Abstract
Regulatory T cells are believed to control the development and progression of autoimmunity by suppressing autoreactive T cells. Decreased numbers of CD4+CD25+ FOXP3+ T cells (Tregs) are associated with impaired immune homeostasis and development of autoimmune diseases. The transcription factors FOXP3 and NFAT1 have key roles in regulatory T-cell development and function. We show that Tregs are decreased at presentation in almost all patients with aplastic anemia; FOXP3 protein and mRNA levels also are significantly lower in patients with aplastic anemia and NFAT1 protein levels are decreased or absent. Transfection of FOXP3-deficient CD4+CD25+ T cells from patients with a plasmid encoding wild-type NFAT1 resulted in increased FOXP3 expression in these cells. By NFAT1 knockdown in CD4+CD25+ T cells, FOXP3 expression was decreased when NFAT1 expression was decreased. Our findings indicate that decreased NFAT1 could explain low FOXP3 expression and diminished Treg frequency in aplastic anemia. Treg defects are now implicated in autoimmune marrow failure.
Introduction
A subpopulation of T cells, termed regulatory T cells (Tregs), have been described in humans and animal models; these cells are important because they suppress autoreactive T cells by direct cell contact.1-4 Phenotypically, Treg are characterized by cell surface expression of the proteins CD4 and CD25 and by intracellular expression of the transcription factor FOXP3. Only CD4+CD25hi+FOXP3+ T cells express suppressor functions. FOXP3 is a member of the forkhead/winged-helix family of transcription regulators (FOXP1–4). In humans, mutations in FOXP3 result in an autoimmune syndrome termed immune dysregulation, polyendocrinopathy, enteropathy, X-linked syndrome.5 The transcription factor NFAT1 induces FOXP3 expression by binding to its promoter.6 In addition, FOXP3 expresses its repressive effects on cytokine expression and its activating effects on CD25 by cooperation with NFAT1.7,8
Acquired aplastic anemia is characterized by destruction of hematopoietic stem cells by cytotoxic T lymphocytes.9 Hematopoietic response and hematologic recovery after successful immunosuppressive treatment represent the most powerful evidence that this rare and complex disease is immune-mediated.10 Increased T-bet protein levels in T cells probably are responsible for the increased interferon-γ levels and the Th1 polarization in patients with aplastic anemia.11
We show that Tregs were decreased in acquired aplastic anemia, as in other autoimmune diseases.12-16 All patients examined had low levels of FOXP3. Mechanistically, FOXP3 down-regulation appeared to be mediated by the transcription factor NFAT1.
Patients, materials, and methods
Patients and control subjects
Informed consent was acquired according to protocols approved by the Institutional Review Board of the National Heart, Lung, and Blood Institute (NHLBI) and was obtained in accordance with the Declaration of Helsinki for all patients (n = 20; age range, 13-52 years) with acquired aplastic anemia examined (Table S1, available on the Blood website; see the Supplemental Materials link at the top of the online article) and healthy volunteers (n = 14; age range, 18-55 years).
Lymphocyte isolation, flow cytometry, and immunoblots
CD4, CD25, and FOXP3 expression was examined in peripheral blood mononuclear cells (PBMC) by 3-color flow cytometry as previously described11 using an APC-antihuman FOXP3 staining kit (eBioscience, San Diego, CA).15 Immunoblot experiments were performed as previously described11 (Document S1).
Quantitative real-time polymerase chain reaction
FOXP3 gene expression was measured in CD4+CD25+ T cells as previously described.15 All polymerase chain reaction assays were performed in duplicate and reported as the mean.
Confocal microscopy and T-cell transfections
NFAT1 and FOXP3 expression was examined by confocal microscopy as previously described17 (Document S1). Transfections were performed18,19 using a GFP-wild-type NFAT1 plasmid20 (a gift from Dr. A. Rao, Harvard University, Cambridge, MA) and examined by confocal laser microscopy with Zeiss 510 confocal system equipped with UV_VIS lasers (Carl Zeiss, Jena, Germany). High-resolution images were obtained with a 63×, water emission objective and deconvolved using Huygens Software (SVI, Hilversun, the Netherlands), and assembled using Imaris 5.0 software (Bitplane AG, Zurich, Switzerland) (Document S1). NFAT1-siRNA was performed in CD4+CD25+ T cells based on the manufacturer's instructions. Interleukin-2 secretion was measured in culture supernatants by enzyme-linked immunosorbent assay (R&D Systems, Minneapolis, MN).
