Abstract
Defects in apoptosis signaling contribute to poor outcome in pediatric acute lymphoblastic leukemia (ALL), and overexpression of antiapoptotic Bcl-2 (Bcl-2 and Bcl-XL) family proteins has been observed in ALL. ABT-737 is a small-molecule BH3-mimetic that inhibits the antiapoptotic Bcl-2 family proteins. We evaluated the cytotoxicity of ABT-737 in combination with vincristine, dexamethasone, and L-asparaginase (VXL) in 7 ALL cell lines. Multilog synergistic cytotoxicity was observed in all 7 cell lines with ABT-737 plus L-asparaginase or vincristine, and in 5 of 7 cell lines with ABT-737 plus dexamethasone or VXL. In leukemia cells, but not in normal lymphocytes, ABT-737 plus L-asparaginase induced greater mitochondrial depolarization (JC-1 staining); mitochondrial cytochrome c release; activation of Bax, Bid, and caspases (immunoblotting); and eventually apoptosis (annexin V staining) than did either drug alone. In mouse xenografts derived from patients with ALL at diagnosis (ALL-7) or at relapse (ALL-19), event-free survival (EFS) was significantly enhanced with ABT-737 plus VXL relative to VXL or ABT-737 alone (P ≤ .02). Thus, ABT-737 synergistically enhanced VXL cytotoxicity in ALL cell lines via a mitochondrial death pathway and enhanced EFS in VXL-treated mice bearing ALL xenografts. Combining VXL with a BH3-mimetic warrants clinical investigation in ALL at relapse and potentially in chemotherapy-resistant ALL subgroups.
Introduction
Significant improvements in primary therapy for childhood acute lymphoblastic leukemia (ALL), including induction therapy using vincristine, L-asparaginase amidohydrase (L-ASP), doxorubicin, and a glucocorticoid, have led to an overall cure rate of approximately 80%.1,2 For patients with ALL who have a slow response to initial therapy, modification of postinduction therapy based on early response to treatment has been shown to improve the survival of children with high-risk ALL.3 Of the 20% of patients who relapse, most die,4–6 and treatment outcome for early bone marrow relapse is especially poor.7 Patients who experience a second relapse have a 3-year survival rate of only 8%, although third remissions are attainable.5 Patients with longer duration of first remission are more likely to achieve remission again, and those who relapse at a site other than bone marrow are more likely to respond to reinduction chemotherapy.6,8–10
Several multiagent regimens, including the combination of vincristine, prednisone, and L-ASP, are reported to provide complete response (CR) rates of approximately 40% in multiple-relapse patients.6 L-ASP, which depletes asparagine and glutamine in leukemic cells,11 is a critical component of therapy for childhood ALL. As a single agent, L-ASP induced complete remissions in 40% to 60% of patients with ALL, and in combination with vincristine and prednisone is associated with an initial remission rate of 95%.12,13 Both in vitro and in vivo resistance to L-ASP has been associated with poor long-term outcome.14–16 In addition, relapsed patients with greater asparagine depletion on day 14 of reinduction were more likely to achieve a second remission in the context of 6-drug therapy.11 However, expression of asparagine synthetase (AS), which may oppose the action of L-ASP by resynthesis of asparagine, has varied widely in clinical ALL samples, but a relationship of AS levels to drug resistance has not been reported.15,17
ABT-737 is a small molecule that binds to and inhibits the Bcl-2 family antiapoptotic proteins Bcl-XL, Bcl-2, and Bcl-w. Similar to the BH3-only “sensitizing” protein Bad, ABT-737 does not directly activate Bax or Bak or induce cytochrome c release. Instead, ABT-737 binds to multidomain antiapoptotic Bcl-2 family proteins, preventing them from sequestering proapoptotic BH3-only proteins.18,19 Overexpression of antiapoptotic Bcl-2 (Bcl-2 and Bcl-XL) family proteins has been observed in acute myeloid leukemia (AML),20–22 ALL,23,24 and other cancers.24,25 Bcl-XL overexpression has been reported as an independent predictor of poor event-free survival (EFS) in pediatric ALL.23 Effective pharmacologic inhibition of the Bcl-2 family of proteins could lower the apoptotic threshold in leukemia cells, resulting in synergy with other chemotherapeutic agents, including drugs commonly used for remission induction in primary and relapsed leukemia, such as vincristine, glucocorticoids, and L-ASP.
Using both in vitro and in vivo models of ALL, we investigated the potential for synergistic activity of ABT-737 in combination with vincristine, L-ASP, and dexamethasone (VXL), drugs commonly used in both induction and reinduction therapy for ALL. As the level of minimal residual disease at the end of induction correlates with long-term outcome,26 identifying new drugs that can enhance the fraction of leukemia cells killed during remission induction should not only achieve more effective salvage rates but would also likely improve treatment outcome for patients who respond to standard chemotherapy. This study evaluated in human ALL cell lines and xenograft models the ability of ABT-737 to enhance the activity of drugs commonly used for remission induction and reinduction in ALL.
