Abstract
Lymph nodes provide specialized stromal microenvironments that support the recruitment and organization of T cells and B cells, enabling them to effectively participate in immune responses. While CD4+3− lymphoid tissue inducer cells (LTic's) are known to play a key role in influencing lymph node (LN) development, the mechanisms that regulate the development of stromal organizer cells are unclear. Here, we define an ontogenetic program of lymph node stromal cell maturation in relation to the requirement for LTic's. We also describe a lymph node reaggregation assay to study cell-cell interactions and lymphocyte recruitment to these organs that reproduces the in vivo events during lymph node development. In addition, analysis of the lymph node anlagen in normal and lymphotoxin a (LTa)–deficient embryos shows that LTa-mediated signaling is required to sustain proliferation and survival of stromal cells in vivo. Our data identify LTa-independent and LTa-dependent stages of lymph node development, and provide direct evidence for the role of LTic's during LN organogenesis.
Introduction
One of the main features of the immune system is the cross-talk interaction between different cell types that ensures full activation of immune responses. The exchange of information between cells of the immune system occurs within specific microenvironments in secondary lymphoid organs such as the spleen, lymph nodes (LNs), and Peyer patches (PPs). Disruption or abnormal development of these microenvironments leads to ineffective adaptive immune responses, resulting in repetitive infections and decrease survival of the host.
LN organogenesis during embryo development depends on successful interactions between bone marrow–derived lymphoid tissue inducer cells (LTic's) and mesenchymal organizer stromal cells.1–3 The signals involved in these interactions are transmitted through ligands and receptors of the tumor necrosis factor family that converge in activation of the NF-κB transcription factors, resulting in marked changes in gene expression.4,5 Genetic approaches had shown the crucial roles of lymphotoxin β receptor (LtβR) and receptor activator of NF-κB (RANK) and their ligands in the development of LNs. For instance, Lta−/− mice and Ltbr−/− mice lack all LNs, Ltb−/− mice form only mesenteric LNs, and Rank−/− and Rank-l−/− mice develop only rudimentary mesenteric LNs.3,6–10 Similarly, mice deficient in the NF-κB family proteins RelA and RelB lack all LNs, while Nfkb2−/− and Ikkaaa knock-in mice present with defects in peripheral LNs.11–16
While LTic's express the membrane-bound LTα1β2 ligand, stromal cells express the LTβR receptor. Engagement of LTβR receptor on stromal cells results in production of chemokines such as CXCL13, CCL21, and CCL19, and cell adhesion molecules that will attract further LTic's that express the chemokine receptors CXCR5 and CCR7.17–19 Subsequent formation of cell clusters between LTic's and organizer cells results in organization of specific areas in the LN anlagen.
Earlier studies by Nishikawa and colleagues had described in great detail the processes involved in PP development, and thus it had been inferred that a similar sequence of events give origin to LNs.20,21 Mebius and colleagues had demonstrated the accumulation of LTic's in LN anlagen, and more recently identified differences in stromal organizer cell populations between mucosal and peripheral LNs in newborn mice.22,23 However, lymphoid tissue stromal cells remain a poorly characterized cell population despite their important function in LN and spleen development.
The significance of studying the cross-talk between LTic's and stromal cells during LN development is based on the similarities between these processes and the interactions between activated lymphocytes and stromal cells that result in formation of ectopic lymphoid tissues in chronic inflammatory diseases such as rheumatoid arthritis and Crohn disease. Indeed, chronic inflammation leads to lymphoid organogenesis as shown in animal models through ectopic overexpression of different cytokines or chemokines.24–28
More strikingly, a recent report demonstrated that all the elements required for the development of secondary or tertiary lymphoid structures were present in LNs of newborn mice, and that formation of the proper architecture of T- and B-cell areas depends on the presence of LTic's during development, or activated lymphocytes in the adult.29 Interestingly, neither stromal cells nor LTic's on their own were able to induce lymphoid structures upon subcutaneous injection in mice. However, the mechanisms regulating LN development during embryogenesis are still poorly understood, in particular how cross-talk interactions between LTic's and stromal organizer cells contribute to LN formation. The difficulty of isolating LNs from early-stage mouse embryos and the lack of in vitro approaches to study LTic–stromal cell interactions had hindered advances in this field.
In this report, we present a novel LN reaggregation assay that, through the isolation of the different cell populations present in LNs from embryos and newborn mice, and subsequent reaggregation and kidney grafting in adult hosts, allows the study of cell-cell interactions during the formation of LN anlagen and recruitment of lymphocytes. We demonstrate that maturation of stromal cells in inguinal LNs (iLNs) takes place between embryonic day (E)15 and E17 and correlates with the accumulation of CD45+ cells, including LTic's, in LN anlagen. Whole-LN kidney graft-transplantation assays show that while E17 iLNs form lymphoid structures upon grafting, E15 iLNs are unable to do so. Addition of fetal LTic to E15 iLNs by LN reaggregation assays rescues this phenotype and results in the formation of organized lymphoid structures populated by host lymphocytes.
In addition, by isolating iLN anlagen from Lta−/− mice, we demonstrate that LTα-mediated signals are required for proliferation and survival of LN stromal cells. In summary, our data provide a model of stromal-cell organizer maturation in the developing iLN, and highlight LTα-independent and LTα-dependent stages of LN organogenesis.
Materials and methods
All the experimental procedures for this manuscript were performed after receiving approval by the University of Birmingham Review Board. In addition, all experimental procedures used during the research presented in this manuscript had been previously approved by the United Kingdom Home Office according to the terms of the Animals (Scientific Procedures) Act of 1986 as specified in project and personal licenses to the authors.
Mice
BALB/c, C57BL6J, and Lta−/− mice (which express normal levels of Tnfa mRNA)30 (B and K Universal, Grimston, United Kingdom) were maintained under specific pathogen–free conditions at the Biomedical Services Unit at the University of Birmingham, according to Home Office regulations. Timed pregnancies were carried out using vaginal plug detection as E0.
Antibodies and immunoconjugates
Primary antibodies used for flow cytometric analysis are: anti-CD3ϵ FITC (145–2C11; BD Pharmingen, San Diego, CA), anti-CD4 PE (GK1.5), anti-CD8a (53-6.7) FITC, anti-CD19 (6D5) PE, anti-CD45.2 (104) FITC, anti-CD45 (30-F11) APC, anti–VCAM-1 (429) biotin, and anti–ICAM-1 (YN1/1.7.4) PE (eBioscience, San Diego, CA). Streptavidin-PE Cy7 (Chemicon, Temecula, CA) was used to visualize biotin-conjugated primary antibodies.
Isolation and analysis of iLN stromal cells
iLNs were isolated from embryos at defined stages of development using a Zeiss Stemi SVII dissecting microscope (Zeiss, Welwyn, United Kingdom) and a Nikon Coolpix 990 camera (Nikon, Melville, NY). Isolated iLNs were washed in Ca2+/Mg2+-free PBS (Sigma, Poole, United Kingdom) and incubated with 0.25% trypsin in 0.02% EDTA at 37°C to produce a single-cell suspension. Cells were labeled with antibodies to CD45, ICAM-1, and VCAM-1, and analyzed using a BD LSR flow cytometer (Becton Dickinson, Franklin Lakes, NJ) with forward- and side-scatter gates set to exclude nonviable cells. In some cases, cell suspensions were depleted of CD45+ cells to isolate stromal cells using multiple rounds of Dynabead sheep anti-rat IgG magnetic beads (Dynal, Wirral, United Kingdom) coated with α-CD45 (M1/9; ATCC, Manassas, VA) as described.31
Analysis of CD45+ cells in developing iLNs
Embryonic and postnatal iLNs were isolated and disaggregated in 2.5 mg/mL collagenase/dispase (R&D Systems, Minneapolis, MN) in PBS at 37°C to produce a single-cell suspension. Cells were labeled with antibodies to CD4 and CD45 and analyzed by fluorescence-activated cell sorter (FACS) as described.
