Abstract
CD8+ T cell-numbers rapidly expand and then contract after exposure to their cognate antigen. Here we show that the sustained frequencies of transgene product-specific CD8+ T cells elicited by replication-defective adenovirus vectors are linked to persistence of low levels of transcriptionally active adenovirus vector genomes at the site of inoculation, in liver, and lymphatic tissues. Continuously produced small amounts of antigen maintain fully active effector CD8+ T cells, while also allowing for their differentiation into central memory cells. The long-term persistence of adenoviral vectors may be highly advantageous for their use as vaccines against pathogens for which T-cell–mediated protection requires both fully activated T cells for immediate control of virus-infected cells and central memory CD8+ T cells that, because of their higher proliferative capacity, may be suited best to eliminate cells infected by pathogens that escaped the initial wave of effector T cells.
Introduction
Preventative viral vaccines provide protection through induction of immunologic memory, most notably circulating neutralizing antibodies.1 For some viruses, such as HIV-1, vaccines have failed to induce protective levels of antibodies and the focus of many of the ongoing HIV-1 vaccine efforts has shifted to T-cell responses.2 Correlates of T-cell–mediated protection to viral infections remain ill-defined because of the not yet fully understood complexity of memory T-cell responses.
Replication-defective adenovirus (Ad) vectors are at the forefront of HIV-1 vaccine research and have entered phase 2 clinical trials.3–5 One of the most remarkable features of Ad-based vaccines is their ability to induce exceptionally high and sustained frequencies of transgene product-specific CD8+ T cells that, unlike those induced by other subunit vaccine carriers such as DNA vaccines or poxvirus vectors, do not contract after the initial activation.6,7
Here we show that replication-defective E1-deleted Ad vector genomes similar to those of Ads acquired by natural infections8,9 persist. Persistent vector was found in muscle at the site of inoculation, in liver, and in lymphatic tissues of experimental animals. Within lymphatic tissues the vector genomes are enriched in T-cells directed to the antigen encoded by the viral vector. The vector's genome remains transcriptionally active, and the continued presence of transgene products appears to maintain high frequencies of activated antigen-specific CD8+ T cells in addition to a pool of resting memory T cells. Although the concept of persisting vaccines may provide challenges for their eventual use for mass vaccination, concomitantly maintaining high frequencies of effector-like T cells and resting memory T cells may provide a solution to the dilemma of vaccines that rely on T-cell–mediated protection.
Materials and methods
Mice
C57Bl/6 and BALB/c mice were purchased at 6 to 8 weeks of age from Charles River Laboratories (Boston, MA). OT1 and P14 mice were bred at the Animal Facility of the Wistar Institute (Philadelphia, PA) and typed by polymerase chain reaction (PCR) for homozygosity. Animals were treated according to guidelines of the Wistar Institute.
Cell lines
HEK 293 and HeLa cells were grown in Dulbecco Modified Eagle medium, supplemented with 10% fetal bovine serum.
Viruses and viral vectors
Ad vectors expressing Gag of HIV-1, the rabies virus glycoprotein or SIINFEKL as a fusion protein with influenza virus nucleoprotein and green fluorescent protein, the glycoprotein of lymphocytic choriomeningitis virus (LCMV), or green fluorescent protein were propagated on HEK 293 cells, purified, and quality-controlled as described previously.10 Vaccinia virus vectors expressing Gag were grown on HeLa cells and titrated as described.11 LCMV strain Armstrong was produced as described.12
Immunization or infection of mice
Mice were immunized intramuscularly at 6 to 10 weeks of age with vectors diluted in 100 μL PBS. Mice were infected with vaccinia virus vectors or LCMV intraperitoneally.
Amplification of vector sequences: rabies virus glycoprotein sequences
Genomic DNA of tissues harvested from vaccinated mice was extracted using DNeasy Tissue kit (Qiagen, Valencia, CA) according to the manufacturer's instructions. The glycoprotein gene of rabies virus (Rab.gp) ERA strain in each tissue was amplified, first by regular polymerase chain reaction (PCR) (5′ and 3′ primers: 5′-CCTGGAGCCCGATTGACATAC-3′ and 5′-ACAAGGTGCTCAATTTCGTC-3′, respectively). The PCR consisted of 30 cycles of 94°C for 50 seconds, 55°C for 50 seconds, and 72°C for 1 minute. The amplicon (0.1 μL) from the first PCR product was then used as template for real-time PCR to semiquantify the Rab.gp gene in different tissues. Nested primers were used to amplify a 266-bp fragment in Rab.gp. The 5′ and 3′ primers were 5′-AAAGCATTTCCGCCCAACAC-3′ and 5′-GGTTAGTGGAGCAGTAGGTAG-3′, respectively. Forty real-time PCR cycles were run as 95°C for 5 seconds, 63°C for 10 seconds, 72°C for 15 seconds, and 85°C for 4 seconds. The copy numbers of Rab.gp in each tissue were normalized by comparison to the glyceraldehyde-3-phosphate dehydrogenase gene (Gapdh) sequences quantified by a real-time PCR as described.13
Gag sequences
DNA and RNA were isolated and RNA was reverse-transcribed. For mouse samples, the housekeeping gene Gapdh was quantified from each sample by real-time PCR. For nonhuman primate samples, quantitation was based on the β-actin gene. Samples were then adjusted to equal amounts of Gapdh or β-actin DNA or cDNA and amplified by PCR, followed by a nested real-time PCR or regular for Gag, resulting in an amplicon of 217 bp.14 The following primers were used for this PCR: external 5′ and 3′primers, 5′-GGAGCTAGAACGATTCGC-3′ and 5′-CTCTTGCCTTATGGCCG-3′, respectively; and nested primers, 5′-AGGGGAAGTGACATA-3′ and 5′-GCTTGCTCGTCGGCTCTTAG-3′, respectively. The standards for Gag (100 to 105 copies) were amplified at the same time. The first PCR consisted of 25 cycles of 94°C for 40 seconds, 52°C for 40 seconds, and 72°C for 40 seconds. The amplicon (0.1 μL) from the first PCR product was then used as template for a second real-time PCR to quantify the Gag gene in different cell fractions. The second PCR was run for 40 cycles at 95°C for 5 seconds, 57°C for 4 seconds, 72°C for 10 seconds, and 84°C for 4 seconds. The copy numbers of Gag in each cell fraction were normalized in comparison to Gapdh or β-Actin sequences quantified by a real-time PCR as described. In same experiments the first PCR was run for 40 cycles and the second PCR was conducted under nonquantitative conditions. A fragment of the hexon encoding sequence was amplified with the following primers: 5′-CTC ATG TAC TAC AAC AGC ACT GGC AA-3′ and 5′-ATT GCG GTG GTG GTT GAA GGG GTT GA-3′. The PCR consisted of 32 cycles of 95°C for 45 seconds, 55°C for 45 seconds, and 72°C for 45 seconds. The amplicon (0.1 μL) from the first PCR product was then used as a template for nested PCR amplification. The 5′ and 3′ primers for the nested PCR were 5′-GGA TGA ACT TCC CAA CTA TTG-3′ and 5′-ATG GCG AAT GGA TTA CCC-3′, respectively, which amplified a 159-bp fragment of the Hexon sequence. The nested PCR cycles were run for 32 cycles at 95°C for 30 seconds, 55°C for 30 seconds, and 72°C for 30 seconds. The Hexon primers correspond to the sequence of AdC68 but cross-react with the corresponding sequence of AdHu5 Hexon.
