in each quadrant indicate the percentage of cells in each region against the total. (D) Total number of live cells present in each thymic lobe from C57BL/6 (WT), CD28-deficient (CD28-), CD28-deficient scurfy [CD28- scurfy], and CD28-sufficient scurfy (scurfy) mice (mean ± SD, n = 3).
State of T cells from CD28 deficient scurfy mice. (A) Lifespan of scurfy (sf), CD28 heterozygous scurfy [sf/CD28(+/−)], and CD28-deficient scurfy [sf/CD28(−/−)] mice (P < .001). (B) Total number of live cells present in inguinal lymph nodes (left panel) and subpopulations of lymph node cells (right panel). CD4+ cells: □, CD8+ cells: ▒, B220+ cells: ■, (mean ± SD, n = 3). (C) CD4/CD8 phenotypes of lymph node cells (top row) and thymocytes (bottom row). Cells from 25-day-old C57BL/6 (WT), CD28-deficient scurfy [CD28(−/−) scurfy], and cd28+/+scurfy (scurfy) mice were stained for CD4 and CD8 and analyzed by flow cytometry. Numbers in each quadrant indicate the percentage of cells in each region against the total. (D) Total number of live cells present in each thymic lobe from C57BL/6 (WT), CD28-deficient (CD28-), CD28-deficient scurfy [CD28- scurfy], and CD28-sufficient scurfy (scurfy) mice (mean ± SD, n = 3).
Despite the increased survival, 3-week-old cd28−/− male scurfy mice exhibited splenomegaly and lymphadenopathy similar to those of age-matched cd28+/+ male scurfy mice (Figure 1B; Figure S1, available on the Blood website; see the Supplemental Materials link at the top of the online article). Substantially increased cellularities were observed in the lymph nodes of cd28−/−scurfy mice relative to cd28+/+scurfy mice, mainly as a result of an increase in the numbers of T and B cells. Although lower percentages of CD4+ cells were present in cd28−/−scurfy mice, cell numbers of CD4+ cells were equivalent between cd28+/+ and cd28−/−scurfy mice (Figure 1B). Similar patterns of elevation in cell numbers also occurred in the spleens (data not shown). These data show that prolonged survival did not correlate with decreased T-cell expansion in cd28−/−scurfy mice.
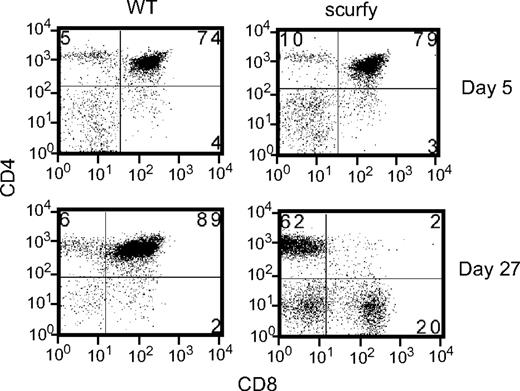
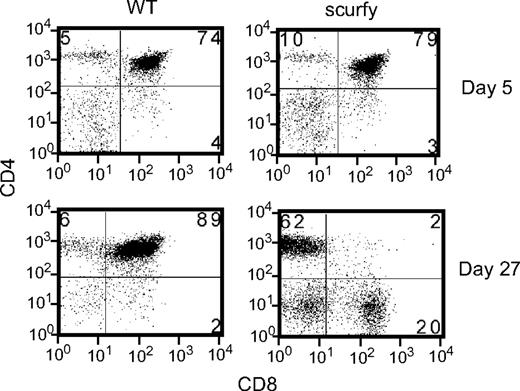
In contrast, the number of thymocytes in 3-week-old cd28−/−scurfy mice showed a profound difference compared with cd28+/+scurfy mice (Figure 1C). cd28+/+scurfy mice thymi were involuted (Figure S1) and had 40-fold lower numbers of cells than in wild-type and cd28−/− mice (Figure 1D). Few CD4+CD8+ cells were present in cd28+/+scurfy mouse thymi (Figure 1C), whereas thymi from cd28−/−scurfy mice displayed the same levels of surface antigen expression as thymi from wild-type mice (Figure 1D). A recent report showed a potential defect in thymic stroma cells of scurfy mice that caused impairment of CD4−CD8− cell thymopoesis.37 We found similar percentages and numbers of CD4+CD8+ cells in 5-day-old cd28+/+scurfy mice as well as in cd28+/+ mice (Figure 2). Thus, the data presented here suggest that FoxP3 does not itself play a role in thymopesis and the absence of CD4+CD8+ cells in 3-week-old scurfy mice may be the result of an effect of the thymic and/or peripheral environment of scurfy mice with advanced autoimmune disease.
Thymocytes from 5- and 27-day-old scurfy and wild-type mice were analyzed for CD4 and CD8 expression. Numbers in each quadrant indicate the percentage of cells in each region against the total.
Thymocytes from 5- and 27-day-old scurfy and wild-type mice were analyzed for CD4 and CD8 expression. Numbers in each quadrant indicate the percentage of cells in each region against the total.
Impaired effector cytokine production by −/−CD4 T cells from cd28−/−scurfy mice
In the periphery of cd28+/+scurfy mice, approximately 50% of CD4 T cells showed downmodulation of CD3, whereas in cd28−/−scurfy mice, this was only 10% of CD4 T cells (Figure 3A). Moreover, more than 50% of cd28+/+scurfy mice CD4 T cells were CD44highCD62L−, consistent with an effector/memory phenotype. In contrast, the CD4 T-cell compartment in cd28−/−scurfy mice contained only 16% of CD44highCD62L− cells, whereas the majority (54%) were found to be CD44lowCD62L−. More than 50% of CD4 T cells from wild-type mice were CD44lowCD62L+ (Figure 3B). Thus, a majority of the population of CD4+ T cells in cd28−/−scurfy mice T cells displayed a phenotype intermediate between activated T cells in cd28+/+scurfy mice and resting naive T cells in wild-type mice.