Fetal spleen organ culture and preparation of CD4+CD3− LTic's
Spleens were isolated from E15 mouse embryos and organ-cultured for 7 days with murine recombinant interleukin-7 (100 ng/mL; R&D Systems) on the surface of 0.8-μm Nuclepore filters (Millipore, Billerica, MA) supported by sponge (Medipost, Weymouth, United Kingdom). Cultured embryonic spleens were then mechanically disaggregated, and resultant suspensions were labeled with antibodies to CD3ϵ and CD4. CD4+CD3− LTic's were sorted to high purity (> 98%) using a MoFlo high-speed cell sorter (Dako Cytomations, Glostrup, Denmark).
Formation of reaggregate organ cultures
E15 iLNs were isolated and disaggregated using 0.25% trypsin, and resultant suspensions were mixed with isolated LTic's at a ratio of 3:1 (total cell number, 120 000). The cell suspension was drawn to a pellet in a 96–V-well plate, and cultured overnight at 37°C. The resultant reaggregate culture was grafted under the kidney capsule of a 6- to 8-week-old C57Bl6 or BALB/c mouse as described.32 After 2 weeks, grafted iLNs were removed and analyzed by FACS or immunohistochemistry, with the host iLNs used as a control.
Immunohistochemistry
iLNs or recovered grafts were isolated and embedded on OCT compound (Tissue Tek, Torrance, CA), then frozen in liquid nitrogen. Sections were cut (5 μm) using a cryostat (Bright, Huntingden, United Kingdom) and mounted onto multispot glass slides. Sections were fixed in acetone for 20 minutes at 4°C and stored at −20°C. Primary antibodies used were: purified anti-CD3 (125-2c11), purified anti-B220 (RA3-6B2), purified anti-PNAd (MECA-79) (BD Pharmingen), and ERTR7 supernatant (kind gift of W. van Ewijk, University of Leiden, the Netherlands). Secondary antibodies used were goat anti-rat IgG strep 488 Alexa Fluor and goat anti-rat IgM strep 594 Alexa Fluor (Molecular Probes, Eugene, OR). Confocal images were obtained on an LSM510 Meta confocal microscope (Zeiss) equipped with either a 10×, 25×, 40×, or 63×/1.4 NA water lens and 405, 488, 543, and 633 lasers using Zeiss LSM software.
Detection of cell proliferation and apoptosis
Embryonic LNs were isolated and disaggregated using collagenase/dispase as described previously. To assay cell proliferation, stromal cells were isolated by magnet bead depletion of CD45+ hematopoietic cells. Stromal cells were resuspended in PBS (containing 9% FCS; 1 × 105 cells/200μL) and placed into a cytospin funnel and centrifuged for 5 minutes at 18.8g. Slides were removed and left to air dry for 10 minutes, then acetone-fixed for 20 minutes at 4°C. Using the method described previously, slides were labeled using anti-Ki67 (kind gift of J. Gerdes, Research Center, Borstel, Germany), antihamster biotin hamster (DAKO, Ely, United Kingdom), and streptavidin Alexa Fluor 488 (Molecular Probes), then visualized using confocal microscopy.
To assess for cells undergoing apoptosis, cells were labeled using anti-CD45 APC, followed by an Annexin V detection kit (BD Pharmingen) according to the manufacturer's instructions. Samples were analyzed by FACS.
RT-PCR analysis
Postnatal day (P) 2 iLN CD45− cell populations were isolated according to their VCAM-1 and ICAM-1 expressions (V−I+, V+I+, and V−I−) by high-speed cell sorting. High-purity cDNA was obtained from purified mRNA using μMacs one-step cDNA synthesis kit, according to the manufacturer's instructions (Miltenyi Biotech, Auburn, CA). Beta-actin was used as a housekeeping gene for sample normalization, prior to amplifying the genes of interest. Reactions were carried out in a Peltier Thermal Cycler PTC-200 (MJ Research, Braintree, United Kingdom) using ABgene ReddyMix (Abgene, Epsom, United Kingom) as described.32 Polymerase chain reaction (PCR) products taken from the exponential phase of the reactions were analyzed by ethidium bromide gel electrophoresis and identified by fragment size on a 2% agarose gel. Densitometrical analysis was determined using Syngene Gel Documentation Gene Tools (Syngene, Cambridge, United Kingdom). Graphs show ratios of mRNA for the genes of interest relative to β-actin. Primers used are described in Document S1, available on the Blood website (see the Supplemental Materials link at the top of the online article). Statistical analysis was performed using the 2-tail t test.
Results
Formation of the conduit system during LN organogenesis
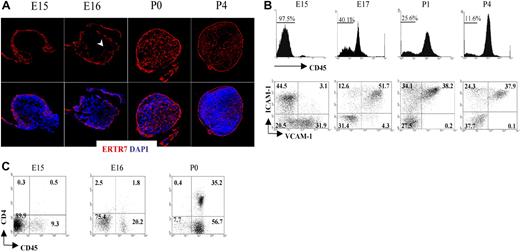
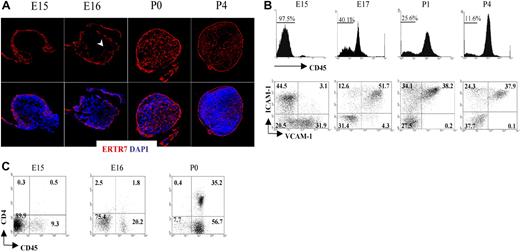
Fibroblastic reticular cells secrete a protein that becomes part of the extracellular matrix and is recognized by the monoclonal antibody ERTR7.33,34 Staining of the LN with ERTR7 highlights the different compartments of the reticular network that form the support structure of these organs. To unravel the formation of the reticular network or conduit system during the initial stages of peripheral LN development, we dissected iLNs from embryonic and neonatal mice, and analyzed them by immunofluorescence staining using ERTR7. As shown in Figure 1A, a reticular ERTR7+ network was visualized in the periphery of the iLN anlagen at E15, while the cells in the center were ERTR7−. By E16, ERTR7+ structures started to develop from the periphery toward the center of the anlagen. At later stages of development and in neonates, the organization of the iLN reticular network had become more complex, with a strong ERTR7 staining of the subcapsular sinus and several vesicular structures inside the organ. Of note, at P4, we observed the presence of high endothelial venules (HEVs), which appeared surrounded by ERTR7+ staining, in addition to the formation of B- and T-cell areas.
Formation of the conduit system during LN organogenesis. (A) Immunofluorescence staining of E15, E16, P0 (40×/1.4 NA water lens), and P4 (25×/1.4 NA) iLN sections showing the development and organization of the ERTR7+ reticular network (red) and nuclear staining DAPI (blue). Note that the cells in the center of the anlagen at E15 were ERTR7− and by E16 ERTR7+ structures were seen toward the center of the organ (arrowhead). (B) Changes in stromal-cell populations during iLN ontogeny. FACS analysis of single-cell suspensions of iLNs at the times indicated showing the recruitment of bone marrow–derived cells as an increased percentage of CD45+ cells and the concomitant changes in the CD45− stromal cells, including the appearance of VCAM-1+ICAM-1+ mature stromal organizer cells and the progressive disappearance of VCAM-1+ICAM-1− cells. Percentages in scatterplots correspond to CD45− stromal cells according to their expression of VCAM-1 and ICAM-1. (C) FACS analysis of single-cell suspensions from E15, E16, and P0 iLNs. Percentages shown in scatterplots correspond to CD3− cells according to their expression of CD45 and CD4. Note that LTic's are CD45+4+, and the largest population present is CD45+4−. Results are representative of 4 independent experiments.