Specificity of amplicons was confirmed by analyses of melting temperature curves, gel electrophoresis of amplicons, and sequencing of some of the samples. Controls included reactions containing no DNA (water control) or samples from naive animals or animals vaccinated with an Ad expressing an unrelated transgene product; RNA samples were amplified without previous reverse-transcription to ensure lack of DNA contamination. Standards were included in each assay. Sensitivity of the assay was determined from a standard curve for Gag and Hexon showing a detection limit of approximately 2 copies of DNA.
Imaging gene expression in muscle
Mice were vaccinated intramuscularly in the lower leg with 1011 viral particles (vp) per mouse of an AdHu5 vector expressing green fluorescent protein. Either 24 hours or 39 days later, mice were killed, and the legs were removed and illuminated with an Illumatool Lighting System (Lightools Research, Encinitas, CA). A Kodak DCS14N digital SLR camera (Kodak, Rochester, NY) with a 60-mm Micro Nikkor lens of f3.5 using a 1:2.2 magnification (Nikon, Tokyo, Japan) was used to take photographs. Raw images were converted to TIFF files and then converted to grayscale and intensity maps were generated using Image-Pro plus, version 6.0 (Media Cybernetics, Silver Spring, MD).
T-cell assays
Intracellular cytokine staining was performed as follows. Splenocytes were cultured at 106 cells/well for 5 hours at 37°C in 96-well round-bottom microtiter plate wells in 2% mixed leukocyte culture Dulbecco Modified Eagle medium in the presence of Brefeldin A (GolgiPlug; Pharmingen, La Jolla, CA) and the antigenic peptide or a control peptide. Cells were then treated with a FITC-labeled antibody to mouse CD8 (Pharmingen), washed, and permeabilized with Cytofix/Cytoperm (Pharmingen). They were washed with Perm/Wash (Pharmingen) and stored at 4°C overnight. The next day, the cells were treated with a phycoerythrin (PE)–labeled antibody to mouse interferon (IFN)–γ (Pharmingen), and in some experiments in addition with an allophyco (APC)–labeled antibody to tumor necrosis factor (TNF)–α. Immunofluorescence was analyzed by 2- or 3-color flow cytometry using an EPICS Elite XL (Beckman-Coulter, Miami, FL) and data were analyzed by WinMDi (version 2.8, copyright J. Trotter, La Jolla, CA), Summit (version 4.2, Dako Cytomation, Fort Collins, CO), or FlowJo (version 7.2.2, Tree Star, Ashland OR) software.
Tetramer staining
Lymphocytes were isolated, red blood cells were lysed with ammonium chloride (ACK) lysis buffer (Gibco, Birmingham, MI) treatment for 3 minutes on ice, washed 3 times with L-15 media (Gibco), and stained with APC-labeled HIV-1 Gag tetramer (National Institutes of Health Tetramer Core Facility at Emory, Atlanta, GA), or LCMV GP33 tetramer (Wherry Lab, Wistar Institute, Philadelphia, PA) peridinin chlorophyll protein (PerCP)-labeled anti-CD8, either PE-labeled anti-CD27, anti-CD127, anti-CD69, or anti-CD62L, and FITC-labeled anti-CD44 for 30 minutes at 4°C. All antibodies used were obtained from BD Biosciences (La Jolla, CA). For flow cytometry, cells were acquired on a BD FACSCalibur (BD Biosciences) and analyzed using FlowJo software version 7.2.2.
51Cr release assay
Target cells (P815) were coated overnight with 5 μg/mL of Gag peptide (Figure 4 filled squares) or an equal concentration of a control peptide (open squares). Before the assay frequencies of Gag-specific CD8+ T-cells were determined by major histocompatibility complex (MHC)–Gag peptide tetramer staining. Lymphocytes were adjusted to equal numbers of the tetramer-positive CD8+ cells and cocultured at serial dilutions with chromium-51 (51Cr)-labeled target cells for 6 hours. Supernatants were harvested and tested in a gamma counter. Percent specific lysis was calculated as described.11
Cell sorting
On lysis of red blood cells, splenocytes were stained with FITC-labeled anti-CD8 (BD Biosciences) and APC-labeled HIV-1 Gag tetramer (National Institutes of Health Tetramer Core Facility at Emory) for 30 minutes at 4°C. Cells were then washed 3 times and sorted using a DakoCytomation MoFlo (DakoCytomation).
Results
Kinetics of CD8+ T-cell responses
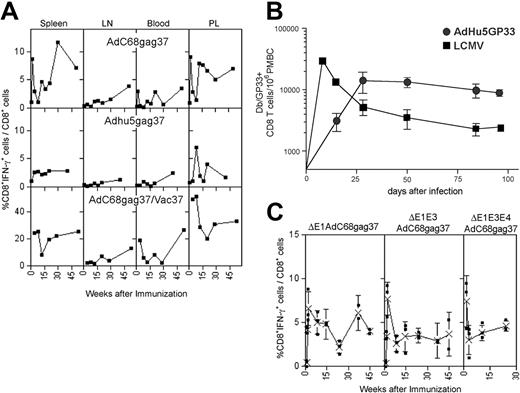
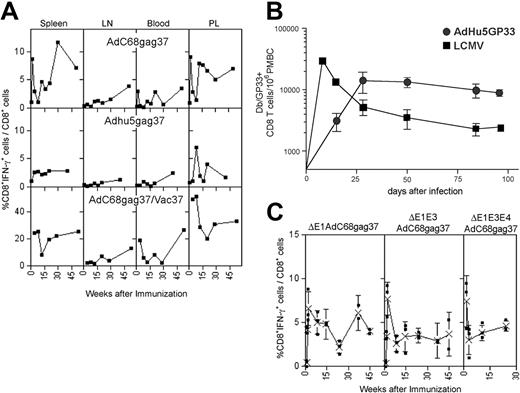
The kinetics of transgene product–specific CD8+ T-cell responses induced by Ad vectors were tested. BALB/c mice were vaccinated once intramuscularly with E1-deleted Ad vectors derived from chimpanzee serotype 68 (AdC68) or human serotype 5 (AdHu5) expressing a truncated Gag of HIV-1 (AdC68gag37, AdHu5gag37). Lymphocytes were isolated from spleens, lymph nodes, blood, and peritoneal lavage at different times and tested for frequencies of Gag-specific CD8+ cells (Figure 1A). High frequencies of IFN-γ–secreting CD8+ T cells developed in spleens and peritoneal lavage by days 10 to 14, with markedly lower frequencies in blood or lymph nodes. Although frequencies fluctuated, there was no overall reduction over the 1-year testing period, and in lymph nodes asteady increase in frequencies was observed, suggestive of a delayed development of central memory CD8 T cells. Similar results were obtained when data were plotted as absolute numbers of antigen-specific T cells per tissue or if animals were vaccinated at different times to allow for analyses of T-cell responses on the same day. A second immunization with a vaccinia virus recombinant expressing the same antigen given 2 months after priming affected an overall increase in gag-specific CD8+ T cells, and again with some fluctuation recall CD8+ T-cell responses failed to contract in most compartments (Figure 1A, bottom panels). To ensure that lack of contraction was not related to the transgene product, we compared induction of CD8+ T-cell responses by an AdHu5 vector expressing the glycoprotein of LCMV to those elicited by LCMV. As shown in Figure 1B, frequencies of CD8+ T cells induced by the AdHu5 vector peaked later than those stimulated by LCMV, but then remained at stable numbers throughout the duration of the experiment, whereas those elicited by LCMV rapidly declined.