Densitometry and statistics
Densitometry of bands of interest was performed as previously described.11 Statistical analysis was performed using the t test (Prism Software, San Diego, CA); P values of less than .05 were considered statistically significant.
Results and discussion
Decreased circulating regulatory T-cell number in patients with acquired aplastic anemia
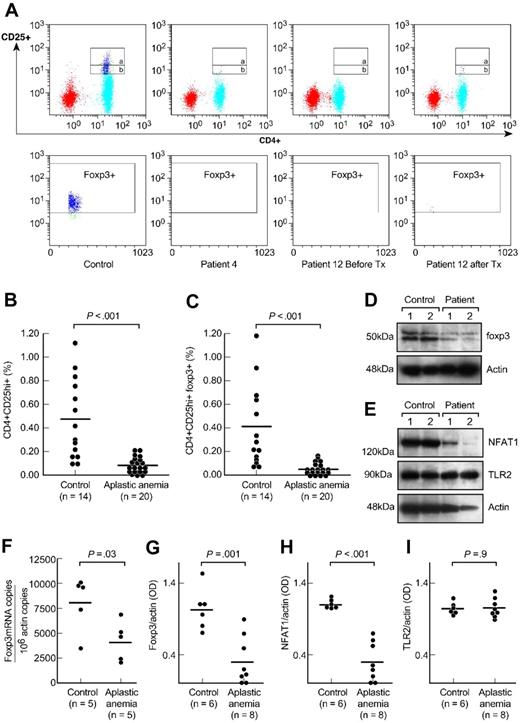
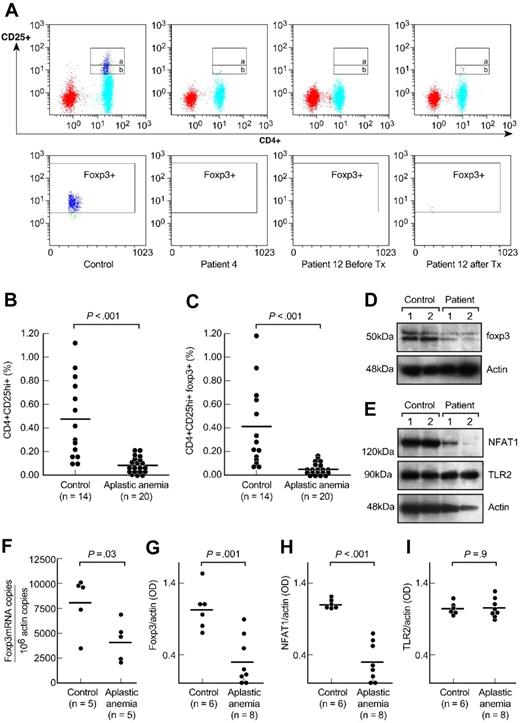
We first measured the frequency of Tregs in aplastic anemia (results are ± SEM). All patients had significantly decreased numbers of CD4+CD25hi+ T cells compared with healthy donors (0.08% ± 0.01% versus 0.46% ± 0.1%, P < .001, Figure 1A,B). The frequency of CD4+CD25hi+FOXP3+ T cells in patients also was much decreased compared with that from the control subjects (0.04% ± 0.01% versus 0.34% ± 0.1%, P < .001, Figure 1A,C). The frequency of CD4+CD25+ T cells (Figure 1A, gate a and b) from patients was also significantly decreased compared with the controls' (0.80% ± 0.32% versus 2.48% ± 0.33%, P < .001, data not shown). Absolute Treg numbers from patients (n = 20) were significantly decreased (median, 0.24 cells/μL; range, 0.05-2.3 cells/μL) compared with the healthy donors' absolute lymphocyte counts (available from 5 healthy control subjects; median, 5.9 cells/μL; range, 1.5-11 cells/μL).