Formation of the conduit system during LN organogenesis. (A) Immunofluorescence staining of E15, E16, P0 (40×/1.4 NA water lens), and P4 (25×/1.4 NA) iLN sections showing the development and organization of the ERTR7+ reticular network (red) and nuclear staining DAPI (blue). Note that the cells in the center of the anlagen at E15 were ERTR7− and by E16 ERTR7+ structures were seen toward the center of the organ (arrowhead). (B) Changes in stromal-cell populations during iLN ontogeny. FACS analysis of single-cell suspensions of iLNs at the times indicated showing the recruitment of bone marrow–derived cells as an increased percentage of CD45+ cells and the concomitant changes in the CD45− stromal cells, including the appearance of VCAM-1+ICAM-1+ mature stromal organizer cells and the progressive disappearance of VCAM-1+ICAM-1− cells. Percentages in scatterplots correspond to CD45− stromal cells according to their expression of VCAM-1 and ICAM-1. (C) FACS analysis of single-cell suspensions from E15, E16, and P0 iLNs. Percentages shown in scatterplots correspond to CD3− cells according to their expression of CD45 and CD4. Note that LTic's are CD45+4+, and the largest population present is CD45+4−. Results are representative of 4 independent experiments.
These results indicate that the initiation of a complex reticular network in iLNs starts to take place as early as E15.34
Maturation of LN stromal cells occurs simultaneously with the arrival of LTic's
We next analyzed colonization of LN anlagen by CD45+ cells and the process of mesenchymal cell maturation to become LN organizer cells. For that purpose, we isolated iLNs from E15 to P4 and analyzed cellular heterogeneity in CD45− stromal cells using the markers VCAM-1 and ICAM-1. Consistent with the notion that the iLN anlagen is colonized by hematopoietic elements at around E15 to E16 of gestation, flow cytometric analysis showed that E15 iLNs typically contained between 2.5% to 8% CD45+ cells (Figure 1B; data not shown). By gating on CD45− cells to analyze the stromal compartment of iLNs, we identified distinct stromal subsets on the basis of VCAM-1 (V+) and ICAM-1 (I+) expression. Interestingly, while V+I− and V−I+ single-positive cell populations were readily detectable at E15, cells of a double-positive V+I+ phenotype, typical of mature LN organizer stromal cells, were notably absent at this stage (Figure 1B). However, in contrast to the E15 LN anlagen, analysis of iLNs at E17 showed the appearance of V+I+ organizer cells, with a parallel decrease in the frequency of single-positive cells, in particular the V+I− cell population (Figure 1B; data not shown). By P1 to P4, the V+I− cells were no longer present, and the V+I+ organizer cells contained the largest population. Concomitant with the appearance of V+I+ organizer cells, an increase in CD45+ cell frequency from 2.5% at E15 to 20% to 60% at E17 was observed (Figure 1B,C; data not shown).
Analysis of CD45+ cells present in E15 iLNs showed that most of these cells were CD4− (Figure 1C). By E16, the CD45+CD4− reached around 18% to 20%, and the CD45+CD4+ LTic's were found at 1.5% to 2.0% of total iLN cells. At the day of birth, CD45+4− cells had increased 2- to 3-fold, and CD45+4+ LTic's by 15- to 20-fold with respect to the frequency found at E16 iLNs. Collectively, these results show that during iLN development, the maturation of stromal cells to become V+I+ organizer cells occurs between E15 and E17, and correlates with a substantial influx of CD45+ cells to the iLN anlagen.
Gene expression analysis of LN stromal-cell populations
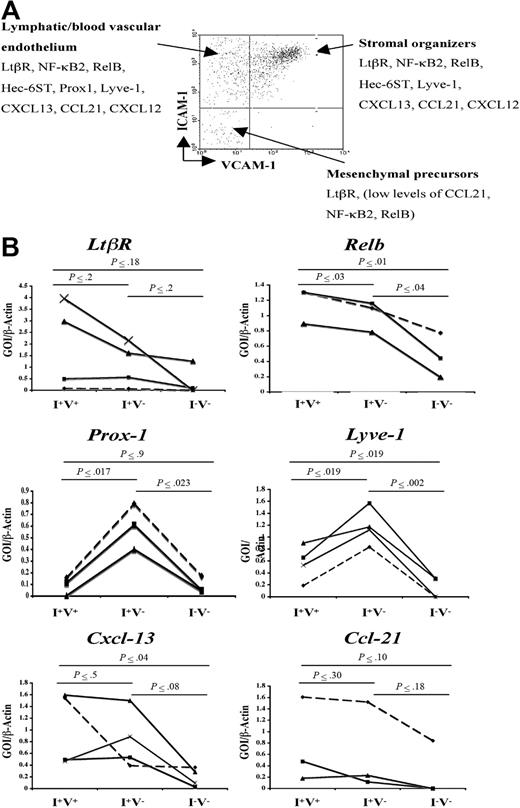
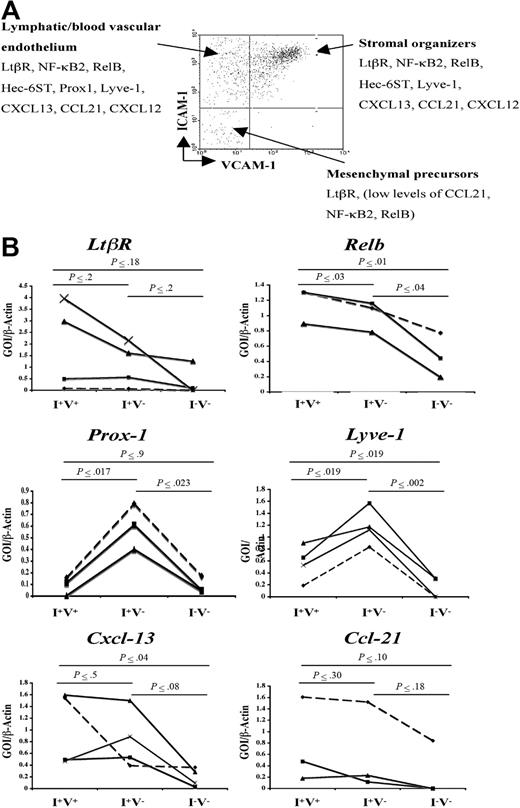
Besides the expression of VCAM-1, ICAM-1, and MAdCAM-1 by LN stromal cells, little is known about the gene expression programs of the different subpopulation of stromal cells. To address this, semiquantitative reverse transcription (RT)–PCR was performed in the main CD45− cell populations present in P2 iLNs according to their VCAM-1 and ICAM-1 expressions (V−I+, V+I+, and V−I−) to assess the expression of cell adhesion, chemokines, and signaling molecules known to have a role during LN development and lymphocyte recruitment. The V+I− cell population contained very few cells to be sorted and analyzed.
Analysis of the expression of Prox-1 and hyaluronan receptor Lyve-1 showed highest levels in the V−I+ subset of stromal cells, indicating that this cell compartment included blood vascular and lymphatic endothelial cells (Figure 2B). While mRNA for Ltbr was present in all cell populations, V+I+ stromal organizer cells and V−I+ cells expressed Cxcl13, Ccl19, Ccl21, Nfkb2, and Relb at similar levels, while V−I− cells expressed low levels of Ccl21, Nfkb2, and Relb (Figure 2B; data not shown). Due to the very low number of stromal cells present in P2 iLNs, protein levels for the genes mentioned here could not be assessed.
Gene expression analysis of newborn LN stromal cells. (A) FACS analysis of P2 iLN CD45− cells stained for VCAM-1 and ICAM-1. Each cell population was isolated to prepare cDNA and assessed for the expression of several genes by semiquantitative PCR. According to their gene expression patterns, the cells were classified in putative mesenchymal precursor cells (V−/I−), stromal organizer cells (V+/I+), and lymphatic/vascular endothelium (V−/I+). (B) Semiquantitative PCR analysis of the Ltbr and Nfkb genes involved in this pathway (Nfkb2, Relb), hyaluronan receptor (Lyve-1 and Prox-1), and chemokines (Cxcl13, Ccl21) in the 3 stromal cell populations present in P2 iLNs. Ratio of gene of interest (GOI) to β-actin is shown. Results shown correspond to 3 to 4 independent cell sorting and PCR experiments (Experiment 1, ♦; Experiment 2, ■; Experiment 3, ▴; and Experiment 4, ×). Statistical analysis was performed using the 2-tail t test. Statistically significant differences, as calculated by the P value, in the expression of specific genes in the 3 cell populations are shown in bold.