Reactivity of T cells from CD28-deficient scurfy mice. (A,B) In vivo activation of cd28−/−scurfy mouse T cells. Lymph node cells from mice indicated above each panel were stained for CD4, CD8, CD3, CD44, and CD62L. Shown are (A) CD3 and (B) CD44 and CD62L staining on CD4+CD8−-gated cells. In panel A, numbers in each subpanel indicate the percentage of cells in each region against the total. In panel B, numbers in or above each quadrant indicate the percentage of cells in each region against the total. (C) Proliferative response of T cells from cd28−/−scurfy mouse. Purified T cells from cd28−/− and cd28+/+ mice were stimulated with anti-CD3 (left panel) or anti-CD3+anti-CD28 (right panel) antibodies. Proliferation of T cells was measured in response to titrated doses of anti-CD3, respectively, by 3H-thymidine incorporation (mean ± SD, n = 3). (D) Cytokine production by T cells from cd28−/−scurfy mice. Mo-Flo sorted TCR+CD4+DX5− cells (2 × 104 cells/well in flat-bottomed 96-well plate) were activated with 1 μg/mL anti-CD3-coated plates in the absence □, or presence ■, of anti-CD28 antibody (0.5 μg/mL). Forty-eight hours later, a portion of culture supernatant was harvested for analysis of cytokines as indicated on the top of the panels and culture supernatant from stimulated T cells were analyzed for cytokines denoted above each panel by enzyme-linked immunosorbent assay. CD4 T cells from wild-type (WT), cd28−/− [28(-)sf)] and cd28+/+scurfy (sf) mice were analyzed (mean ± SD, n = 2).
Reactivity of T cells from CD28-deficient scurfy mice. (A,B) In vivo activation of cd28−/−scurfy mouse T cells. Lymph node cells from mice indicated above each panel were stained for CD4, CD8, CD3, CD44, and CD62L. Shown are (A) CD3 and (B) CD44 and CD62L staining on CD4+CD8−-gated cells. In panel A, numbers in each subpanel indicate the percentage of cells in each region against the total. In panel B, numbers in or above each quadrant indicate the percentage of cells in each region against the total. (C) Proliferative response of T cells from cd28−/−scurfy mouse. Purified T cells from cd28−/− and cd28+/+ mice were stimulated with anti-CD3 (left panel) or anti-CD3+anti-CD28 (right panel) antibodies. Proliferation of T cells was measured in response to titrated doses of anti-CD3, respectively, by 3H-thymidine incorporation (mean ± SD, n = 3). (D) Cytokine production by T cells from cd28−/−scurfy mice. Mo-Flo sorted TCR+CD4+DX5− cells (2 × 104 cells/well in flat-bottomed 96-well plate) were activated with 1 μg/mL anti-CD3-coated plates in the absence □, or presence ■, of anti-CD28 antibody (0.5 μg/mL). Forty-eight hours later, a portion of culture supernatant was harvested for analysis of cytokines as indicated on the top of the panels and culture supernatant from stimulated T cells were analyzed for cytokines denoted above each panel by enzyme-linked immunosorbent assay. CD4 T cells from wild-type (WT), cd28−/− [28(-)sf)] and cd28+/+scurfy (sf) mice were analyzed (mean ± SD, n = 2).
Despite the surface antigen phenotypes of mild activation, T cells from cd28−/−scurfy mice responded to anti-CD3 stimulation in vitro as vigorously as those from age-matched cd28+/+scurfy mice (Figure 3C). Addition of anti-CD28 antibody did not enhance the proliferation of T cells from either cd28−/− or cd28+/+scurfy mice but did increase the proliferation of T cells from wild-type mice. Moreover, when stimulated with anti-CD3, CD4 T cells from cd28−/−scurfy mice produced much higher levels of IL-2 compared with CD4 T cells from cd28+/+scurfy mice (Figure 3D). Elevation of IL-2 production was also observed in CD8 and natural killer T (NKT) cells from cd28−/−scurfy mice (Figure S2).
Most notably, anti-CD3 Ab–stimulated CD4 T cells from cd28−/−scurfy mice produced levels of IL-4 and IL-10 comparable to those from wild-type mice but exhibited a more than 500-fold reduction compared with CD4 T cells from cd28+/+scurfy mice. Engagement of CD28 on cd28+/+scurfy mice T cells did not change the level of IL-4 production and only slightly enhanced IL-10 production, indicating that these cells are already CD28-independent in terms of IL-4 and IL-10 production. CD4 T cells from cd28−/−scurfy mice also produced much less IFN-γ compared with CD4 T cells from cd28+/+scurfy mice.