Kinetics of the Gag-specific CD8+ T-cell response. (A) Top panels: Groups of 5 BALB/c mice injected intramuscularly with 1011 vp of AdC68gag37 or 5 × 1011 vp of AdHu5gag37 vector were killed at different times. Lymphocytes isolated from indicated compartments were tested for Gag-specific IFN-γ–producing CD8+ cells by intracellular cytokine staining and analyzed by 2-color flow cytometry.6 Results show frequencies of CD8+ cells producing IFN-γ as a percentage of all CD8+ cells from a representative experiment (top panels). Frequencies of Gag-specific CD8+ T cells in BALB/c mice immunized with 5 × 1011 vp of AdC68gag37 and boosted 2 months later with 106 plaque forming units (pfu) of a recombinant vaccinia virus expressing gag (VVgag). Weeks after immunization refer to the boost. Each experiment was conducted 2 to 4 times and the data shown are representative. (B) The same experiment was conducted with cells from mice immunized with 5 × 1011 vp of Adhu5gag37 vector. C57Bl/6 mice were infected with 2 × 105 pfu of LCMV intraperitoneally or immunized with 1010 vp of Adhu5 expressing LCMV glycoprotein intramuscularly. Db/GP33 tetramer-positive CD8 T cells were enumerated in the peripheral blood mononuclear cells by MHC tetramer staining at the indicated time points. n is 3 for LCMV and n is 2 for Adhu5-GP33. Similar results were observed for Adhu5-GP given intravenously or intraperitoneally (data not shown). (C) Mice were vaccinated with 1011 vp of E1-deleted, E1-deleted, and E3-deleted or E1-deleted, E3-deleted, and E4-deleted AdC8 vector expressing Gag37. T-cell frequencies of spleens of individual mice were analyzed at different times. The graphs show frequencies of Gag-specific CD8+ T cells for individual mice (■) and mean frequencies (X) (± SD).
Kinetics of the Gag-specific CD8+ T-cell response. (A) Top panels: Groups of 5 BALB/c mice injected intramuscularly with 1011 vp of AdC68gag37 or 5 × 1011 vp of AdHu5gag37 vector were killed at different times. Lymphocytes isolated from indicated compartments were tested for Gag-specific IFN-γ–producing CD8+ cells by intracellular cytokine staining and analyzed by 2-color flow cytometry.6 Results show frequencies of CD8+ cells producing IFN-γ as a percentage of all CD8+ cells from a representative experiment (top panels). Frequencies of Gag-specific CD8+ T cells in BALB/c mice immunized with 5 × 1011 vp of AdC68gag37 and boosted 2 months later with 106 plaque forming units (pfu) of a recombinant vaccinia virus expressing gag (VVgag). Weeks after immunization refer to the boost. Each experiment was conducted 2 to 4 times and the data shown are representative. (B) The same experiment was conducted with cells from mice immunized with 5 × 1011 vp of Adhu5gag37 vector. C57Bl/6 mice were infected with 2 × 105 pfu of LCMV intraperitoneally or immunized with 1010 vp of Adhu5 expressing LCMV glycoprotein intramuscularly. Db/GP33 tetramer-positive CD8 T cells were enumerated in the peripheral blood mononuclear cells by MHC tetramer staining at the indicated time points. n is 3 for LCMV and n is 2 for Adhu5-GP33. Similar results were observed for Adhu5-GP given intravenously or intraperitoneally (data not shown). (C) Mice were vaccinated with 1011 vp of E1-deleted, E1-deleted, and E3-deleted or E1-deleted, E3-deleted, and E4-deleted AdC8 vector expressing Gag37. T-cell frequencies of spleens of individual mice were analyzed at different times. The graphs show frequencies of Gag-specific CD8+ T cells for individual mice (■) and mean frequencies (X) (± SD).
Splenocytes from AdHu5gag37 or AdC68gag37 vector–immunized mice, when tested 2 months after a single intramuscular dose, spontaneously secreted large amounts of interleukin (IL)-2 (Table 1) and IFN-γ (not shown) without further addition of the cognate antigen. In contrast, splenocytes from mice immunized with a different vaccine, such as a DNA vaccine, only secreted cytokines in vitro if antigen was added to the culture. The sustained secretion of cytokines without further addition of antigen together with the profile of Gag-specific CD8+ T-cell frequencies in Ad vector-vaccinated mice is suggestive of antigen persistence. The antigen could not have originated from replicating virus; Ads from humans or chimpanzees do not replicate in mice and vector preparations were free of replication-competent virus particles.
Some of the E1-deleted AdHu5 vectors that are currently in clinical trials are deleted in E3 or in E3 and E4.10 The E3 domain encodes polypeptides that subvert immune responses,15–17 and previous studies have implicated that this allows for viral persistence on natural infections.17 E4 gene products are essential for Ad replication.18 Deletion of E4 reduces the outgrowth of replication competent Ad vectors and has thus been used in some of the vaccine vectors that are in clinical trials. The kinetics of Gag-specific CD8+ T-cell frequencies induced by E1-deleted and E3-deleted or E1-deleted, E3-deleted, and E4-deleted AdC68gag37 vectors mirrored those by E1-deleted AdC68gag37 vectors (Figure 1C), indicating that gene products from these domains are not essential for the observed longevity of the CD8+ T-cell response.