Gene expression analysis of newborn LN stromal cells. (A) FACS analysis of P2 iLN CD45− cells stained for VCAM-1 and ICAM-1. Each cell population was isolated to prepare cDNA and assessed for the expression of several genes by semiquantitative PCR. According to their gene expression patterns, the cells were classified in putative mesenchymal precursor cells (V−/I−), stromal organizer cells (V+/I+), and lymphatic/vascular endothelium (V−/I+). (B) Semiquantitative PCR analysis of the Ltbr and Nfkb genes involved in this pathway (Nfkb2, Relb), hyaluronan receptor (Lyve-1 and Prox-1), and chemokines (Cxcl13, Ccl21) in the 3 stromal cell populations present in P2 iLNs. Ratio of gene of interest (GOI) to β-actin is shown. Results shown correspond to 3 to 4 independent cell sorting and PCR experiments (Experiment 1, ♦; Experiment 2, ■; Experiment 3, ▴; and Experiment 4, ×). Statistical analysis was performed using the 2-tail t test. Statistically significant differences, as calculated by the P value, in the expression of specific genes in the 3 cell populations are shown in bold.
These results show that Ltbr, the components of the alternative NF-κB pathway, and its target genes encoding chemokines and cell adhesion molecules are expressed at the mRNA level in V+I+ stromal organizer cell compartment and in V−I+ lymphatic/blood vascular endothelial cells at comparatively higher levels than in the V−I− cell population.
Direct evidence of an essential role for LTic's in LN organogenesis
We and others have shown that grafting of LNs from neonatal mice under the kidney capsule of adult recipient mice results in the formation of lymphoid tissue structures containing host lymphocytes organized in B- and T-cell areas almost indistinguishable from adult LNs (Figure 3A-E).13,35–37 To assess the role of LTic's during LN development and lymphocyte recruitment, we used kidney capsule graft assays to test whether there were any differences in LN formation and organization between E15 iLN anlagen, which holds a very low frequency of LTic and organizer cells, and E17 iLNs containing both a higher frequency of LTic's and a large population of stromal organizer cells than at the previous stage.
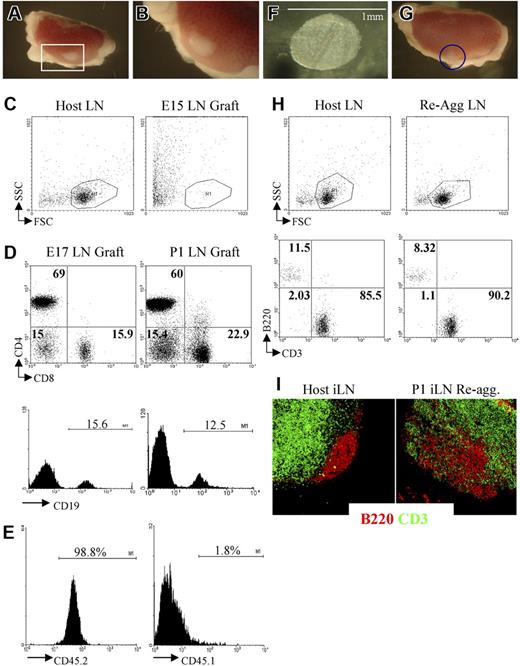
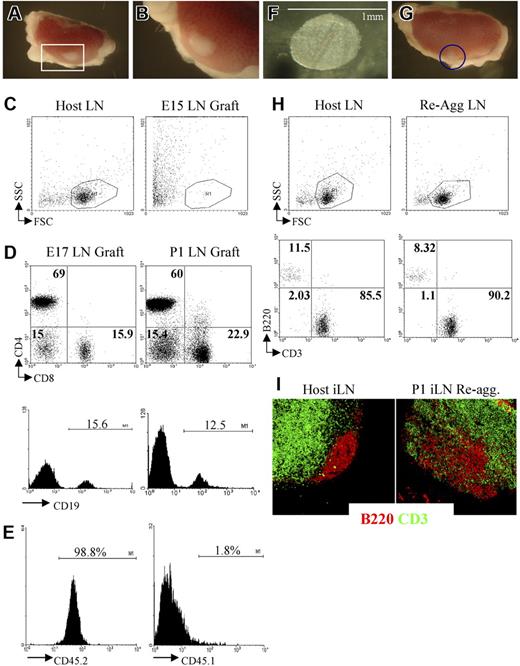
Grafting of iLNs or reaggregate LNs results in the formation of lymphoid structures. (A,B) Grafting of a P1 iLN under the kidney capsule of an adult mouse results in the formation of a lymphoid structure after 2 weeks. The red rectangle in panel A (25×/1.4) contains the graft that is enlarged in panel B (40×/1.4). (C) FACS analysis of an E15 embryonic iLN grafted for 2 weeks under the kidney capsule of an adult mouse and the iLN of the host. As shown by forward/side-scatter profile, the E15 grafts contained only dead cells. Results are representative of 6 independent experiments. (D) E17 and P1 iLN grafts contained CD4+ and CD8+ T cells as well as CD19+ B cells. Percentages shown in dot plots correspond to single positive CD4, CD8, and CD4−8− cells. Histograms show the percentage of CD19+ B cells. Results shown are representative of 5 independent assays. (E) Bone marrow–derived cells colonizing the kidney-grafted iLNs are of host origin. Single-cell suspensions of wild-type congenic (CD45.1) iLNs grafted for 2 weeks under the kidney capsule of C57Bl6J (CD45.2) mice were analyzed for the expression of CD45.1/CD45.2 markers to assess their origin. Percentages shown correspond to donor iLN CD45.1+ cells and host CD45.2+ cells. As shown here, more than 98% of the cells are of host origin. (F) Reaggregate iLNs before being grafted under the kidney capsule of an adult recipient mouse (40×/1.4). (G) The iLN reaggregate (blue circle) shown on the kidney 2 weeks after grafting (25×/1.4). (H) Reaggregate iLNs recruited B and T cells similar to the LNs of the host. Percentages shown correspond to B220+ B cells and CD3+ T cells in host iLNs and kidney-grafted reaggregate iLNs. (I) Reaggregate iLNs were colonized by lymphocytes that migrated to specific areas in the organ. Immunofluorescence staining of sections of an adult host iLN and a P1 reaggregate iLN that had been grafted for 2 weeks under the kidney capsule of the host showing B220+ B-cell follicular areas (red) in the cortex and the CD3+ T-cell areas (green) located in the paracortex. The organization of B- and T-cell areas appeared similar between the host iLN and the reaggregate organ, although B cells appear less packed in the case of the latter (10×/1.4 NA). Results shown are representative of 6 to 8 independent experiments. (A,B,F,G,I) Images were viewed with a Zeiss Stemi SVII dissecting microscope.