CD4+CD25+ T cells in patients with aplastic anemia and in healthy control subjects. (A) Peripheral blood mononuclear cells from patients with aplastic anemia and healthy control subjects were stained with anti-CD4 and anti-CD25 antibodies followed by intracellular anti-FOXP3 antibodies. The upper gate on the dot plots (gate a) represents CD4+CD25hi+ T cells and the lower gate (gate b) the CD4+CD25+ T cells. FOXP3 expression was gated on CD4+CD25hi+ T cell population (gate a). Representative dot plots are shown from a healthy control subject, a patient with aplastic anemia, and another patient before and after immunosuppressive treatment. Tx, treatment. (B,C) Relative numbers of CD4+CD25hi+ T and CD4+CD25hi+ FOXP3+ T cells from all healthy control subjects and patients with aplastic anemia examined are shown. All patients were examined before receiving any immunosuppressive treatment. Differences between patients and control subjects were statistically significant (P < .001). (D) CD4+CD25+ T cells' nuclear extracts from healthy volunteers and patients with aplastic anemia were analyzed for FOXP3 expression. All patients examined had significantly decreased FOXP3 protein levels compared with healthy donors (P < .001). All patients' samples were analyzed side-by-side with healthy donors' samples. (E) Cytoplasmic extracts from CD4+CD25+ T cells from patients and healthy donors were analyzed by immunoblot for NFAT1 expression. All patients examined showed diminished or undetectable NFAT1 protein levels compared with healthy control subjects (P < .001). All patients' samples were run side-by-side with control subjects' samples. There were no differences in TLR2 expression between patients and control subjects. (F) RNA from CD4+CD25+ T cells from patients with aplastic anemia and control subjects was analyzed for FOXP3 gene expression in quantitative polymerase chain reaction experiments. Patients with aplastic anemia showed decreased FOXP3 mRNA/actin copies compared with healthy control subjects (P = .03). Horizontal lines represent mean values. (G-I) The densitometric intensities of immunoblot results from all the subjects studied for FOXP3, NFAT1, and TLR2 expression are collectively presented. Horizontal lines represent mean values. Results are plus or minus standard error of the mean (± SEM).
CD4+CD25+ T cells in patients with aplastic anemia and in healthy control subjects. (A) Peripheral blood mononuclear cells from patients with aplastic anemia and healthy control subjects were stained with anti-CD4 and anti-CD25 antibodies followed by intracellular anti-FOXP3 antibodies. The upper gate on the dot plots (gate a) represents CD4+CD25hi+ T cells and the lower gate (gate b) the CD4+CD25+ T cells. FOXP3 expression was gated on CD4+CD25hi+ T cell population (gate a). Representative dot plots are shown from a healthy control subject, a patient with aplastic anemia, and another patient before and after immunosuppressive treatment. Tx, treatment. (B,C) Relative numbers of CD4+CD25hi+ T and CD4+CD25hi+ FOXP3+ T cells from all healthy control subjects and patients with aplastic anemia examined are shown. All patients were examined before receiving any immunosuppressive treatment. Differences between patients and control subjects were statistically significant (P < .001). (D) CD4+CD25+ T cells' nuclear extracts from healthy volunteers and patients with aplastic anemia were analyzed for FOXP3 expression. All patients examined had significantly decreased FOXP3 protein levels compared with healthy donors (P < .001). All patients' samples were analyzed side-by-side with healthy donors' samples. (E) Cytoplasmic extracts from CD4+CD25+ T cells from patients and healthy donors were analyzed by immunoblot for NFAT1 expression. All patients examined showed diminished or undetectable NFAT1 protein levels compared with healthy control subjects (P < .001). All patients' samples were run side-by-side with control subjects' samples. There were no differences in TLR2 expression between patients and control subjects. (F) RNA from CD4+CD25+ T cells from patients with aplastic anemia and control subjects was analyzed for FOXP3 gene expression in quantitative polymerase chain reaction experiments. Patients with aplastic anemia showed decreased FOXP3 mRNA/actin copies compared with healthy control subjects (P = .03). Horizontal lines represent mean values. (G-I) The densitometric intensities of immunoblot results from all the subjects studied for FOXP3, NFAT1, and TLR2 expression are collectively presented. Horizontal lines represent mean values. Results are plus or minus standard error of the mean (± SEM).
In 13 cases, Treg and FOXP3 expression was reexamined 3 to 6 months after the first sampling; 6 of 7 patients who showed hematologic response to immunosuppressive treatment had slightly higher Treg numbers and FOXP3 expression compared with numbers obtained before treatment (data not shown). Three further patients in complete remission after immunosuppressive treatment had increased CD4+CD25hi+ T cells compared with treatment-naive patients (data not shown).