E1-deleted Ad vectors persist in mice and primates
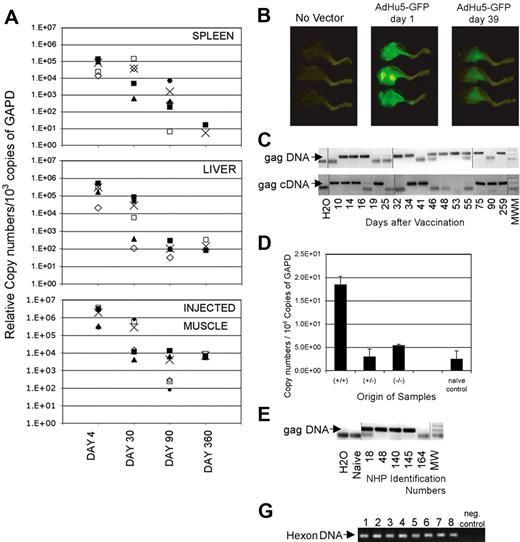
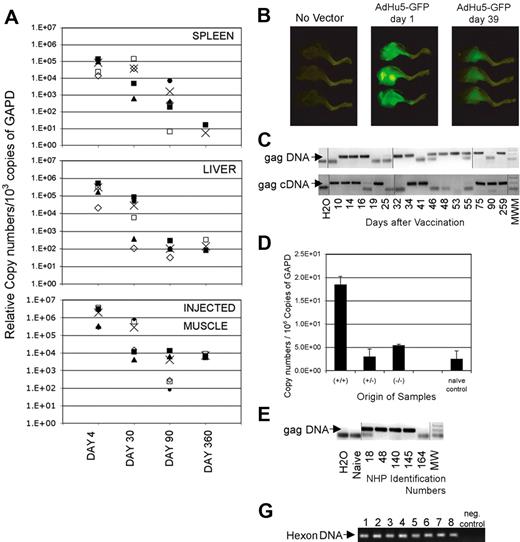
To formally demonstrate whether the E1-deleted Ad vector genome persists, tissues of mice were tested for presence of vector sequences 4, 30, 90, and 360 days after intramuscular immunization with an AdC68 vector by a nested real-time PCR (Figure 2A).19 Vector sequences could not be detected from most tissues such as ovaries, muscle distant from the inoculation site, lung, heart, brain, stomach, nasal associated lymphoid tissue, or trachea (not shown). Four days after vaccination with an AdC68 vector, transgene sequences could be detected in liver, spleen, and the inoculated muscle. By days 30 and 90, vector sequences remained detectable in most animals in these tissues but gradually declined 102 to 103-fold. After 1 year, relative copy numbers in liver and the injected muscles were comparable with those seen on day 90, whereas a further decline was seen in spleens. To ensure that protein was produced by the transgene, we immunized mice with an AdHu5 vector expressing green fluorescent protein. As shown in Figure 2B, the inoculated leg muscles of vaccinated mice were brightly green fluorescent 1 day later. When tested 5 weeks after inoculation, green fluorescent protein had declined in intensity but was nevertheless still clearly visible. Similar studies were conducted with an AdC68 green fluorescent protein vector, which expresses less fluorescent protein compared with the AdHu5green fluorescent protein vector. With the AdC68 green fluorescent protein vector, the signal was lost after 6 weeks (data not shown), although in separate experiments samples remained clearly positive for transgene sequences tested for by a highly sensitive nested PCR.
Persisting presence of vector sequences. (A) Adult female outbred (ICR) mice received 1011v.p.AdC68rab.gp vector in saline, given once intramuscularly. On days 4, 30, 90, and 360 after vector application, mice were killed and perfused with cold PBS. Tissues were harvested from individual mice. DNA was isolated and the Gapdh sequences were amplified by a real-time PCR. The samples were adjusted to 103 copies of Gapdh and the rabies virus glycoprotein gene was amplified by a real-time nested PCR. (B) Mice were vaccinated intramuscularly in the lower leg with 1011 particles of an Ad vector expressing green fluorescent protein. Mice were killed 1 day and 39 days after vaccination and legs were illuminated with an Illumatool Lighting System. Digital photographs were taken using a Kodak DCS14N SLR camera with a 60-mm Micro Nikkor lens (Nikon). (C) DNA and RNA were isolated from spleens of mice immunized at different times previously with 1011 vp of AdC8gag37. RNA was reverse-transcribed. Gapdh was quantified from each sample by real-time PCR.17 Samples were adjusted before amplification to 6 × 107 or 1.5 × 109 copies of Gapdh DNA or cDNA, respectively, and amplified by a nested PCR. After amplification by the internal real-time PCR, each sample was analyzed by gel electrophoresis. The lower graph shows results for Gag DNA or cDNA. The arrow indicates the anticipated size of the Gag amplicon. These results are from a single experiment in which we ran multiple gels to accommodate the samples. Lines were added to show where the lanes were cut. (D) Mice were immunized with AdC68gag37. Twenty months later they were boosted with 106 pfu of a vaccinia virus vector expressing Gag to increase frequencies of Gag-specific CD8+ T cells. In pre-experiments it was shown that Gag sequences from the vaccinia virus vector could not be amplified from spleens as of 1 week after inoculation. Five weeks after the boost, splenocytes were isolated. Cells were stained with a Gag-specific tetramer (tet) and an antibody to CD8. Cells were sorted into CD8+tet− cells, CD8−tet− cells and CD8+tet+ cells. Total cellular RNA was isolated and purified from each cell fraction. Complementary DNA was synthesized. The HIV Gag gene in each cell fraction was amplified first by regular PCR. The amplicon from the first PCR product was then used as template for a second real-time PCR to quantify the Gag gene in different cell fractions. The copy numbers of Gag in each cell fraction were normalized in comparison to Gapdh sequences quantified by a real-time PCR from the same samples. (E) Monkeys 18, 48, 140, and 145 were immunized intramuscularly with 1012 vp of AdC7gag37 vector and boosted 8 months later with 1012 vp of AdC6gag37 vector. Animal 164 was injected at the same time with the same vector backbone expressing the rabies virus glycoprotein.21 DNA from peripheral blood mononuclear cells harvested 99 days after the boost was tested for gag DNA as described in panel A on adjustments of samples to 3 × 104 β-actin molecules. These results are from a single experiment; the lanes were rearranged to change the order of the samples. Lines were added to show where the lanes were cut. (F) The graph shows the gels from a hexon-specific nested PCR that was used to amplify vector sequences from peripheral blood mononuclear cells of vaccinated rhesus macaques. Lanes 1 and 2 show results from peripheral blood mononuclear cells harvested 6 (1) and 17 (2) weeks after intramuscular vaccination of a monkey with 1011 vp of a mixture of 4 AdC68 vectors expressing HIV-1 Gag, gp140, 5′pol, or 3′pol+nef. Lanes 3 and 4 show results from peripheral blood mononuclear cells from a monkey harvested 6 (3) and 17 (4) weeks after intramuscular immunization with 1011 vp of 4 AdHu5 vectors expressing the same antigens as used for results in lanes 1 and 2. Lanes 5 through 8 show results from peripheral blood mononuclear cells from 2 animals harvested 2 (5,7) and 14 (6,8) weeks after immunization with 1011 vp of an AdHu5 vector expressing Gag. Lanes 9 and 10 show the negative control of the PCR reactions.