Grafting of iLNs or reaggregate LNs results in the formation of lymphoid structures. (A,B) Grafting of a P1 iLN under the kidney capsule of an adult mouse results in the formation of a lymphoid structure after 2 weeks. The red rectangle in panel A (25×/1.4) contains the graft that is enlarged in panel B (40×/1.4). (C) FACS analysis of an E15 embryonic iLN grafted for 2 weeks under the kidney capsule of an adult mouse and the iLN of the host. As shown by forward/side-scatter profile, the E15 grafts contained only dead cells. Results are representative of 6 independent experiments. (D) E17 and P1 iLN grafts contained CD4+ and CD8+ T cells as well as CD19+ B cells. Percentages shown in dot plots correspond to single positive CD4, CD8, and CD4−8− cells. Histograms show the percentage of CD19+ B cells. Results shown are representative of 5 independent assays. (E) Bone marrow–derived cells colonizing the kidney-grafted iLNs are of host origin. Single-cell suspensions of wild-type congenic (CD45.1) iLNs grafted for 2 weeks under the kidney capsule of C57Bl6J (CD45.2) mice were analyzed for the expression of CD45.1/CD45.2 markers to assess their origin. Percentages shown correspond to donor iLN CD45.1+ cells and host CD45.2+ cells. As shown here, more than 98% of the cells are of host origin. (F) Reaggregate iLNs before being grafted under the kidney capsule of an adult recipient mouse (40×/1.4). (G) The iLN reaggregate (blue circle) shown on the kidney 2 weeks after grafting (25×/1.4). (H) Reaggregate iLNs recruited B and T cells similar to the LNs of the host. Percentages shown correspond to B220+ B cells and CD3+ T cells in host iLNs and kidney-grafted reaggregate iLNs. (I) Reaggregate iLNs were colonized by lymphocytes that migrated to specific areas in the organ. Immunofluorescence staining of sections of an adult host iLN and a P1 reaggregate iLN that had been grafted for 2 weeks under the kidney capsule of the host showing B220+ B-cell follicular areas (red) in the cortex and the CD3+ T-cell areas (green) located in the paracortex. The organization of B- and T-cell areas appeared similar between the host iLN and the reaggregate organ, although B cells appear less packed in the case of the latter (10×/1.4 NA). Results shown are representative of 6 to 8 independent experiments. (A,B,F,G,I) Images were viewed with a Zeiss Stemi SVII dissecting microscope.
At 2 weeks after grafting under the kidney capsule, E15 iLNs were found to have generated poorly defined fibrous tissue at the graft site, which upon further analysis contained only dead cells (Figure 3C). In contrast, grafting of E17 or P1 iLNs for a similar period resulted in the formation of cellular lymphoid structures containing host-derived B and T lymphocytes that were organized into specific areas in a manner comparable to unmanipulated host LNs (Figure 3D).6 Taken together with the paucity of CD45+4+ cells at E15 compared with E17, these findings support the notion that a critical threshold of both LTic's and mature stromal organizer cells are required for normal iLN development.
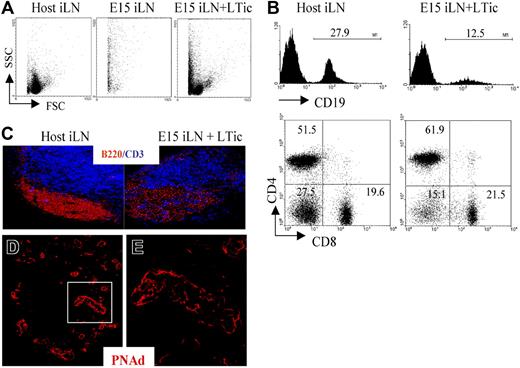
To provide direct evidence of a role for LTic's in the development of the iLN anlagen, we adapted the reaggregate organ cultures in combination with kidney capsule transplantation (Figure 3F,G). While reaggregate grafts from P1 iLN tissue were found to contain host lymphocytes and showed evidence of T/B segregation (Figure 3G-I), the grafting of reaggregate organ cultures initiated from several E15 iLNs once again resulted in the generation of fibrous tissue containing only dead cells (Figure 4A). Importantly however, when reaggregate cultures were formed from a mixture of E15 iLN stromal cells and CD4+CD3−IL7Ra+ LTic's isolated from fetal spleens cultured in IL-7, after 2 weeks, lymphoid grafts were detectable that had recruited host T and B cells, although the latter at a lower frequency than in wild-type iLNs (Figure 4B,C). Moreover, immunofluorescence staining of these lymphoid structures showed that they contained the classical architecture of wild-type LNs with specific B- and T-cell areas and a PNAd+ HEV network (Figure 4C-E). The culture of fetal spleens in IL-7 to isolate LTic's was necessary to expand such cell population.
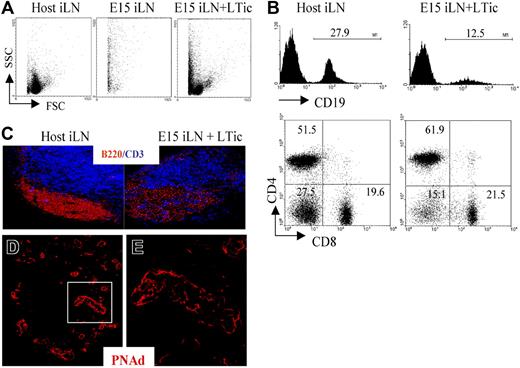
Addition of fetal LTic is sufficient to rescue the impaired formation of lymphoid aggregates by E15 iLN upon kidney graft. (A) Forward- and side-scatter profiles of a host iLN, a reaggregate E15 iLN, and an E15 iLN that receive LTic's were grafted under the kidney capsule of adult recipients and analyzed for their cellular content 2 weeks after transplantation. Reaggregated E15 iLNs formed a mass of dead cells, while the reaggregated E15 iLNs that receive LTic's contained live cells. (B) Reaggregated E15 iLN + LTic contained T and B cells. Numbers above brackets correspond to percentages of CD19+ B cells. Percentages of single positive CD4, CD8 and CD4−8− are shown in each quadrant of dot plots. (C) B and T cells in reaggregated iLNs were organized in specific areas similar to adult LNs as seen by immunofluorescence staining with anti-CD3 (blue) and anti-B220 (red) mAbs (10x). (D,E) HEV development in reaggregate iLNs. Reaggregated E15 iLN + LTic structures contain a network of HEVs. Immunofluorescence staining of sections of E15 iLN + LTic reaggregated LN grafts with MECA 79 mAb (red) that recognizes a sugar moiety present in several peripheral node addressins (PNAd's) that are expressed in HEVs. The white rectangle in panel D (25×/1.4 NA) is enlarged in panel E (40×/1.4 NA water lens). Results shown are representative of 6 independent assays.
Addition of fetal LTic is sufficient to rescue the impaired formation of lymphoid aggregates by E15 iLN upon kidney graft. (A) Forward- and side-scatter profiles of a host iLN, a reaggregate E15 iLN, and an E15 iLN that receive LTic's were grafted under the kidney capsule of adult recipients and analyzed for their cellular content 2 weeks after transplantation. Reaggregated E15 iLNs formed a mass of dead cells, while the reaggregated E15 iLNs that receive LTic's contained live cells. (B) Reaggregated E15 iLN + LTic contained T and B cells. Numbers above brackets correspond to percentages of CD19+ B cells. Percentages of single positive CD4, CD8 and CD4−8− are shown in each quadrant of dot plots. (C) B and T cells in reaggregated iLNs were organized in specific areas similar to adult LNs as seen by immunofluorescence staining with anti-CD3 (blue) and anti-B220 (red) mAbs (10x). (D,E) HEV development in reaggregate iLNs. Reaggregated E15 iLN + LTic structures contain a network of HEVs. Immunofluorescence staining of sections of E15 iLN + LTic reaggregated LN grafts with MECA 79 mAb (red) that recognizes a sugar moiety present in several peripheral node addressins (PNAd's) that are expressed in HEVs. The white rectangle in panel D (25×/1.4 NA) is enlarged in panel E (40×/1.4 NA water lens). Results shown are representative of 6 independent assays.
Taken together, these results show that by means of reaggregate LN assays, the single addition of LTic's to the E15 iLN anlagen induces a program of development leading to the formation of organized lymphoid tissue capable of recruiting host lymphocytes.