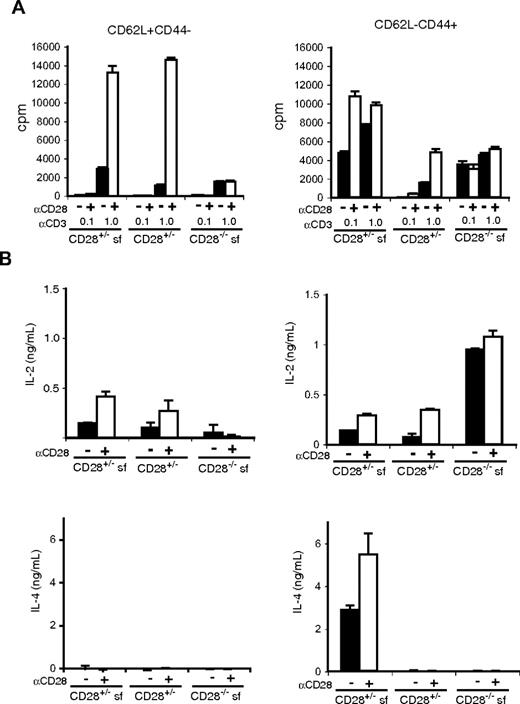
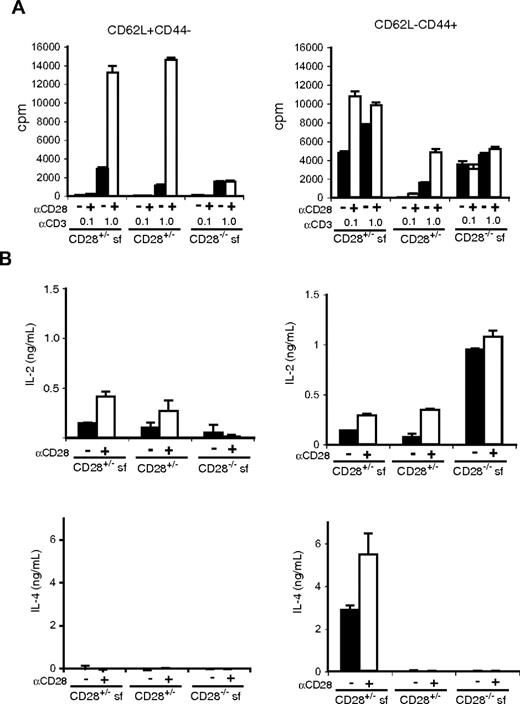
To determine whether subpopulations of CD4 T cells have differences in their ability to produce effector cytokines, we compared TCR-induced responses of CD62L+CD44− (naive phenotype) and CD62L−CD44+ (effector/memory phenotype) CD4+CD25− T cells from wild-type, scurfy, and cd28−/−scurfy mice (Figure 4). We used groups of cd28+/−, cd28+/−scurfy, and cd28−/−scurfy mice because they can be obtained as littermates. Anti-CD3 stimulation induced substantial proliferation of naive (CD62L+CD44−) CD4 T cells from all genotypes and we observed no hyperreactivity in cells from cd28−/−scurfy mice (Figure 4A). CD28 costimulation augmented the anti-CD3 induced proliferation of naive CD4 T cell from both wild-type and scurfy mice by 6- to 10-fold, whereas it had no effect on the proliferation of cd28−/−scurfy naive T cells (Figure 4A). In contrast, effector/memory type CD4+ T cells (CD62L−CD44+) from cd28+/− and cd28−/−scurfy mice were hyperresponsive to anti-CD3 stimulation compared with their wild-type counterparts. Addition of anti-CD28 induced a 2.2-fold increase in proliferation of CD4 T cells from scurfy mice in the presence of 0.1 μg/mL anti-CD3 and a 3.1-fold increase was observed in CD4 T cells from wild-type mice activated with 1 μg/mL anti-CD3 antibody (Figure 4A).
Responses of naive and activated CD4 T cells from CD28−/−scurfy mice. CD4+CD25− NK1.1− populations from mice as indicated were sorted into CD62L+CD44− and CD62L−CD44+ populations. Sorted cells (1.5 × 104 cells/well in flat-bottomed 96-well plate) were activated in triplicate wells coated with 0.1 or 1 μg/mL of anti-CD3 in the presence or absence of anti-CD28 antibody (0.5 μg/mL). (A) Proliferation (mean ± SD, n = 3) and (B) cytokine production (mean ± SD, n = 2) was determined as in Figure 3.
Responses of naive and activated CD4 T cells from CD28−/−scurfy mice. CD4+CD25− NK1.1− populations from mice as indicated were sorted into CD62L+CD44− and CD62L−CD44+ populations. Sorted cells (1.5 × 104 cells/well in flat-bottomed 96-well plate) were activated in triplicate wells coated with 0.1 or 1 μg/mL of anti-CD3 in the presence or absence of anti-CD28 antibody (0.5 μg/mL). (A) Proliferation (mean ± SD, n = 3) and (B) cytokine production (mean ± SD, n = 2) was determined as in Figure 3.
Next we analyzed cytokine production by naive and effector/memory CD4 T cells. When stimulated in vitro with anti-CD3, naive CD4 T cells from cd28−/−scurfy mice produced less IL-2 than those from cd28+/− wild-type or cd28+/−scurfy mice (Figure 4B). CD28 costimulation clearly augmented IL-2 production by cd28+/−scurfy mouse T cells indicating that CD28 plays a critical role in IL-2 production even with the scurfy background. In contrast, effector/memory CD4 T cells from cd28−/−scurfy mice made significantly higher levels of IL-2 compared with CD4 T cells from cd28+/−scurfy and cd28+/− wild-type mice. Although the levels of IL-2 production by CD4 T cells from cd28+/−scurfy and wild-type mice were augmented by anti-CD28 stimulation, they did not reach the levels of IL-2 production by CD4 T cells from cd28−/−scurfy mice.
As expected, naive CD4 T cells from all genotypes failed to produce IL-4, and effector/memory CD4 T cells from cd28+/−scurfy mice produced significantly higher amounts of IL-4 (Figure 4B) and IL-10 (Figure S2) than CD4 T cells from cd28+/− or cd28−/−scurfy mice. A small elevation was observed in the level of IFN-γ production by naive and effector/memory T cells from cd28+/−scurfy mice compared with that by T cells from cd28+/− wild-type and cd28−/−scurfy mice (Figure S2). Taken together, effector/memory (CD62L−CD44+) CD4 T cells from cd28−/−scurfy mice showed patterns of cytokine production clearly distinct from those of T cell from cd28+/−scurfy mice. These data indicate that T cells from cd28−/− mice are defective in the differentiation process from the IL-2–producing stage into the Th2 (and Th1 to a lesser degree) type cytokine-producing cells.
NK T cells from cd28−/−scurfy mice also produced less than 10% IL-4 and IL-10 compared with NK T cells from cd28+/+scurfy mice (Figure S3). On the other hand, IFN-γ production by CD8 T cells and NK T cells was similar between cd28−/− and cd28+/+scurfy mice compared with wild-type mice.