Decreased FOXP3 and NFAT1 protein in CD4+CD25+ T cells from patients with acquired aplastic anemia
All patients examined (n = 8) had significantly decreased FOXP3 protein levels (FOXP3/actin OD: 0.31 ± 0.1 versus 1.03 ± 0.1, P = .001, Figure 1D,G and Figure S2) and FOXP3 mRNA/106 actin copies (n = 5, P = .03, Figure 1F) compared with healthy controls' CD4+CD25+ T cells. The basal promoter of FOXP3 contains at least 6 NFAT1 and AP-1 binding sites, which positively regulate the transactivation of FOXP3 transcription.6 NFAT1 also forms cooperative complexes with FOXP3; these complexes affect repressive effects on cytokine gene expression and their activating effects on Treg marker genes, CTLA4 and CD25.7,8 Based on the relationship between NFAT1 expression and FOXP3 established in vitro and in animal models,6-8 we examined this regulator's expression in aplastic anemia; patients' CD4+CD25+ T cells contained significantly decreased or absent NFAT1 protein levels compared with control subjects (NFAT1/actin OD: 0.29 ± 0.09 versus 1.1 ± 0.02, P < .001, Figure 1E,H, and Figure S2). The NFAT1 levels in CD4+CD25− T cells were comparable between patients and control subjects (Figure 2C). No differences were detected between patients and controls' TLR2 protein levels21 (TLR2/actin OD: 1.06 ± 0.04 versus 1.05 ± 0.03, P = .9, Figure 1E,I).
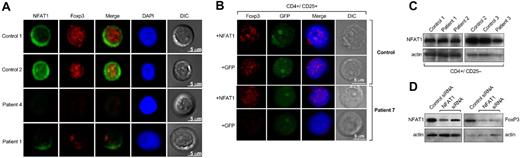
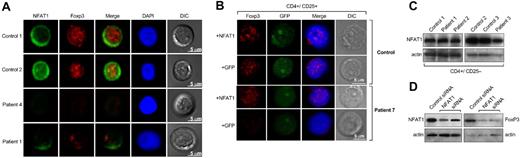
FOXP3 and NFAT1 in primary CD4+CD25+ T cells. (A) Purified CD4+CD25+ T cells from patients and healthy donors were analyzed by confocal microscopy for NFAT1 and FOXP3 expression. NFAT1 resided in the cytoplasm in unstimulated cells from control subject and FOXP3 was localized in the nucleus. All patients with aplastic anemia who were examined showed decreased or absent NFAT1 and FOXP3. Representative results are shown from at least 4 different experiments. In all experiments, patients' and control subjects' cells were stained in parallel. DIC indicates differential interference contrast; DAPI, 4′-6-diamidino-2-phenylindole. (B) Purified CD4+CD25+ T cells from patients and healthy donors were analyzed by confocal microscopy for FOXP3 expression after transient transfection with a wild-type NFAT1 construct. CD4+CD25+ T cells from healthy donors did not show any difference in FOXP3 expression after transfection with the NFAT1 construct. CD4+CD25+ T cells from patients showed significantly increased FOXP3 expression after the transfection. Representative results are shown from at least 4 different experiments. Patients' and control subjects' cells were transfected and stained in parallel. (C) NFAT1 levels in CD4+CD25− T cells were comparable between patients and control subjects. (D) Cytoplasmic and nuclear extracts from CD4+CD25+ T cells (from healthy donors, n = 2) transfected with NFAT1-siRNA were analyzed by immunoblot for NFAT1 and FOXP3 expression, respectively. The NFAT1-knockdown CD4+CD25+ T cells showed decreased FOXP3 expression compared with the cells that were transfected with a control small inhibitory RNA. See “Patients, materials and methods, Confocal microscopy and T-cell transfections” for detailed image acquisition information.
FOXP3 and NFAT1 in primary CD4+CD25+ T cells. (A) Purified CD4+CD25+ T cells from patients and healthy donors were analyzed by confocal microscopy for NFAT1 and FOXP3 expression. NFAT1 resided in the cytoplasm in unstimulated cells from control subject and FOXP3 was localized in the nucleus. All patients with aplastic anemia who were examined showed decreased or absent NFAT1 and FOXP3. Representative results are shown from at least 4 different experiments. In all experiments, patients' and control subjects' cells were stained in parallel. DIC indicates differential interference contrast; DAPI, 4′-6-diamidino-2-phenylindole. (B) Purified CD4+CD25+ T cells from patients and healthy donors were analyzed by confocal microscopy for FOXP3 expression after transient transfection with a wild-type NFAT1 construct. CD4+CD25+ T cells from healthy donors did not show any difference in FOXP3 expression after transfection with the NFAT1 construct. CD4+CD25+ T cells from patients showed significantly increased FOXP3 expression after the transfection. Representative results are shown from at least 4 different experiments. Patients' and control subjects' cells were transfected and stained in parallel. (C) NFAT1 levels in CD4+CD25− T cells were comparable between patients and control subjects. (D) Cytoplasmic and nuclear extracts from CD4+CD25+ T cells (from healthy donors, n = 2) transfected with NFAT1-siRNA were analyzed by immunoblot for NFAT1 and FOXP3 expression, respectively. The NFAT1-knockdown CD4+CD25+ T cells showed decreased FOXP3 expression compared with the cells that were transfected with a control small inhibitory RNA. See “Patients, materials and methods, Confocal microscopy and T-cell transfections” for detailed image acquisition information.