Comparison of wild-type and Lta−/− mouse embryos identifies LTα-independent and -dependent stages of iLN organogenesis
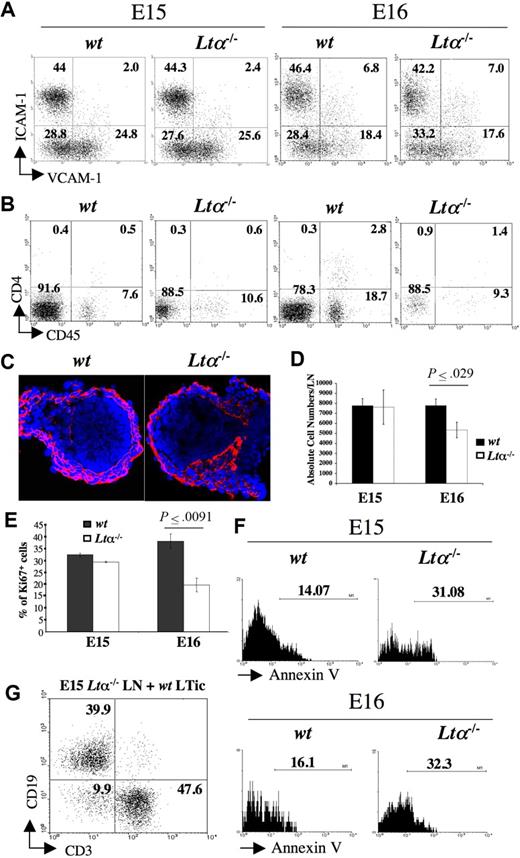
We next investigated the involvement of LTα in both early- and late-stage iLN organogenesis. ILNs from E15 and E16 Lta−/− and wild-type mice were isolated and analyzed by FACS to assess the presence of organizer stromal and CD45+ cells. E15 iLNs could be readily isolated from both wild-type and Lta−/− mice, and showed no differences with respect to their stromal and inducer cell populations (Figure 5A,C). At E16, approximately only 50% of the iLNs from Lta−/− mice could be isolated due to their reduced size as compared with wild-type mice, and by E17, Lta−/− iLNs could not be properly identified (Table 1; data not shown). Moreover, by E16, while a 3- to 6-fold increase in the frequency of LTic's was seen in wild-type iLNs, just a 2-fold increase was consistently recorded in Lta−/− iLNs with respect to their E15 counterparts (Figure 5A,B). A similar defect was observed in CD45+4− cells between wild-type and Lta−/− iLNs, probably reflecting a decreased influx of both cell populations.
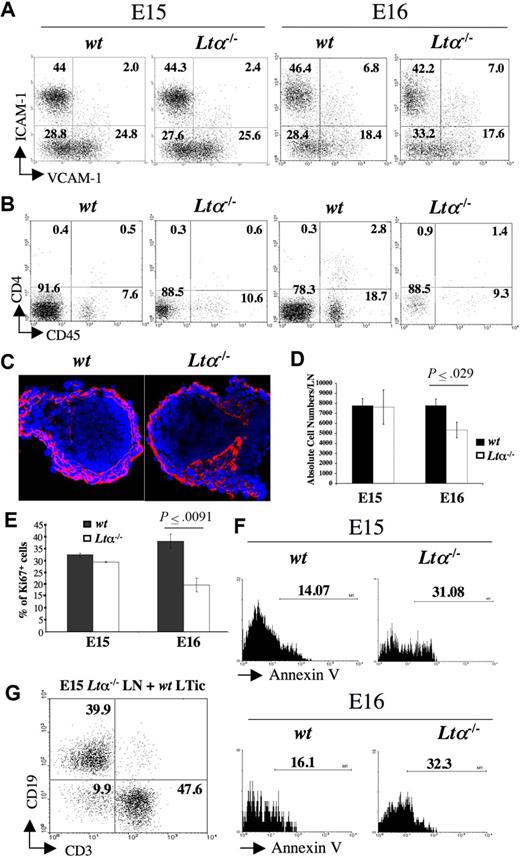
Analysis of LN development in Lta−/− mice. (A) FACS analysis of CD45− stromal cell from E15 and E16 wild-type and Lta−/− iLNs with mAbs for VCAM-1 and ICAM-1. Percentages of CD45− cells according to their expression of VCAM-1 and ICAM-1 are shown in each quadrant. (B) FACS analysis of cell suspensions of E15 and E16 wild-type and Lta−/− iLNs with mAbs for CD4 and CD45. Numbers on each quadrant correspond to percentages of CD3− cells expressing CD4 and CD45. (C) Immunofluorescence staining of E15 wild-type and Lta−/− iLN sections with ERTR7 (red) and DAPI (blue) (10×/1.4 NA). (D) A statistically significant reduction in total cell numbers in iLN of Lta−/− E16 embryos compared with their wild-type counterparts. Results shown are wild-type: 7780 cells SEM 657, Ltα−/−: 5339 cells SEM 778, unpaired t test P < .03. (E) Statistically significant decrease in percentages of cycling CD45− stromal cells from E16 Lta−/− iLNs with respect to their wild-type counterparts as measured by Ki67 staining. Results shown are Ki67+ cells wild-type: 38% SEM 3, Ltα−/−: 19.6% SEM 2.9, unpaired t test P < .009. (F) Annexin V staining of CD45− stromal cell from E15 and E16 Lta−/− and wild-type iLNs. Note the increased frequency of cells undergoing apoptosis (Annexin V+) in the Ltα−/− cells compared to their wild-type counterparts. Numbers above brackets correspond to percentages of Annexin V+ cells. (G) The addition of wild-type LTic's rescued the defect of E15 Lta−/− reaggregate iLNs to form a lymphoid aggregate upon kidney capsule grafting that recruited CD19+ B cells and CD3+ T cells. Numbers on the quadrants correspond to percentages of either CD3+ T cells, CD19+ B cells, or double negative cells. Results shown here are from 1 representative assay out of 5 independent experiments. Error bars in panels D,E represent SEM.
Analysis of LN development in Lta−/− mice. (A) FACS analysis of CD45− stromal cell from E15 and E16 wild-type and Lta−/− iLNs with mAbs for VCAM-1 and ICAM-1. Percentages of CD45− cells according to their expression of VCAM-1 and ICAM-1 are shown in each quadrant. (B) FACS analysis of cell suspensions of E15 and E16 wild-type and Lta−/− iLNs with mAbs for CD4 and CD45. Numbers on each quadrant correspond to percentages of CD3− cells expressing CD4 and CD45. (C) Immunofluorescence staining of E15 wild-type and Lta−/− iLN sections with ERTR7 (red) and DAPI (blue) (10×/1.4 NA). (D) A statistically significant reduction in total cell numbers in iLN of Lta−/− E16 embryos compared with their wild-type counterparts. Results shown are wild-type: 7780 cells SEM 657, Ltα−/−: 5339 cells SEM 778, unpaired t test P < .03. (E) Statistically significant decrease in percentages of cycling CD45− stromal cells from E16 Lta−/− iLNs with respect to their wild-type counterparts as measured by Ki67 staining. Results shown are Ki67+ cells wild-type: 38% SEM 3, Ltα−/−: 19.6% SEM 2.9, unpaired t test P < .009. (F) Annexin V staining of CD45− stromal cell from E15 and E16 Lta−/− and wild-type iLNs. Note the increased frequency of cells undergoing apoptosis (Annexin V+) in the Ltα−/− cells compared to their wild-type counterparts. Numbers above brackets correspond to percentages of Annexin V+ cells. (G) The addition of wild-type LTic's rescued the defect of E15 Lta−/− reaggregate iLNs to form a lymphoid aggregate upon kidney capsule grafting that recruited CD19+ B cells and CD3+ T cells. Numbers on the quadrants correspond to percentages of either CD3+ T cells, CD19+ B cells, or double negative cells. Results shown here are from 1 representative assay out of 5 independent experiments. Error bars in panels D,E represent SEM.