Persisting presence of vector sequences. (A) Adult female outbred (ICR) mice received 1011v.p.AdC68rab.gp vector in saline, given once intramuscularly. On days 4, 30, 90, and 360 after vector application, mice were killed and perfused with cold PBS. Tissues were harvested from individual mice. DNA was isolated and the Gapdh sequences were amplified by a real-time PCR. The samples were adjusted to 103 copies of Gapdh and the rabies virus glycoprotein gene was amplified by a real-time nested PCR. (B) Mice were vaccinated intramuscularly in the lower leg with 1011 particles of an Ad vector expressing green fluorescent protein. Mice were killed 1 day and 39 days after vaccination and legs were illuminated with an Illumatool Lighting System. Digital photographs were taken using a Kodak DCS14N SLR camera with a 60-mm Micro Nikkor lens (Nikon). (C) DNA and RNA were isolated from spleens of mice immunized at different times previously with 1011 vp of AdC8gag37. RNA was reverse-transcribed. Gapdh was quantified from each sample by real-time PCR.17 Samples were adjusted before amplification to 6 × 107 or 1.5 × 109 copies of Gapdh DNA or cDNA, respectively, and amplified by a nested PCR. After amplification by the internal real-time PCR, each sample was analyzed by gel electrophoresis. The lower graph shows results for Gag DNA or cDNA. The arrow indicates the anticipated size of the Gag amplicon. These results are from a single experiment in which we ran multiple gels to accommodate the samples. Lines were added to show where the lanes were cut. (D) Mice were immunized with AdC68gag37. Twenty months later they were boosted with 106 pfu of a vaccinia virus vector expressing Gag to increase frequencies of Gag-specific CD8+ T cells. In pre-experiments it was shown that Gag sequences from the vaccinia virus vector could not be amplified from spleens as of 1 week after inoculation. Five weeks after the boost, splenocytes were isolated. Cells were stained with a Gag-specific tetramer (tet) and an antibody to CD8. Cells were sorted into CD8+tet− cells, CD8−tet− cells and CD8+tet+ cells. Total cellular RNA was isolated and purified from each cell fraction. Complementary DNA was synthesized. The HIV Gag gene in each cell fraction was amplified first by regular PCR. The amplicon from the first PCR product was then used as template for a second real-time PCR to quantify the Gag gene in different cell fractions. The copy numbers of Gag in each cell fraction were normalized in comparison to Gapdh sequences quantified by a real-time PCR from the same samples. (E) Monkeys 18, 48, 140, and 145 were immunized intramuscularly with 1012 vp of AdC7gag37 vector and boosted 8 months later with 1012 vp of AdC6gag37 vector. Animal 164 was injected at the same time with the same vector backbone expressing the rabies virus glycoprotein.21 DNA from peripheral blood mononuclear cells harvested 99 days after the boost was tested for gag DNA as described in panel A on adjustments of samples to 3 × 104 β-actin molecules. These results are from a single experiment; the lanes were rearranged to change the order of the samples. Lines were added to show where the lanes were cut. (F) The graph shows the gels from a hexon-specific nested PCR that was used to amplify vector sequences from peripheral blood mononuclear cells of vaccinated rhesus macaques. Lanes 1 and 2 show results from peripheral blood mononuclear cells harvested 6 (1) and 17 (2) weeks after intramuscular vaccination of a monkey with 1011 vp of a mixture of 4 AdC68 vectors expressing HIV-1 Gag, gp140, 5′pol, or 3′pol+nef. Lanes 3 and 4 show results from peripheral blood mononuclear cells from a monkey harvested 6 (3) and 17 (4) weeks after intramuscular immunization with 1011 vp of 4 AdHu5 vectors expressing the same antigens as used for results in lanes 1 and 2. Lanes 5 through 8 show results from peripheral blood mononuclear cells from 2 animals harvested 2 (5,7) and 14 (6,8) weeks after immunization with 1011 vp of an AdHu5 vector expressing Gag. Lanes 9 and 10 show the negative control of the PCR reactions.
An additional kinetics experiment was conducted with splenocytes. Gag-specific DNA and reverse-transcribed mRNA could be amplified consistently from spleens of AdC68gag37 vaccinated mice for up to 2 years, although they could not be amplified at each time point from pooled splenocytes (Figure 2C shows data for up to 259 days). On testing spleens from individual mice, Gag DNA or cDNA could be detected at a given time point from some but not all of the animals, indicating that levels fluctuated or were at the lower limit of our detection method. Sequences of the adenovirus hexon and the left inverted terminal repeat (ITR) could also be amplified, suggesting that the vector genome remained intact, and similar results were obtained with AdHu5 vector and vectors based on AdC7, which is a different chimpanzee adenovirus (data not shown). Booster immunization with a Gag-expressing poxvirus vector did not result in clearance of persistently transduced lymphocytes. In mice, Gag DNA and transcripts were detected mainly in T-lymphocytes and a pronounced enrichment of Gag transcripts was found in Gag-specific CD8+ T cells (Figure 2D). The Ad vectors also persisted in blood mononuclear cells of vaccinated rhesus macaques tested in one experiment 3 months (peripheral blood mononuclear cells) after vaccination (Figure 2E), and in a separate experiment 2 and 14 or 6 and 17 weeks after a single dose of AdC68gag or AdHu5gag (Figure 2F).
Vector persistence drives continued proliferation of transgene product-specific T cells
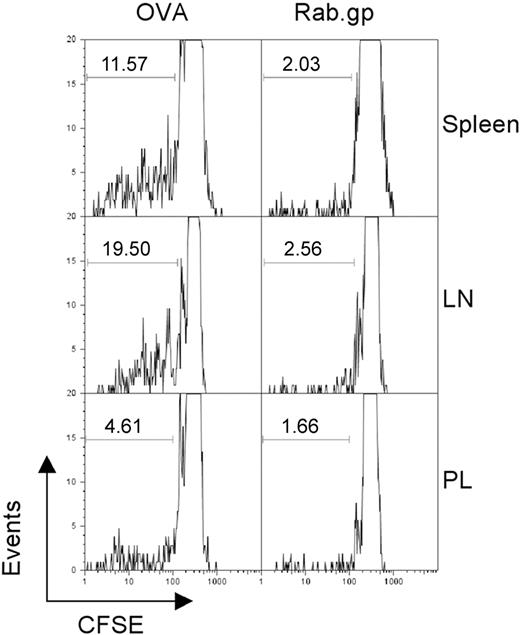
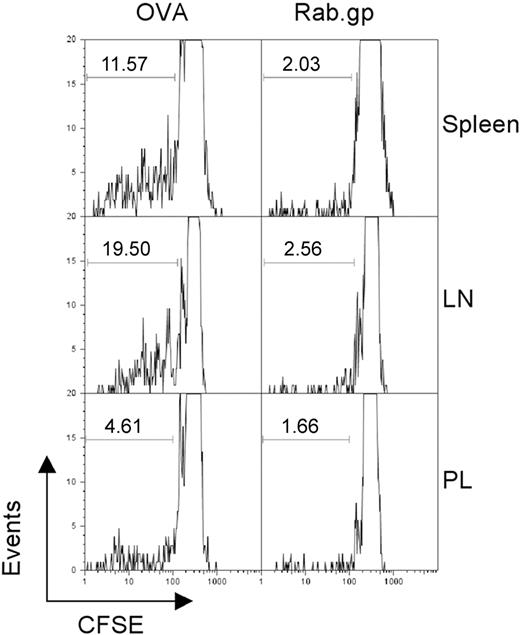
To examine whether persistence of Ad vectors provided sufficient antigen to stimulate T cells, we conducted adoptive transfer experiments with splenocytes from OT-1 mice. These mice are transgenic for a T-cell receptor that is specific for the SIINFEKL epitope of ovalbumin. Recipient C57Bl/6 Thy1.1+ mice were immunized with an AdC68 vector expressing SIINFEKL or the rabies virus glycoprotein. Four months later, mice were transfused with CFSE-labeled splenocytes from Thy1.2+ OT-1 mice under the assumption that continued production of SIINFEKL by a persisting Ad vector would drive proliferation of the SIINFEKL-specific CD8+ donor T cells, resulting in loss of CSFE. When analyzed 7 days after the adoptive transfer, up to 20% of SIINFEKL-specific donor CD8+ T cells showed loss of carboxyfluorescein diacetate succinimidyl ester (CFSE) fluorescence in AdC68-SIINFEKL–immunized mice and had hence proliferated. Proliferation was most pronounced with OT-1 CD8+ T cells isolated from lymph nodes and spleen in comparison to those from peritoneal lavage (Figure 3). OT-1 T cells transferred into mice vaccinated with the Ad vector encoding the rabies virus glycoprotein showed markedly less proliferation. Comparable results were obtained on transfer of Gag-specific CD8+ T cells into mice previously immunized with the AdC68gag37 vector (not shown).