Differences between CD28-deficient and -sufficient scurfy mice in vivo
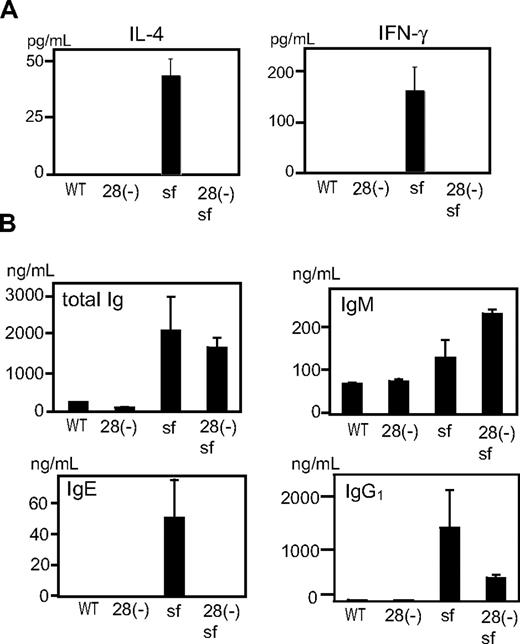
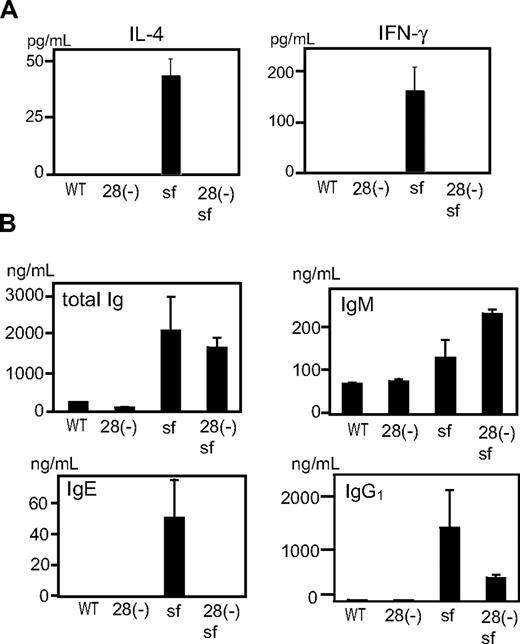
To test whether differences in cytokine production reflected the state of T cells in vivo, we examined sera from cd28−/− and cd28+/+scurfy mice. Whereas sera from cd28+/+scurfy mice showed extremely high levels of IL-4 and IFN-γ, these cytokines were undetectable in sera from both wild-type and cd28−/−scurfy mice (Figure 5A). Moreover, as observed in patients with immune dysregulation, polyendocrinopathy, enteropathy, X-linked syndrome (IPEX),4 IgE and IgG1 levels were highly elevated in the sera of cd28+/+scurfy mice compared with cd28−/−scurfy and wild-type mice (Figure 5B). Although IgM levels were significantly higher in cd28−/−scurfy mice than in cd28+/+scurfy mice, levels of total Ig were comparable.
Analysis of in vivo lymphocyte functions of cd28−/−scurfy mice. Serum from cd28−/− and cd28+/+scurfy mice were analyzed for (A) interleukin-4 and interferon-γ, and (B) immunoglobulin isotypes. WT: C57BL/6, 28(-): cd28−/− mice with C57BL/6 background, 28(-) sf: cd28−/−scurfy mice, sf: cd28+/+scurfy mice (mean ± SD, n = 3).
Analysis of in vivo lymphocyte functions of cd28−/−scurfy mice. Serum from cd28−/− and cd28+/+scurfy mice were analyzed for (A) interleukin-4 and interferon-γ, and (B) immunoglobulin isotypes. WT: C57BL/6, 28(-): cd28−/− mice with C57BL/6 background, 28(-) sf: cd28−/−scurfy mice, sf: cd28+/+scurfy mice (mean ± SD, n = 3).
In addition to elevated cytokine production, the disease of scurfy mice is characterized by multiorgan tissue destruction by infiltrating lymphocytes.1 Mononuclear infiltrates were present in the lungs (Figure S4A) and livers (Figure S4B) of 3-week-old cd28+/+scurfy mice, whereas no major infiltrates were detected in either of these organs from cd28−/−scurfy mice.
CTLA4-Ig injection rescues scurfy mice from early death
The data presented here demonstrated that CD28 plays a pivotal role in disease development in scurfy mice. This predicts that blockade of CD28 signaling in scurfy mice will protect them from lethal disease. To test this prediction, we examined whether injection of soluble CD152 (CTLA4)-Ig into scurfy mice blocks progression of disease. CTLA4-Ig is a fusion protein that has been used successfully to block CD28 function by inhibiting interactions between B7 molecules and CD28 both in vitro and in vivo.38 Litters of scurfy mice of unknown phenotype were injected with CTLA4-Ig (100 μg) or PBS every 4 days from day 7 until day 19. After treatment, litters of mice were either killed at day 22 for analysis or monitored further without any additional injections.
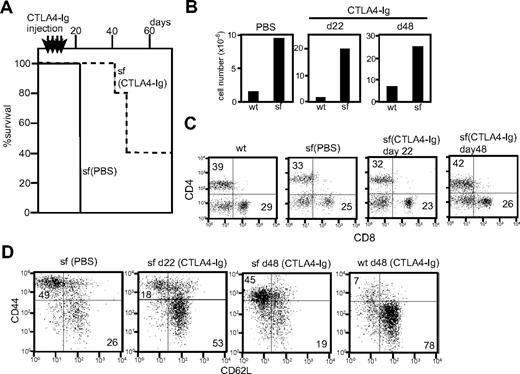
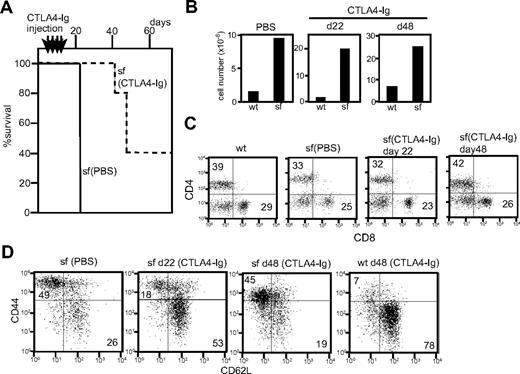
Scurfy mice (n = 5) injected with CTLA-4-Ig showed a markedly increased lifespan (Figure 6A). No visible symptoms were observed at day 22 and the first scurfy mouse to become moribund was killed at day 42. Two of the remaining mice became moribund at day 48 and 2 others remained healthy past day 70. Because CTLA4-Ig injection was stopped at day 19, the data suggest that continuous costimulatory blockade is important to prevent lethality of scurfy disease.