Total cell counts revealed a statistically significant reduction in cell numbers in Lta−/− iLNs compared with their wild-type counterparts at E16 (wild-type: 7780 cells SEM ± 657; Lta−/−: 5339 cells SEM ± 778; unpaired t test, P < .03) (Figure 5D). One possibility for the reduction in size of Lta−/− iLNs compared with their wild-type counterparts is that in the absence of LTα-induced signaling, stromal cells might stop proliferating and undergo programmed cell death. To test this hypothesis, we stained single-cell suspensions from E15 and E16 iLNs with the Ki-67 mAb and showed a statistically significant reduction in the frequency of cycling cells from Lta−/− embryos compared with their wild-type counterparts at E16 (Ki67+ cells, wild-type: 38% SEM ± 3%; Lta−/−: 19.6% SEM ± 2.9%; unpaired t test, P < .009) (Figure 5D). Annexin V staining of CD45− stromal cells showed a 2-fold increase in the percentage of cells undergoing apoptosis in E15 and E16 Lta−/− iLNs compared with wild-type (Figure 5E). In contrast, CD45+ cells from Lta−/− and wild-type mice showed no differences in the frequency of Annexin V+ cells (data not shown). Overall, these observations indicate that initial stages of organogenesis leading to the formation of the iLN anlagen occurs via a LTα-independent mechanism, while later developmental events require LTα-induced signaling to sustain stromal cell viability.
Can wild-type LTic's rescue lymphoid organ formation by Lta−/− iLN stromal cells?
We next investigated whether addition of wild-type LTic's to E15 Lta−/− iLN cells would rescue the defect in reaggregate LN organ cultures. Single-cell suspensions of E15 Lta−/− iLN cells were reaggregated with wild-type LTic's and grafted as described in “Results, Direct evidence of an essential role for LTic's in LN organogenesis.” Grafts were recovered after 2 weeks and shown to contain host T and B cells similar to grafts from wild-type iLNs (Figure 5F). Conversely, grafting of reaggregate organ cultures between E15 wild-type iLNs and Lta−/− LTic's either fail to generate a graft or gave origin to fibrous tissue devoid of lymphocytes in 4 independent experiments.
These results indicate that LTic's expressing LTα1β2 ligand were able to support the formation of lymphoid aggregates by Lta−/− stromal cells upon kidney grafting.
Discussion
Proper generation of secondary lymphoid organs requires the coordinated expression of ligands and receptors that mediate the complex cross-talk interactions between LTic's and stromal cells. The ontogeny of peripheral LN development from the arrival of CD45+ cells to the embryonic LN anlagen to the colonization of the organs by lymphocytes in newborn mice remains a poorly defined process.
In order to further understand the early events in peripheral LN development, we carried out a characterization of iLNs from E15 to P4 wild-type mice to assess structural changes and the presence of stromal organizer cells and LTic's in these organs. We showed that initiation of the reticular network could be detected in the cortex of E15 iLN anlagen and by E16 onwards, ERTR7+ structures start to form toward the center of these organs, developing progressively in the intricate organization of the conduit system as seen in iLNs of adult mice.
We were able to show a significant accumulation of CD45+ cells between E15 and E17 that correlated with major changes taking place in stromal cells, including the appearance of V+I+ mature stromal organizer cells.38 A similar sequence of events takes place during PP development, although lower levels of V+I+ cells were observed.20
Analysis of CD45+ cells in iLNs on E15 to E16 and P0 showed that at least 2 cell populations, CD45+4+ LTic's and CD45+4− cells, were present in these organs during development. Importantly, the frequency of CD45+4− cells was consistently higher than that of LTic's. Further studies will be required to assess whether the CD45+4− cells shown here are similar to the ones recently described that have an important function during the early stages of PP development.39
Based on these results, we propose that V+I− cells upon cell-cell interactions with LTic's become mature V+I+ organizer cells, although we cannot rule out that part of the V−I+ cell population may also become organizer cells.
A gene expression profile of the different stromal cell populations in P2 iLNs was performed, showing that V+I+ organizer cells and V−I+ cells expressed approximately similar mRNA levels of LtbR, Relb, Nfkb2, and target genes of this pathway. The expression of Prox-1 and Lyve-1 in the V−I+ cell population indicated that this compartment contained mainly blood vascular-lymphatic endothelial cells. We hypothesize that the V−I− cell population represent the mesenchymal precursor cells that give origin to the V+I− cell and V+I+ cell compartments.20
Inducer cell function during LN development
Previous attempts to deplete LTic's from embryos or newborn mice to assess their function had failed.23 Recent reports have demonstrated the importance of RORγt in the development of LTi cells and LN organogenesis, and have elegantly shown LTic's in situ in the developing LN anlagen.23,38,40
Here, we took 2 approaches to dissect the function of LTic's during LN development. First, we used whole E15 iLNs that had a very low frequency of both LTic's and mature stromal organizer cells and E17 and P1 iLNs that contained a significantly higher frequency of both cell types than at E15 to graft under the kidney capsule of adult mice. Grafted LNs showed that while the E15 iLNs formed fibrous tissue containing dead cells, the E17 and P1 iLNs formed lymphoid structures that were indistinguishable from adult LNs.
Second, we developed LN reaggregate assays and demonstrated that an E17 iLN single-cell suspension formed a tissue mass in culture that upon kidney grafting in adult mice originated a lymphoid structure. Such reaggregate LNs contained host B and T lymphocytes organized in specific areas as seen in wild-type LNs.
We next showed that addition of a defined cell population such as purified fetal IL-7–cultured LTic's to a cell suspension of E15 iLNs was capable of inducing the formation of lymphoid structures upon kidney capsule graft. These assays indicate that E15 iLNs are at an immature stage and thus are unable to express the proper molecules to be colonized by lymphocytes, and signals provided by LTic's are capable of inducing the maturation of stromal cell precursors, resulting in the formation of lymphoid tissues. We cannot distinguish whether the failure of E15 iLNs to form lymphoid tissue upon grafting is due to the paucity of mature stromal organizer cells or LTic's. We cannot rule out that adult CD4+CD3− cells play a role in the maintenance of the reaggregate lymphoid grafts although they are not sufficient to initiate the process as shown in grafted E15 iLNs.41,42 Our results clearly suggest a strong requirement for a critical mass of CD45+ cells to induce a large number of stromal cells to become mature organizer cells leading to successful LN formation, similar to the community effect needed to induce activation of the stroma that had been previously postulated by Choi and colleagues8 and Eberl and Littman.38
Our findings are in agreement with a previous report showing that adoptive transfer of wild-type LTic's on the day of birth into Id2−/ − mice, which lack LNs, PPs, and nasal-associated lymphoid tissue (NALT), were able to induce the latter.43 Similarly, transfer of fetal splenic wild-type LTic's to Cxcr5−/− mice that lack LNs and PPs resulted in restoration of PP formation, although a large number of LTic- and VCAM-1+–activated mesenchymal cells were already present in the intestine of these mutant mice, and thus may have contributed to the rescue.44,45
LTα-dependent and -independent stages of LN organogenesis
It had been previously shown that sustained LTβR stimulation is required to maintain spleen architecture and follicular dendritic cell (FDC) networks in adult mice.46,47 More recent studies had demonstrated that intrinsic LTβR signaling on lymphoid tissue DCs is necessary for in vivo proliferation of the CD8− DC population or to induce a precursor cell to become a CD8− DC.48 Also, constitutive LTβR signaling is required for the expression of addressins and the maintenance of HEV-differentiated endothelium in LNs during resting and reactive states.49 Globally, these reports clearly show that indeed LTβR signaling has a homeostatic function in different cellular systems and contexts.
Yet, we still do not know with certainty whether LNs start to form during Lta−/− embryo development, and why these organs are not present in newborn mice from these and other mouse mutants in components of the LTβR pathway.
We analyzed iLNs from E15 Lta−/− and wild-type mice and showed that they were indistinguishable from each other, indicating that the initial arrival of LTic's to the iLN anlagen is independent of LTα, which is consistent with previous findings by Eberl et al38 and Yoshida et al.40 However, LTic's and CD45+4− cells were present at a reduced frequency in E16 Lta−/− iLNs compared with their wild-type counterparts, likely reflecting an impaired recruitment of both cell populations in the absence of LTα-induced signals. Stromal-cell populations from E16 Lta−/− iLNs showed a statistically significant reduction in both absolute cell numbers and frequency of cycling cells compared with wild-type iLNs. Furthermore, Lta−/− cells were undergoing programmed cell death at twice the frequency of their wild-type counterparts.