Vector persistence drives proliferation of antigen-specific T cells. C57Bl/6 Thy1.1 mice were immunized with 1010 vp of either AdC68NPOVA green fluorescent protein or AdC68rab.gp vector (3 mice per group). After 4 months, 3 × 107 CFSE-labeled splenocytes isolated from OT1 mice were transferred into each of the immunized mice. One week after transfer the mice were killed and lymphocytes were isolated from the indicated tissues, stained with anti-CD8 and anti-Thy1.2 to identify donor cells, and the amount of CFSE in those cells was determined by flow cytometry. The numbers within the graphs show the percent of cells with low CFSE staining as indicated by the gates.
Vector persistence drives proliferation of antigen-specific T cells. C57Bl/6 Thy1.1 mice were immunized with 1010 vp of either AdC68NPOVA green fluorescent protein or AdC68rab.gp vector (3 mice per group). After 4 months, 3 × 107 CFSE-labeled splenocytes isolated from OT1 mice were transferred into each of the immunized mice. One week after transfer the mice were killed and lymphocytes were isolated from the indicated tissues, stained with anti-CD8 and anti-Thy1.2 to identify donor cells, and the amount of CFSE in those cells was determined by flow cytometry. The numbers within the graphs show the percent of cells with low CFSE staining as indicated by the gates.
Vector persistence maintains an activated cohort of transgene product-specific T cells
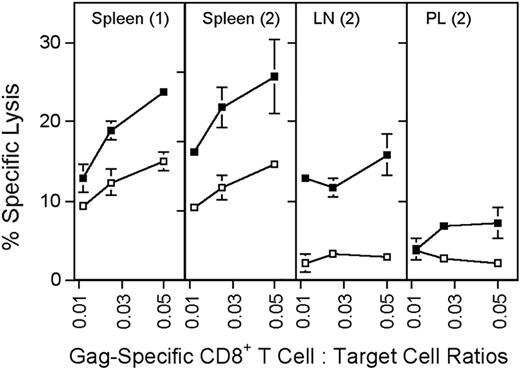
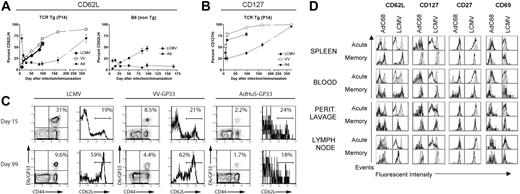
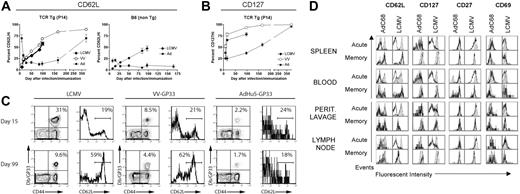
Sustained antigen-driven proliferation of CD8+ T cells would be expected to result in continued recall of memory CD8+ T cells into the effector T-cell pool. Gag-specific CD8+ T cells isolated from spleens, peritoneal lavage, and lymph nodes of mice immunized several months earlier with the AdC68gag37 vector lysed Gag-expressing target cells when tested directly ex vivo (Figure 4). Analyses of phenotypic markers on T cells induced by an AdHu5 vector or a vaccinia vector expressing the gp33 epitope of LCMV or LCMV showed a difference in kinetics of differentiation of CD62Llow effector CD8+ T cells into CD62Lhigh central memory cells (Figure 5A,C). The experiment was conducted in C57Bl/6 mice that had or had not been transferred with T cells from P14 mice, which are transgenic for the gp33-specific T-cell receptor. As shown (Figure 5A,C), gp33-specific T-cell receptor transgenic or nontransgenic CD8+ T cells from vaccinia virus or LCMV-immunized mice rapidly downregulated CD62L but then showed a gradual increase. By 100 days after antigen exposure, approximately 50% of gp33-specific CD8+ T cells expressed high levels of CD62L. In contrast, in Ad-GP immunized mice, expression of CD62L was initially downregulated with a delay and then stayed low in more than 80% of cells for at least 175 days. By day 405, approximately 70% of the AdHu5-induced CD8 T cells were CD62Lhigh. LCMV or vaccinia virus–induced gp33-specific CD8+ T cells rapidly upregulated CD127 after immunization; by day 14 after immunization, 70% to 75% of cells were CD127high. This percentage increased further to nearly 100% by day 405, as is shown for cells from mice that received the vaccinia virus vector. Ad-GP vector–induced gp33 CD8+ T cells showed a delay in CD127 upregulation that mirrored that of CD62L expression (Figure 5B).
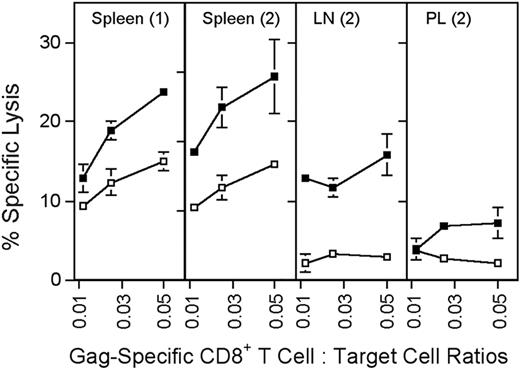
Cytolytic activity of Gag-specific CD8+ T cells. Groups of BALB/c mice were immunized with 5 × 1011 vp of AdC68gag37 and tested 2 months later. (1) Additional mice were primed with 5 × 1010 vp of AdC68gag37 and boosted with 5 × 1010 vp of AdHu5gag37 and tested 3 months after the boost (2). Target cells (P815) were coated overnight with 5 μg/mL Gag peptide (■) or an equal concentration of a control peptide (□). Before the assay, frequencies of Gag-specific CD8+, T cells were determined by MHC-gag peptide tetramer staining. Lymphocytes were adjusted to equal numbers of the tetramer-positive CD8+ cells and cocultured at serial dilutions with 51Cr-labeled target cells for 6 hours. Supernatants were harvested and tested in a gamma counter. Percent specific lysis was calculated as described.9 The origin of the lymphocytes is indicated in the upper part of the graphs. LN, lymph nodes; PL, peritoneal lavage. Error bars show standard deviations for the percent of specific lysis tested in 3 separate samples.