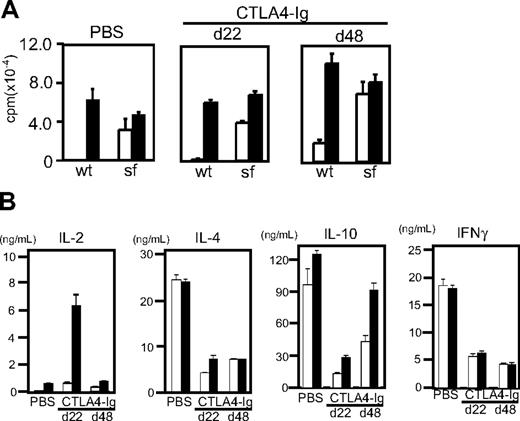
Effect of CTLA4-Ig injection in scurfy mice. (A) Lifespan of CTLA4-Ig–injected (dotted line, n = 5) and phosphate-buffered saline–injected (solid line, n = 3) scurfy mice. Intraperitoneal CTLA4-Ig injection took place every 4 days from day 7 until day 19 after birth (shown above the panel) (P < .001). (B) Total cell number of inguinal lymph nodes from phosphate-buffered saline– and CTLA4-Ig–injected scurfy (sf) and wild-type (wt) mice. (C,D) Surface antigen analysis of T cells from CTLA4-Ig–injected scurfy mice. Numbers in each quadrant indicate the percentage of cells in each region against the total. (C) CD4 and CD8 and (D) CD44 and CD62L (CD4 gated) surface phenotypes of inguinal lymph node cells from wild-type and scurfy mice. Cells were isolated from CTLA4-Ig–injected scurfy mice at day 22 (d22) and day 48 (d48) after birth. As a control, data from phosphate-buffered saline–injected scurfy mice at day 22 are shown.
Effect of CTLA4-Ig injection in scurfy mice. (A) Lifespan of CTLA4-Ig–injected (dotted line, n = 5) and phosphate-buffered saline–injected (solid line, n = 3) scurfy mice. Intraperitoneal CTLA4-Ig injection took place every 4 days from day 7 until day 19 after birth (shown above the panel) (P < .001). (B) Total cell number of inguinal lymph nodes from phosphate-buffered saline– and CTLA4-Ig–injected scurfy (sf) and wild-type (wt) mice. (C,D) Surface antigen analysis of T cells from CTLA4-Ig–injected scurfy mice. Numbers in each quadrant indicate the percentage of cells in each region against the total. (C) CD4 and CD8 and (D) CD44 and CD62L (CD4 gated) surface phenotypes of inguinal lymph node cells from wild-type and scurfy mice. Cells were isolated from CTLA4-Ig–injected scurfy mice at day 22 (d22) and day 48 (d48) after birth. As a control, data from phosphate-buffered saline–injected scurfy mice at day 22 are shown.
CTLA4-Ig-injected scurfy mice killed on day 22 showed phenotypes in their T-cell compartment comparable with those of cd28−/−scurfy mice. Lymphoid organs of CTLA4-Ig-injected scurfy mice (day 22) were enlarged and the cell numbers were highly elevated compared with wild-type (Figure 6B). Analysis of the surface antigens showed that CTLA4-Ig injection did not alter the CD4/CD8 ratio but reduced the numbers of CD44highCD62L− T cells in scurfy mice compared with PBS-injected scurfy mice (Figures 6C,D). At day 48, however, T cells from CTLA4-Ig-injected scurfy mice were predominantly CD44high CD62L− as was also observed in PBS-injected scurfy mice, indicating that self-reactive T cells are fully activated by this time.
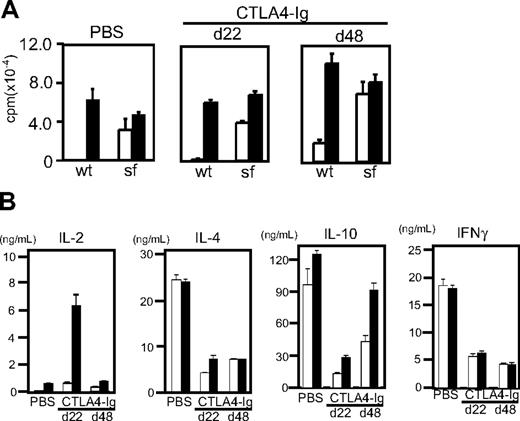
T cells from CTLA4-Ig–injected and PBS-injected scurfy mice were equally reactive to anti-CD3 stimulation and proliferated vigorously without CD28 stimulation (Figure 7A). Yet cytokine production by T cells from day 22 CTLA4-injected scurfy mice showed clear differences from those of PBS-injected scurfy mouse T cells (Figure 7B). IL-2 production was elevated significantly compared with PBS-injected scurfy mice. Conversely, IL-4, IL-10, and IFN-γ production was clearly reduced in CTLA4-Ig–injected scurfy mice compared with PBS-injected mice. In addition, histology showed a clear absence of infiltration by mononuclear cells in the lungs of CTLA4-Ig–injected scurfy mice at day 22 (Figure S5).
Functional analysis of T cells from CTLA4-Ig–injected mice. CD4 T cells were isolated from CTLA4-Ig–injected scurfy mice at day 22 (d22) and day 48 (d48) after birth. As a control, data from a phosphate-buffered saline–injected scurfy mouse at day 22 are shown. T cells were stimulated with anti-CD3 (□) or anti-CD3 and CD28 (■). (A) 3H-thymidine uptake of T cells after 72 hours of stimulation (proliferation) (mean ± SD, n = 3). (B) Interleukin-2, interleukin-4, interleukin-10, and interferon-γ production after 48 hours are shown. Results are representative of 3 CTLA4-Ig–injected mice (mean ± SD, n = 2).
Functional analysis of T cells from CTLA4-Ig–injected mice. CD4 T cells were isolated from CTLA4-Ig–injected scurfy mice at day 22 (d22) and day 48 (d48) after birth. As a control, data from a phosphate-buffered saline–injected scurfy mouse at day 22 are shown. T cells were stimulated with anti-CD3 (□) or anti-CD3 and CD28 (■). (A) 3H-thymidine uptake of T cells after 72 hours of stimulation (proliferation) (mean ± SD, n = 3). (B) Interleukin-2, interleukin-4, interleukin-10, and interferon-γ production after 48 hours are shown. Results are representative of 3 CTLA4-Ig–injected mice (mean ± SD, n = 2).