In our hands, iLNs from E17 Lta−/− mice appeared significantly smaller than at E15, making them very difficult to isolate. A similar impairment on LTic recruitment to mesenteric LNs had been shown in Il7ra−/− mice.50 Concurrently, Yoshida et al had shown that IL-7R–induced stimulation of LTic's results in high expression levels of LTα1β2.40
Reaggregate LN grafting assays showed that addition of wild-type LTic's to E15 Lta−/− iLNs resulted in the formation of lymphoid structures containing host lymphocytes. These results, together with the failure to form lymphoid structures by Lta−/− LTic's and E15 wild-type iLNs reaggregates, suggest a maturation/cell viability function for LTα-induced signaling in stromal cells.
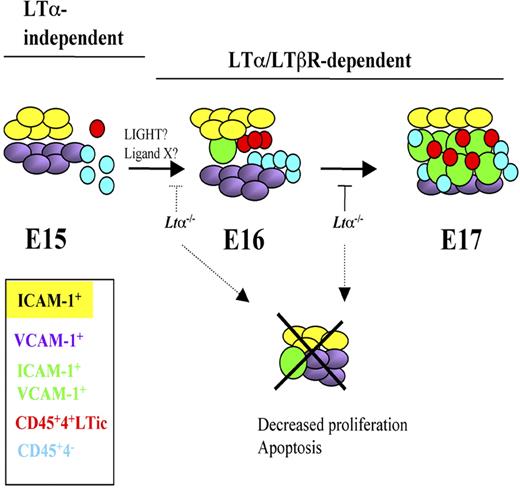
Our current model to explain the interactions between LTic's and stromal cells during iLN development is shown in Figure 6. E15 iLNs contain mostly single-positive V+ and I+ cells and very few V+I+ organizer cells. Most of the CD45+ cells present in anlagen are CD4−. Around E16 to E17, a marked increase in both LTic's and CD45+4− cells takes place that correlates with a massive appearance of V+I+ cells. Clustering and cell-cell interactions between LTic's and stromal cells induce the maturation of the latter to V+I+ cells.
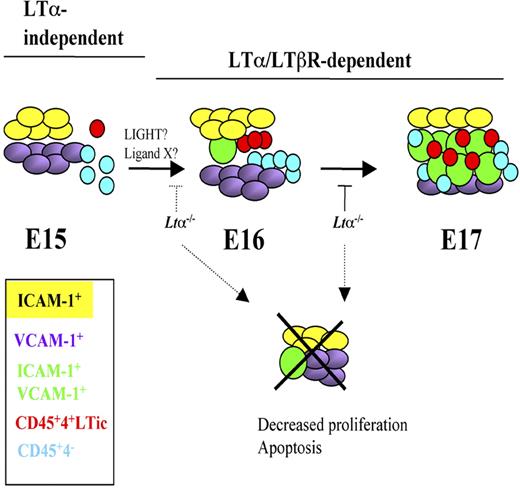
Model of iLN development and stromal cell maturation from E15 to E17 highlighting LTα-independent and -dependent stages. E15 iLNs contain mostly single-positive V+ and I+ cells and very few V+I+ mature organizer cells. The CD45+ cells present at this stage are in their majority CD4−. Between E16 and E17, a marked increase in both LTic's and CD45+4− cells takes place that correlates with a massive appearance of V+I+ cells. Cell-cell interactions between LTic's and stromal cells induce the maturation of the latter to V+I+ cells. LTα1β2/LTβR signals seem to be required from E15 onwards for the substantial emergence of V+I+ mature stromal cells and the recruitment of large numbers of LTic's and CD45+4− cells seen at E17.
Model of iLN development and stromal cell maturation from E15 to E17 highlighting LTα-independent and -dependent stages. E15 iLNs contain mostly single-positive V+ and I+ cells and very few V+I+ mature organizer cells. The CD45+ cells present at this stage are in their majority CD4−. Between E16 and E17, a marked increase in both LTic's and CD45+4− cells takes place that correlates with a massive appearance of V+I+ cells. Cell-cell interactions between LTic's and stromal cells induce the maturation of the latter to V+I+ cells. LTα1β2/LTβR signals seem to be required from E15 onwards for the substantial emergence of V+I+ mature stromal cells and the recruitment of large numbers of LTic's and CD45+4− cells seen at E17.
LTα1β2/LTβR signaling is required between E15 and E16 to ensure viability of stromal cells for the substantial emergence of V+I+ mature organizer cell population and the recruitment of large numbers of LTic's and CD45+4− cells. In the absence of LTα, iLN organogenesis grinds to a halt around E15 to E17.51 Interestingly, lack of LTα1β2 binding to LTβR does not appear to have an effect on the presence of V+ and I+ single-positive stromal cells, suggesting that either expression of these cell adhesion molecules is LTα1β2 independent, or that there is a functional redundancy between LTα1β2 and LIGHT (lymphotoxin-like inducible protein that competes with glycoprotein D for herpes virus entry on T cells) or other unknown LTβR ligands and in the absence of the former, binding of LIGHT/other ligands to LTβR is sufficient to induce expression of these molecules as shown in mesenteric LN development in Ltb−/− mice.52 Indeed, fetal LTic's express Light mRNA.53
Lack of RANK/TRAF-6 signals that up-regulate LTα1β2 in LTic's had been shown to be required for the presence of VCAM-1+ stromal cells in LN anlagen.40 Further studies are necessary to elucidate between these mechanisms, as well as to discover whether LTα1β2/LTβR signals are required only to maintain stromal cells into cycle or cooperate to drive cell proliferation and/or prevent programmed cell death. In this regard, Mackay and colleagues had shown that engagement of LTβR by an agonistic mAb in a fibroblastoid cell line induced proliferation in a dose-dependent manner.54
Our findings that expression of VCAM-1 in iLN anlagen seems independent of LTα are in contrast with the lack of expression of this molecule reported by Yoshida et al in Lta−/− mice.40 Differences in levels of TNFα in the Lta−/− mouse strains used, time of analysis, or LN specificity could be some of the reasons for this apparent discrepancy.
We propose that lack of LTβR-induced activation of both NF-κB classical (p50/RelA) and alternative (p52/RelB) pathways in Lta−/− stromal cells results in decreased proliferation and apoptosis.55–57 Our results may explain the requirement for LTβR signals to induce secondary lymphoid tissues formation and integrity through different mechanisms (1) by participating in the homeostasis of stromal cells, and (2) by inducing the expression of cell adhesion molecules and chemokines involved in recruitment of bone marrow–derived cells and the organization of specific cell areas in these tissues.13,14,49,58
The results presented here emphasize the importance of lympho-stromal interactions during the development of secondary lymphoid tissues. LN reaggregate assays are an ideal system to study the function of the different cell types that participate in the process of LN development and during the formation of ectopic lymphoid tissues in patients with rheumatoid arthritis, Sjogren syndrome, and other chronic inflammatory diseases.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We are indebted to the personnel of the Biomedical Services Unit of the University of Birmingham for excellent care of the animal colonies. We are also thankful to Francesco Falciani for help with statistical analysis.
This work was supported by a Biotechnology and Biological Sciences Research Council project grant to J.C. and G.A., the MRC Center for Immune Regulation of the University of Birmingham Medical School, and the Medical Research Council. A.W. and D.C. were supported by MRC PhD studentships.
Authorship
Contribution: A.W., D.C., S.P., G.A., and J.C. designed and performed the research, collected and analyzed the data, and wrote the paper. A.M., N.P., P.L., and E.J. contributed to performing the research.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: J. H. Caamaño, IBR-MRC Centre for Immune Regulation, University of Birmingham Medical School, Birmingham B15 2TT, United Kingdom; e-mail: j.caamano@bham.ac.uk.