Cytolytic activity of Gag-specific CD8+ T cells. Groups of BALB/c mice were immunized with 5 × 1011 vp of AdC68gag37 and tested 2 months later. (1) Additional mice were primed with 5 × 1010 vp of AdC68gag37 and boosted with 5 × 1010 vp of AdHu5gag37 and tested 3 months after the boost (2). Target cells (P815) were coated overnight with 5 μg/mL Gag peptide (■) or an equal concentration of a control peptide (□). Before the assay, frequencies of Gag-specific CD8+, T cells were determined by MHC-gag peptide tetramer staining. Lymphocytes were adjusted to equal numbers of the tetramer-positive CD8+ cells and cocultured at serial dilutions with 51Cr-labeled target cells for 6 hours. Supernatants were harvested and tested in a gamma counter. Percent specific lysis was calculated as described.9 The origin of the lymphocytes is indicated in the upper part of the graphs. LN, lymph nodes; PL, peritoneal lavage. Error bars show standard deviations for the percent of specific lysis tested in 3 separate samples.
Kinetics of memory CD8 T-cell differentiation. (A-C) Mice were either infected with LCMV Armstrong (2 × 105 pfu intraperitoneally), or VV-GP33 (2 × 106 pfu intraperitoneally), or immunized with 1010 vp of AdHu5-GP33 intramuscularly, intravenously, or intraperitoneally (similar results were observed by all routes). Two groups of mice were used. In the first group, a small number of naive splenocytes from P14 mice (≈5 × 104 P14 cells) was adoptively transferred to naive B6 mice. The next day these P14 chimeras were infected or immunized. These are designated T-cell receptor Tg (P14). A second group of wild-type B6 mice was also used for confirmation of the P14 results (B6 non-Tg). Db/GP33-specific CD8 T-cell responses were monitored in the peripheral blood mononuclear cells of individual mice over time by MHC tetramer staining in conjunction with staining for CD62L and CD127. (A,B) The kinetics of CD62L and CD127 reversion on the population of Db/GP33 tetramer-positive CD8 T cells is plotted for each condition including P14 chimeras and B6 mice. For each group, n is 2 to 9. Error bars in panels A and B show standard deviations between samples from individual nice. (C) Examples of individual Db/GP33 tetramer staining and CD62L expression on Db/GP33 tetramer+ CD8 T cells are shown for each condition at 2 time points. The numbers show percent double positive cells over all cells positive for a given marker for the dot blots and percent of cells that were expressed high levels of a given marker as indicated by the brackets in the histograms. (D) Groups of BALB/c mice were immunized intramuscularly with 1010 vp of AdC68HIVgag37 for the acute cohort and 5 × 1011 vp for the memory cohort; they were killed at day 10 (acute) or 15 months (memory) after immunization. Groups of C57BL/6 mice were immunized intraperitoneally with 2 × 105 pfu LCMV Armstrong and killed on day 8 (acute) and 7 months (memory) after immunization. Lymphocytes isolated from indicated tissues were stained with a HIVgag tetramer for the AdC68HIVgag37–immunized mice and a GP33 tetramer for the LCMV mice. Cells were also stained with anti-CD8 and the indicated surface markers, and analyzed by flow cytometry. Results shown are gated on tetramer-positive cells (black line) or naive cells (filled curve).
Kinetics of memory CD8 T-cell differentiation. (A-C) Mice were either infected with LCMV Armstrong (2 × 105 pfu intraperitoneally), or VV-GP33 (2 × 106 pfu intraperitoneally), or immunized with 1010 vp of AdHu5-GP33 intramuscularly, intravenously, or intraperitoneally (similar results were observed by all routes). Two groups of mice were used. In the first group, a small number of naive splenocytes from P14 mice (≈5 × 104 P14 cells) was adoptively transferred to naive B6 mice. The next day these P14 chimeras were infected or immunized. These are designated T-cell receptor Tg (P14). A second group of wild-type B6 mice was also used for confirmation of the P14 results (B6 non-Tg). Db/GP33-specific CD8 T-cell responses were monitored in the peripheral blood mononuclear cells of individual mice over time by MHC tetramer staining in conjunction with staining for CD62L and CD127. (A,B) The kinetics of CD62L and CD127 reversion on the population of Db/GP33 tetramer-positive CD8 T cells is plotted for each condition including P14 chimeras and B6 mice. For each group, n is 2 to 9. Error bars in panels A and B show standard deviations between samples from individual nice. (C) Examples of individual Db/GP33 tetramer staining and CD62L expression on Db/GP33 tetramer+ CD8 T cells are shown for each condition at 2 time points. The numbers show percent double positive cells over all cells positive for a given marker for the dot blots and percent of cells that were expressed high levels of a given marker as indicated by the brackets in the histograms. (D) Groups of BALB/c mice were immunized intramuscularly with 1010 vp of AdC68HIVgag37 for the acute cohort and 5 × 1011 vp for the memory cohort; they were killed at day 10 (acute) or 15 months (memory) after immunization. Groups of C57BL/6 mice were immunized intraperitoneally with 2 × 105 pfu LCMV Armstrong and killed on day 8 (acute) and 7 months (memory) after immunization. Lymphocytes isolated from indicated tissues were stained with a HIVgag tetramer for the AdC68HIVgag37–immunized mice and a GP33 tetramer for the LCMV mice. Cells were also stained with anti-CD8 and the indicated surface markers, and analyzed by flow cytometry. Results shown are gated on tetramer-positive cells (black line) or naive cells (filled curve).
To ensure that the differences in expression of differentiation markers were not linked to the specificity of the CD8+ T cells and could be detected in T cells isolated from compartments other than spleen, an additional experiment was conducted comparing effector and late memory CD8+ T cells from mice immunized with an Ad vector–expressing Gag or with LCMV (Figure 5D).
Acutely after antigen exposure, a higher percentage of Ad vector than LCMV-induced CD8+ T cells from all compartments expressed CD127; expression of CD69 was also more pronounced, mainly in spleens and peripheral blood mononuclear cells. After 15 months, levels of expression of CD127 and CD69 were comparable. A larger fraction of Ad than of LCMV-induced CD8+ T cells remained CD62Llow when tested 15 months after immunization. This was particularly pronounced in cells isolated from blood and peritoneal lavage. In blood, a population of CD27intermediate Ad vector-induced CD8+ T cells could be detected that was not present in LCMV-immunized mice. In lymph nodes, CD69 was higher on Ad-induced cells tested at 15 months. Chronic exposure to antigen can result in loss of CD8+ T-cell functions. This was not the case as T cells induced by the AdC68gag37 vector maintained the ability to secrete cytokines such as IFN-γ and TNF-α (Table 2).