At day 48, CTLA4-Ig–injected mice showed overt symptoms of weight loss, skin lesions, and lack of mobility. CD4 T cells from 48-day-old CTLA4-Ig–injected scurfy mice produced elevated levels of IL-10 compared with their 22-day-old counterparts, whereas the levels of IL-4 and IFN-γ were comparable between day 22 and 48 CTLA4-Ig–injected scurfy mice (Figure 7B). CD4 T cells from CTLA4-Ig–treated scurfy mice, regardless of their age, produced less IL-4 and IFN-γ than those from PBS-treated scurfy mice (Figure 7B). However, the amounts of IL-4 and IL-10 produced by CD4 T cells from CTLA4-Ig–treated scurfy mice were still several-fold higher than those produced by CD4 T cells from CTLA4-Ig–injected littermate wild-type mice (data not shown). In contrast, IFN-γ production by CD4 T cells from CTLA4-Ig–injected scurfy mice was mildly increased compared with CTLA4-Ig–treated wild-type mice (data not shown). Both the PBS-injected scurfy mice (day 22) and CTLA4-Ig–injected scurfy mice (day 48) showed significant mononuclear cell infiltration in their lungs (Figure S5). These data suggest that production of IL-4, IL-10, and/or infiltration by mononuclear cells may contribute to the progression of scurfy disease.
Discussion
Our data show that CD28 plays a pivotal role in the progression of lethal autoimmune disease in scurfy mice and that survival of cd28−/−scurfy mice is unrelated to aberrant T-cell proliferation. Indeed, CD62L−CD44+ CD4 T cells from cd28−/−scurfy mice produced more IL-2 than cd28+/−scurfy mouse CD4 T cells and lack of CD28 did not impair their ability to proliferate after CD3 stimulation. The data demonstrated that in scurfy mice, CD4 T cells expand without committing to become effectors when CD28 is absent. Moreover, CD28 is also required for the process leading to tissue infiltration by lymphocytes.
Importantly, naive CD4 T cells (CD62L+CD44−) from scurfy and wild-type mice responded in a similar manner to anti-CD3 and anti-CD28 stimulation, whereas naive CD4 T cells from cd28−/−scurfy mice showed reduced ability to produce IL-2. In contrast, effector/memory CD4 T cells (CD62L−CD44+) from cd28+/− and cd28−/−scurfy mice were hyperproliferative in response to anti-CD3 stimulation. Collectively, these data suggest that hypersensitivity of scurfy CD4 T cells is not a cell intrinsic property of the scurfy background, but is most likely the result of the presence of large number of preactivated T cells caused by lack of nTregs.
In scurfy mice, activated CD4 T cells differentiate into effectors and produce IL-4, IL-10, and IFN-γ. Surprisingly, activated CD4 T cells from cd28−/−scurfy mice produced increased amounts of IL-2, decreased IFN-γ, and virtually no IL-4 and IL-10. The data demonstrate that even in the absence of nTregs, CD28 signaling plays a critical role in commitment of activated CD4 T cells into Th2 or, to a lesser extent, Th1 effectors. Failure of CD4 T cells from cd28−/−scurfy mice to produce effector cytokines is consistent with published reports (reviewed in Salomon and Bluestone7 ).
In contrast to IL-4 and IL-10, IL-2 production was much up-regulated in cd28−/−scurfy mice. Increased amount of IL-2 production by CD4 T cells from cd28−/−scurfy mice is not caused by a cell intrinsic abnormality of naive T cells in scurfy mice because they show no sign of enhanced IL-2 production. The reason why cd28−/− effector T cells make enhanced levels of IL-2 is currently unclear. Although beyond the scope of this article, one question remains to be answered: how naive CD4 T cells become activated in scurfy mice to produce IL-2 in the absence of CD28.
Previous reports showed that CD28 costimulation is not always required for T-cell proliferation and IL-2 production, especially when strong and/or sustained antigen receptor signal is provided. Thus, one potential explanation for CD28-independent IL-2 production is that naive T cells receive prolonged antigenic stimulation in the absence of nTregs. Another mutually nonexclusive possibility is the contribution from homeostatic expansion. During the neonatal period, T cells undergo homeostatic proliferation in response to lymphopenia.39 Homeostatic expansion of T cells is less dependent on CD28 than antigen-induced proliferation of T cells and can promote polyclonal proliferation (reviewed in Khoruts and Fraser40 ). Thus, the lymphopenic environment of the neonate may initiate CD28-independent activation of self-reactive CD4 T cells in scurfy mice.
The data presented here exhibit interesting similarities and differences between scurfy and ctla4−/− mice.23,24,41,42 Like scurfy mice, ctla4−/− mice develop a lymphoproliferative disorder by 3 weeks of age and die around the same time.43 cd28−/−ctla4−/− mice, b7–1−/−b7–2−/−ctla4−/− mice, and ctla4−/− mice injected with CTLA4-Ig all show dramatically extended lifespans and reduced levels of cytokine production by T cells.23,24,41,42 However, CD28-deficient and CTLA4-Ig–treated ctla4−/− mice show striking differences from CD28-deficient and CTLA4-Ig–treated scurfy mice. For example, when CD28-B7 interactions are blocked, T cells from ctla4−/− mice do not show any lymphoproliferative disorders and exhibited decreased T-cell responsiveness in vivo and in vitro. cd28−/−ctla4−/− T cells are hypoproliferative at levels comparable with those of cd28−/− mouse T cells.24 Thus, hyperproliferation of T cells caused by loss of CTLA4 requires CD28, but hyperproliferation of T cells from scurfy mice is independent of CD28. It was also shown that nTregs from CTLA4-deficient mice have uncompromised suppressive activity.44 Thus, in the absence of CD28, these nTregs may dominate the reactivity of nonregulatory T cells, whereas lack of Foxp3 may cause complete loss of suppressive function.
