In this issue of Blood, Hulstein and colleagues demonstrate down-regulation of platelet GPIb interaction with von Willebrand factor (VWF) by β-2 glycoprotein I (β2 GPI) and the inhibition of this down-regulation by autoantibodies to β2 GPI that are present in patients with antiphospholipid syndrome (APS).
Platelet adhesion to surface-bound plasma VWF is mediated through the platelet glycoprotein, GPIb-complex (GPIb-IX-V).1,2 Although shear plays an important role in regulating this interaction, there are known mutations in either GPIb (platelet-type von Willebrand disease [VWD]) or in VWF (type 2B VWD) that up-regulate this interaction, and mutations in VWF (type 2M VWD) that diminish this interaction.3 Although thrombosis is a widely recognized complication associated with APS, and plasma VWF is often increased in the plasma of these patients, a regulatory mechanism affecting platelet adhesion has not been previously defined. Hulstein and colleagues demonstrate that β2 GPI physiologically dampens the interaction of GPIb with VWF and this physiologic inhibition is abrogated by the presence of antibodies to β2 GPI that are present in the plasma of patients with APS.
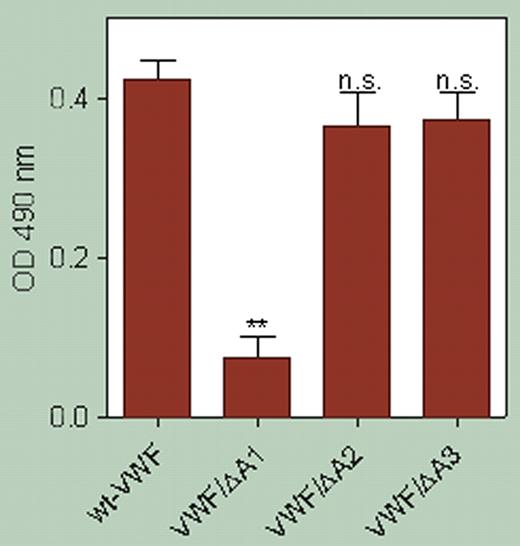
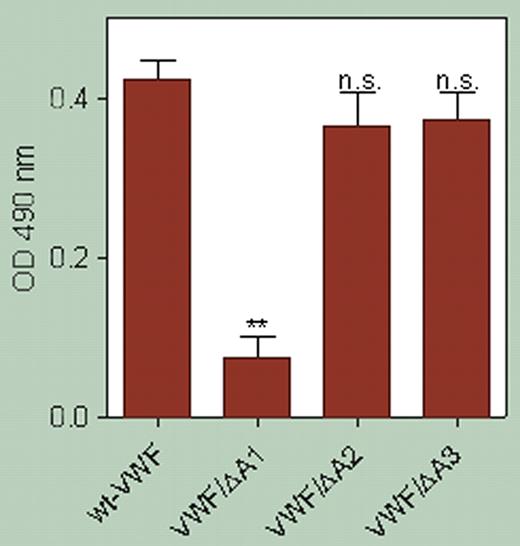
Furthermore, they demonstrate (see figure) that binding of β2 GPI to VWF involves the A1 loop of VWF, because VWF that is missing the A1 domain fails to bind. These authors go on to provide further evidence that the β2 GPI autoantibodies in these patients bind β2 GPI and abrogate this inhibition, resulting in the circulation of activated VWF. The level of activated VWF in patients with lupus anticoagulant and autoantibodies to β2 GPI matches that seen in patients with thrombotic thrombocytopenic purpura (TTP).
β2 GPI interacts with the GpIb-binding conformation of the A1 domain of VWF. **P = .003. Data represent the mean ± SD of 3 experiments. See the complete figure in the article beginning onpage 1483.
β2 GPI interacts with the GpIb-binding conformation of the A1 domain of VWF. **P = .003. Data represent the mean ± SD of 3 experiments. See the complete figure in the article beginning onpage 1483.
Another mechanism that could add to the complexity of this mechanism suggests that the complex of β2 GPI with anti–β2 GPI antibodies can activate platelets.4 Shi et al4 suggest that the effect is mediated by β2 GPI/anti–β2 GPI directly complexed with platelet GPIb, but they only indirectly speculate on its involvement with VWF. The article by Hulstein and colleagues, however, demonstrates direct binding with VWF. Could both the mechanism described by Hulstein et al in this issue of Blood and the one suggested by Shi et al4 be operating simultaneously? First, β2 GPI would dampen the interaction of VWF with platelet GPIb. The autoantibody to β2 GPI would block this dampening, resulting in activated VWF, and secondly, the formed complex between β2 GPI and anti–β2 GPI would bind to platelet GPIb and activate platelets. Obviously, further studies will be required to clarify these two mechanisms.
The thrombosis seen in patients with autoimmune diseases is undoubtedly multifactorial, but the observation of Hulstein and colleagues is novel and may be yet another cause for the increased propensity for thrombosis in this clinical setting. While a variety of antithrombotic approaches against platelets have begun to be exploited for therapeutic purposes, the interruption of the initiation phase of platelet adhesion has not been as aggressively explored, even though teliologically this step would appear to be a promising site for early intervention. Depending on the relative contribution of this current mechanism to hypercoagulability in APS, antibody or peptide inhibition of either platelet GPIb or plasma VWF could be advantageous.
Conflict-of-interest disclosure: The author declares no competing financial interests. ■