Discussion
Neutralizing antibodies are the primary correlate of protection against many viral infections. However, in the case of HIV infections, the envelope protein, which is the primary target for neutralization, has evolved multiple pathways to escape elimination by antibodies.20 As such, efforts to develop envelope-based HIV-vaccines that elicit high and sustained titers of broadly cross-neutralizing antibodies have been unsuccessful. HIV-1-specific CD8+ T cells may reduce morbidity after infections with HIV-1, as suggested by data gained on simian-human immunodeficiency virus (SHIV) or simian immunodeficiency virus (SIV) infection of nonhuman primates, in which T-cell–inducing vaccines were shown to provide some protection against disease14,21,22 and in which depletion of CD8+ T cells increases viral loads and accelerates progression to disease.23,24 In humans, a role for T-cell–mediated control of HIV-1 replication is evidenced by the decline of viremia after acute infections that follows expansion of HIV-specific T cells,25 and by the rapid development of viral escape variants with mutated CD8+ T-cell epitopes.14 In HIV-1–infected individuals, lack of progression to disease was furthermore shown to correlate with multifunctionality of HIV-1–specific CD8+ T-cells.26
Although these studies suggest that high frequencies of multifunctional HIV-1–specific CD8+ T cells can control a chronic HIV-1 infection, it remains unclear what type of vaccine-induced T cells are best suited to provided resistance against a primary infection. Frequencies and functionality of CD8+ T cells are largely determined by the vaccine carrier and can be modulated by repeated immunizations.12,27 Vaccines, similar to infections, cause an initial expansion of specific CD8+ T cells. Once the antigen has been removed, the majority of effector CD8+ T cells undergo apoptotic cell death and only a subset differentiates into long-lasting memory CD8+ T cells, whose numbers are maintained by homeostatic proliferation.28 In mice as well as in humans, memory CD8+ T cells are heterogeneous and can be broadly classified into central memory cells that express CD62Lhigh and reside primarily in lymphatic tissues and into CD62low effector memory cells that circulate in peripheral tissues including the mucosa of the genital tract and the intestine,29,30 which are the primary port of entry for HIV-1 and the primary target for early viral replication,31 respectively. Effector memory T cells can immediately assume effector functions such as cell lysis but lack the proliferative capacity of central memory CD8+ T cells. Unlike central memory CD8+ T cells that are maintained at stable levels for the lifespan of an individual, frequencies of effector memory CD8+ T cells appear to decline over time presumably by their differentiation into central memory CD8+ T cells.32
The role of different CD8+ T cells in providing protection against viral infections remains controversial. Some have argued that only effector cells that are fully active and present in high numbers can protect against viral infections.33 However, effector T cells are short-lived and could only be maintained long-term by traditional vaccines through constant booster immunizations. Others have made the case that effector memory CD8+ T cells provide resistance against viral infection by immediately acting at the port of viral entry.34,35 While effector memory T cells can persist longer than effector cells and have the advantages of peripheral localization and rapid effector functions, these cells have a low proliferative potential that may limit their effectiveness should an infection spread beyond the primary site of entry. Yet a third group has shown that central memory CD8+ T cells are more effective than effector memory CD8+ T cells to reduce viral burden.,36,37 Vaccines that are rapidly cleared and aim to protect through cell-mediated immunity rely on long-term protection through central memory T cells. Central memory CD8+ T cells have the advantages of robust re-expansion into effectors and long-term persistence, but their absence at the portals of entry for pathogens may be a deficiency in their ability to protect against infections such as with HIV that can rapidly escape immune surveillance through mutations unless controlled early at the port of entry. Vaccines, such as replication-defective Ad vectors that persist at low levels and continue to produce antigen, maintain a cohort of activated effector memory/effector T cells. It would appear that the low level of antigen-persistence achieved with the Ad vectors also allows the development of central memory CD8+ T cells as evidence by the eventual appearance of a cohort of cells that expresses high levels of CD62L. Intermittent antigen-driven reactivation of central memory T cells into effector T cells and in part to a shift toward effector memory T cells may occur because of repeated internal boosts. Thus, immunization with Ad vectors may achieve a crucial mixture of effector, effector memory, and central memory CD8+ T cells.
E1-deleted Ad vectors have been shown to induce high frequencies of transgene product-specific CD8+ T cells in primates that, unlike those induced by other vaccine modalities such as MVA vectors, fail to contract over time.38–40 In addition, our studies showed that, although Ad vectors induce potent CD8+ T-cell responses in spleen and blood, only modest numbers of vaccine induced CD8+ T cells home to lymph nodes,41 which again supports the notion that CD8+ T cells elicited by Ad vectors show a delay in progression toward central memory cells that, because of upregulation of CD62L, home to lymph nodes. This is also supported by our repeated findings that most Ad vector–induced nonhuman primate CD8+ T cells produce IFN-γ but not IL-2 in response to their cognate antigen. Transgene product–specific CD8+ T cells induced by Ad vectors remain CD45RAlow and are thus distinct from those induced by persistent herpes virus infections.42,43
The mechanism of Ad vector persistence remains to be elucidated. Ads acquired by natural infections persist for years in lymph nodes and tonsils.44 E1-deleted Ad vectors derived from human or chimpanzee serotypes appear to share this trait, persisting at the site of inoculation in liver and in lymphatic tissues. Within spleens, Ad vectors persist preferentially in T cells activated in response to Ad-transduced cells, which would be expected to rapidly eliminate Ad vector–transduced cells.45 The enrichment of vector genome copies in transgene product-specific CD8+ T cells may in part reflect the relative resistance of activated T cells to CD8+ T-cell–mediated cytolysis. Considering the natural turnover rates of effector and memory T cells,46 one would nevertheless expect a steady attrition of Ad genome copy numbers over time followed by a complete loss. Our data clearly show that vector genome copies decline during the first year after Ad vector injection and, although we were able to detect Ad vector genome of up to 2 years in spleens of mice (not shown), we cannot rule out that Ad vector–transduced cells would eventually be eliminated in a longer-lived species. It is also feasible that the vector genome undergoes some replication during cell proliferation. This may reflect either integration into the host cell genome, which would be atypical for an Ad genome, or, more likely, episomal gene duplication facilitated by the host cells' transcription machinery.17,47 Persistence in muscle and liver may either reflect that continued presence of vectors in nonreplicating hepatocytes and muscle cells or, alternatively, their presence in T cells that homed to these tissues.
Products of the viral E3 domain, which are nonessential for viral proliferation but which facilitate viral survival by subverting CD8+ T-cell responses, have been implicated to promote persistence of Ads acquired by natural infections.17 Our results show that the genomes of E3-deleted or E3-deleted and E4-deleted vectors remain detectable for months after a single inoculation (not shown), indicating that neither gene product is essential for the observed vector persistence.
In summary, Ad vectors induce exceptionally sustained transgene product–specific CD8+ T-cell responses. As demonstrated here, this reflects persistence of transcriptionally active Ad vector genomes. A vaccine that induces sustained frequencies of partly activated CD8+ T cells may be of enormous benefit to ward off pathogens such as HIV-1.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgment
This work was supported by National Institutes of Health grant P01 AI052271.
National Institutes of Health
Authorship
Contribution: N.T., S.E.H., and E.J.W. designed and performed research, analyzed data, and wrote the paper. J.C.F. and A.R.-S. designed and performed research, and analyzed data. K.C.H.M., D.Z., S.-W.L., and Z.Q.X. performed research and analyzed data. A.B., A.I., and C.L.-C. performed research. H.C.J.E. designed research, analyzed data, and wrote the paper.
N.T., J.C.F., and A.R.-S. contributed equally to this work.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Hildegund C. J. Ertl, Wistar Institute, 3601 Spruce St, Philadelphia, PA 19104; e-mail: ertl@wistar.upenn.edu.