Our data show a potential connection between effector T-cell response and lethality in scurfy disease. CD4 T cells from CD28-deficient scurfy mice showed no sign of IL-4, IL-10, and IFN-γ hyperproduction. Similarly, a potential connection between Th2 response and lethality of scurfy mice was previously reported with cd4−/−scurfy mice.6 Moreover, levels of IL-4, IL-10, and IFN-γ are highly elevated in patients with IPEX.3,4 Thus, uncontrolled cytokine production by scurfy T cells may be responsible for lethal disease development in scurfy mice.
An intriguing observation was made in the CTLA4-Ig–injected scurfy mice when killed on day 48. These mice showed clear symptoms of scurfy disease. Because CTLA4-Ig injection was discontinued at day 19 after birth, the data suggest that continuous blockade of B7 molecules may be required to attenuate progression of scurfy disease. Alternatively, it may reflect the difference of the mode by which CTLA4-Ig works from deficiency of CD28 expression. Several reports of B7-CTLA-4 pathways of immunoregulation have been published and not all depend on B7-CD28-mediated costimulation (reviewed in Alegre and Fallarino45 ). We are currently in the process of distinguishing among the potential pathways that may lead to the observed phenotype.
Except for IL-10 levels in 48-day-old mice (which were elevated), the amounts of IL-4, IL-10, and IFN-γ produced by CD4 T cells from CTLA4-Ig–treated scurfy mice were reduced compared with CD4 T cells from PBS-injected scurfy mice. However, cytokine levels were still much higher than those produced by CD4 T cells from CTLA4-Ig–injected wild-type mice. This may be enough over time to induce disease in CTLA4-Ig–injected scurfy mice at a later time point.
In addition, tissue infiltration by lymphocytes may play a critical role in the disease development. Unlike IL-4 and IFN-γ production, scurfy mice injected with CTLA4-Ig showed significant mononuclear cell infiltration in the lung and liver at day 48. The data indicate that CD28 is required for T cells to initiate tissue invasion. This mononuclear cell infiltration may be the direct effect of a CD28 signal to activated T cells or an indirect effect through up-regulation of cytokine/chemokine signaling and/or induction of other coactivators such as ICOS.
Together, the data demonstrate the significance of CD28 for critical development of autoimmune disorder in scurfy mice. Although the mechanisms underlying the autoimmune pathology caused by mutations of human and mouse Foxp3 may be different, intervention of CD28-B7 interaction may prove beneficial in treating patients with IPEX. Such possibilities need to be explored in future analysis of the human systems.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This study was supported in part by grants from NIH (AI055022 and AI049398) and JDRF (5–2006-388) (M.I.) and from the Arthritis Foundation (Y.S.).
We thank Dr Jeff Lee for advice, MCG Flow Cytometry Core and Histology Core for support, and Joyce Wilson and Doris McCool for assistance.
National Institutes of Health
Authorship
Contribution: N.S., A.M., and M.I. designed the research; N.S., P.C., Y.S., B.B., M.T., and D.K. performed the experiments; N.S. and M.I. analyzed the data; D.H.M. and C.P.L. contributed vital reagents; and N.S. and M. I. wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Makio Iwashima, CN4155, Immunotherapy Center, MCG, 1120 15th St, Augusta, GA 30912; e-mail: miwashima@mcg.edu.

![Figure 1. State of T cells from CD28 deficient scurfy mice. (A) Lifespan of scurfy (sf), CD28 heterozygous scurfy [sf/CD28(+/−)], and CD28-deficient scurfy [sf/CD28(−/−)] mice (P < .001). (B) Total number of live cells present in inguinal lymph nodes (left panel) and subpopulations of lymph node cells (right panel). CD4+ cells: □, CD8+ cells: ▒, B220+ cells: ■, (mean ± SD, n = 3). (C) CD4/CD8 phenotypes of lymph node cells (top row) and thymocytes (bottom row). Cells from 25-day-old C57BL/6 (WT), CD28-deficient scurfy [CD28(−/−) scurfy], and cd28+/+ scurfy (scurfy) mice were stained for CD4 and CD8 and analyzed by flow cytometry. Numbers in each quadrant indicate the percentage of cells in each region against the total. (D) Total number of live cells present in each thymic lobe from C57BL/6 (WT), CD28-deficient (CD28-), CD28-deficient scurfy [CD28- scurfy], and CD28-sufficient scurfy (scurfy) mice (mean ± SD, n = 3).](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/110/4/10.1182_blood-2006-10-054585/5/m_zh80150705590001.jpeg?Expires=1765906994&Signature=iufWzBoIGmg7GfQQixs-p81okK83-nWK63Ez7H2GC701nMQYGCpeNroWywSpKHm5-aYhEphLad0-GzSy5U83F7DtOxmKOkhTIuvCjSWpSlNFFjpAEKyWIWWzWhh~3aicRwXetStBbSKb5PZdjwUC92YPmzFQprLAel6GJOw1eLJsJgN1sIqcc1Zj7sF5BjX2lc7JObiQ2Is-jIhreuqtODtMnZ~gD6qQwQzpD2CYO0JH2g43hcWRcr1DkH-nTCV7b7br4wpUzNjWrRMhfj6-VTS4a3J6rPtGYP4B8fC5EhwRQWmsK~QlUtz~J1173Wytg~v7UKi~UMaUNGxky9QRug__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Figure 3. Reactivity of T cells from CD28-deficient scurfy mice. (A,B) In vivo activation of cd28−/− scurfy mouse T cells. Lymph node cells from mice indicated above each panel were stained for CD4, CD8, CD3, CD44, and CD62L. Shown are (A) CD3 and (B) CD44 and CD62L staining on CD4+CD8−-gated cells. In panel A, numbers