These data were confirmed by confocal microscopy: CD4+CD25+ T cells from control subjects (n = 4) showed the expected pattern of NFAT1 and FOXP3 expression and localization. In patients, the results were consistent with the immunoblot and quantitative polymerase chain reaction experiment data; all patients examined (n = 5) had significantly decreased or absent FOXP3 and NFAT1 expression (Figure 2A).
To examine whether decreased FOXP3 correlated with NFAT1 expression, CD4+CD25+ T cells from patients and healthy control subjects were transiently transfected with an expression plasmid encoding for the wild-type NFAT1 gene. CD4+CD25+ T cells from patients previously examined and shown not to express any FOXP3 after transfection with the NFAT1 plasmid demonstrated increased levels of FOXP3 (Figure 2B). No difference in FOXP3 expression was observed in CD4+CD25+ T cells from healthy control subjects after transfection. CD4+CD25− T cells from patients and control subjects also showed aberrant expression of FOXP3 after transfection compared with cells that were transfected with an empty GFP-plasmid as control (Figure S3). The transfected and the purified CD4+CD25+ and CD4+CD25− T cells were stimulated with aCD3 mAb or unperturbed and analyzed for interleukin-2 production. CD4+CD25− T cells produced 4.5-fold more interleukin-2 after stimulation compared with the untreated population, whereas CD4+CD25+ and the transfected CD4+CD25− T cells did not produce interleukin-2 after stimulation (data not shown). In cultures with CD25− T cells, the transfected cells could not suppress interleukin-2 production from CD4+CD25− T cells (data not shown). From these results, we inferred that FOXP3+CD25− cells generated after transient transfection were of an “anergic” phenotype as described for naturally occurring CD4+CD25+FOXP3+ T cells, but they were not suppressive.22
To confirm that decreased NFAT1 levels related to FOXP3 levels, we used CD4+CD25+ T cells from healthy control subjects in NFAT1-knockdown experiments. The NFAT1-knockdown CD4+CD25+ T cells showed decreased FOXP3 expression compared with the cells that were transfected with a control siRNA (Figure 2D), recapitulating a possible mechanism in patients' cells by dysregulation of this critical regulator.
In summary, CD4+CD25+FOXP3+ regulatory T cells are decreased in most patients with aplastic anemia, possibly as a result of altered transcriptional regulation; decreased NFAT1 could explain low FOXP3 expression and Treg frequency. A role for NFAT1 might also be explored in other immune-mediated diseases in which Tregs have been implicated. Of course, alterations in other transcription factors that could influence FOXP3 expression are not excluded by our experiments and might also contribute to the decreased Treg numbers.
A Treg defect may help explain the increased autoreactive T cells and the development of the aplastic anemia phenotype.
The online version of this manuscript contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This study was supported by the Intramural Research Program of the National Institutes of Health.
We thank Dr A. Rao for providing the NFAT1-GFP plasmid, our research nurses, Olga Nunez and Barbara Weinstein, for collection of samples, and our laboratory manager, Spencer Green, for technical assistance.
National Institutes of Health
Authorship
Contribution: E.E.S. designed and performed the research, analyzed data, and wrote the paper; K.R. and S.M. performed research, analyzed data, and revised the paper; D.M. collected and analyzed data and revised the paper; K.K. provided technical support in flow cytometry experiments; V.V. and S.K. provided technical support; A.J.B. analyzed data and revised the paper; and N.S.Y. designed the research and wrote the paper.
K.R., S.M., and D.M. contributed equally to this study.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Elena E. Solomou, National Institutes of Health, Hematology Branch, Building 10, CRC, Room 3E5216, 10 Center Drive, Bethesda, MD 20892; e-mail: solomoue@nhlbi.nih.gov, elenasolomou@hotmail.com.