in each subpanel indicate the percentage of cells in each region against the total. In panel B, numbers in or above each quadrant indicate the percentage of cells in each region against the total. (C) Proliferative response of T cells from cd28−/− scurfy mouse. Purified T cells from cd28−/− and cd28+/+ mice were stimulated with anti-CD3 (left panel) or anti-CD3+anti-CD28 (right panel) antibodies. Proliferation of T cells was measured in response to titrated doses of anti-CD3, respectively, by 3H-thymidine incorporation (mean ± SD, n = 3). (D) Cytokine production by T cells from cd28−/− scurfy mice. Mo-Flo sorted TCR+CD4+DX5− cells (2 × 104 cells/well in flat-bottomed 96-well plate) were activated with 1 μg/mL anti-CD3-coated plates in the absence □, or presence ■, of anti-CD28 antibody (0.5 μg/mL). Forty-eight hours later, a portion of culture supernatant was harvested for analysis of cytokines as indicated on the top of the panels and culture supernatant from stimulated T cells were analyzed for cytokines denoted above each panel by enzyme-linked immunosorbent assay. CD4 T cells from wild-type (WT), cd28−/− [28(-)sf)] and cd28+/+ scurfy (sf) mice were analyzed (mean ± SD, n = 2).](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/110/4/10.1182_blood-2006-10-054585/5/m_zh80150705590003.jpeg?Expires=1765906994&Signature=TlqxMSp-IipywJZSSjt24dqp92aCEri9Q-2Kg5ZgUCVAZnb~ojw0UM5kD4SPBPiS~1KEQCLWzQRfww-~dOvobq4UkJeF7hQoQh4fXZgKLMCD3eyu7tCCL6pRHE9dJingosSYQtKC4vHVXBybDt6ualzfbLqL0wJYZc~TPtuJ6oac65g~0L2OTIoTwX4x1BtEadJy6rtyC~w3C4YUfQv5VwhpQeTsgVoToqmfPhR8XeZYMt3rB9IQ3ACDfuzCPcRCO~sYifTzjd~2WZQptM8UvwzUWqYQ17Hj2oJmWXCUTd67lRMqwBEWnrB3Uv-X96XLGlGoVClP7CVkR7LgflFbFg__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)

![Figure 1. State of T cells from CD28 deficient scurfy mice. (A) Lifespan of scurfy (sf), CD28 heterozygous scurfy [sf/CD28(+/−)], and CD28-deficient scurfy [sf/CD28(−/−)] mice (P < .001). (B) Total number of live cells present in inguinal lymph nodes (left panel) and subpopulations of lymph node cells (right panel). CD4+ cells: □, CD8+ cells: ▒, B220+ cells: ■, (mean ± SD, n = 3). (C) CD4/CD8 phenotypes of lymph node cells (top row) and thymocytes (bottom row). Cells from 25-day-old C57BL/6 (WT), CD28-deficient scurfy [CD28(−/−) scurfy], and cd28+/+ scurfy (scurfy) mice were stained for CD4 and CD8 and analyzed by flow cytometry. Numbers in each quadrant indicate the percentage of cells in each region against the total. (D) Total number of live cells present in each thymic lobe from C57BL/6 (WT), CD28-deficient (CD28-), CD28-deficient scurfy [CD28- scurfy], and CD28-sufficient scurfy (scurfy) mice (mean ± SD, n = 3).](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/110/4/10.1182_blood-2006-10-054585/5/m_zh80150705590001.jpeg?Expires=1765906995&Signature=fuHlcn7KGpF4a6pAisjZfQY-gFmdUzXpe3yNALWLhn5z1Vj7A2HIm3nT5X1PhzNdpj-azp3pxruI1NdeKB4UpNGNgmkjGz-dZwgXxz7F1rQUZGx8f6hjBWHcIIuqJFTfANSyE~hKNOw-xgiko4x9OebYikiUK2MTo8waALLZ7NEjTSXCJOvXTSGniQB0Skk0SW5IbvUVL8bHgjTWyYcZdaF2VhphHSoB9uFnfR4IzYLZg1TWnsaHqZwZqEbu5kfiLHB6aK0ACgUWScbG2Nh4vLvEVNuhkZ~8vCaoY8pf8oEHQ4Egg6Iju7WQlZNC4dWy9FQBLXxpYulNPtfmg-gYbA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Figure 3. Reactivity of T cells from CD28-deficient scurfy mice. (A,B) In vivo activation of cd28−/− scurfy mouse T cells. Lymph node cells from mice indicated above each panel were stained for CD4, CD8, CD3, CD44, and CD62L. Shown are (A) CD3 and (B) CD44 and CD62L staining on CD4+CD8−-gated cells. In panel A, numbers in each subpanel indicate the percentage of cells in each region against the total. In panel B, numbers in or above each quadrant indicate the percentage of cells in each region against the total. (C) Proliferative response of T cells from cd28−/− scurfy mouse. Purified T cells from cd28−/− and cd28+/+ mice were stimulated with anti-CD3 (left panel) or anti-CD3+anti-CD28 (right panel) antibodies. Proliferation of T cells was measured in response to titrated doses of anti-CD3, respectively, by 3H-thymidine incorporation (mean ± SD, n = 3). (D) Cytokine production by T cells from cd28−/− scurfy mice. Mo-Flo sorted TCR+CD4+DX5− cells (2 × 104 cells/well in flat-bottomed 96-well plate) were activated with 1 μg/mL anti-CD3-coated plates in the absence □, or presence ■, of anti-CD28 antibody (0.5 μg/mL). Forty-eight hours later, a portion of culture supernatant was harvested for analysis of cytokines as indicated on the top of the panels and culture supernatant from stimulated T cells were analyzed for cytokines denoted above each panel by enzyme-linked immunosorbent assay. CD4 T cells from wild-type (WT), cd28−/− [28(-)sf)] and cd28+/+ scurfy (sf) mice were analyzed (mean ± SD, n = 2).](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/110/4/10.1182_blood-2006-10-054585/5/m_zh80150705590003.jpeg?Expires=1765906995&Signature=C9KYjwPHjuxlg8QHjKcIAq7NVpelvYZtU8Dw8hDnU1OUE~8lzNyH2p-XItxzZvpZU7WoWllW8DWJGTA23mvLEh~O2489E0CQ-9l0rYUiPVOGDVOPeylYQDs-tRYrqBcE39rYPIfFfLuIenJonICeIbhhVrehIT~BLLk1apkfB6qKfV0PU0mTYacjMLA51jIN3xmBZyf4ptHG49kEyuc6PEXwmihtBrB3N-89gTilFIF1w9Cf5Oj8XKFT8AhRsXvh6BlKo3PwHztFy3rkysSOry19hpp4cQg5oZIwIDCNpYz~xiAXb6-wOshYPmUCyEFeUw2XDQvPLuQU0G2YDGkqSg__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)