Materials and methods
Materials
ABT-737 was provided by Abbott laboratories (Abbott Park, IL). Vincristine and dexamethasone were from Sigma-Aldrich (St Louis, MO), rabbit antihuman Bax and Bid antibodies were from BD Biosciences (San Diego, CA), anti-Bad (C-7) and cytochrome c (7H8) antibodies were from Santa Cruz Biotechnology (Santa Cruz, CA), anti–caspase-9 (C9), anti–caspase-8 (1C12), and anti–caspase-3 (8G10) antibodies were from Cell Signaling Technology (Danvers, MA), JC-1 mitochondrial membrane potential probe was from Molecular Probes (Eugene, OR), and the annexin V/FITC kit was from Invitrogen (Carlsbad, CA). For L-ASP, we evaluated the efficacy of the drug from 2 different sources (Sigma-Aldrich and Merck, West Point, PA), and found that the activity was approximately equivalent.
Cells in culture
Human ALL cell lines COG-LL-317 (human T-cell leukemia established from a patient at second relapse) and COG-LL-319 (human pre-B leukemia established at diagnosis prior to therapy) were maintained in Iscove modified Dulbecco medium (IMDM; Cambrex, Walkersville, MD) supplemented with 3 mM l-glutamine, 5 μg/mL insulin, and 20% heat-inactivated fetal bovine serum (FBS).27 Other ALL cell lines used were NALM-6 (pre-B ALL) from Deutsche Sammlung von Mikroorganismen und Zellkulturen (DSMZ, German Collection of Microorganisms and Cell Cultures, Braunschweig, Germany), RS4-11 (pre-B ALL), and T-cell ALL cell lines (CEM, MOLT-3, and MOLT-4) from American Type Culture Collection (ATCC, Manassas, VA), and were cultured in RPMI-1640 medium (Mediatech, Herndon, VA) supplemented with 10% heat-inactivated FBS.28,29 Preparation of peripheral blood mononuclear cells (PBMCs) was carried out by diluting blood 1:1 with phosphate-buffered saline (PBS) and centrifuging over Ficoll (375g for 30 minutes at room temperature). The mononuclear cell layer was collected, and washed twice in PBS and resuspended in IMDM supplemented with 20% FBS. All of the cell lines used were mycoplasma free, and were cultured and treated with drugs in a 37°C incubator with 5% O2 (bone marrow–level hypoxia),30,31 5% CO2, and 90% N2. Cell line identities were confirmed by short tandem repeat (STR) genotyping using the AmpF STR Identifiler kit (Applied Biosystems, Foster City, CA).32 STR profiles were unique for all cell lines, except MOLT-3 and MOLT-4, which were established from the same patient and showed highly similar profiles, but remained distinguishable by the STR assay. Studies using human specimens were approved by the Childrens Hospital Los Angeles committee for protection of human subjects.
Cytotoxicity assay
The cytotoxicity of ABT-737, VXL, and their combinations was determined using the DIMSCAN digital imaging microscopy assay system (Childrens Hospital Los Angeles, CA).33,34 Drug concentration ranges used for assays are specified in the figure legends, and combination cytotoxicity was determined employing a fixed-ratio of concentrations. The concentrations for cytotoxicity assays were selected to include clinically achievable concentrations for VXL.35–39 Cells (15 000 in 100 μL/well) were seeded in 96-well plates 16 to 24 hours before 100 μL of drugs were added to each well. After a 48-hour incubation with drugs, fluorescein diacetate (FDA), and eosin Y at a final FDA concentration of 10 μg/mL were added. After a 15-minute incubation, fluorescence was measured, and the fractional survival of treated versus control cells was determined.
Assessment of apoptosis by flow cytometry
Apoptosis was evaluated by assessing annexin V surface positivity by flow cytometry. COG-LL-317 cells were incubated with ABT-737, L-ASP, or the combination, washed twice with PBS, and resuspended in binding buffer (10 mM HEPES/NaOH [pH 7.4], 140 mM NaCl, and 25 mM CaCl2) at a density of 0.5 × 106 cells per 50 μL. Annexin V/FITC (10 μL) was added to the cell suspension and incubated for 10 minutes at room temperature. Then, cells were washed and resuspended in binding buffer. Prior to flow cytometric analysis, propidium iodide (PI) stock solution was added for counter staining. A BD LSR II flow cytometer was used (BD Biosciences, San Jose, CA), operated with DiVA (version 4.1.2) software. Bandpass filters were 525 (± 25) nm for FITC, and 610 (± 10) nm for PI.
Determination of mitochondrial membrane depolarization (Δψm)
Cells were treated with ABT-737, L-ASP, or the combination, collected, and resuspended in 1 mL medium containing 10 μg/mL JC-1 (5,5′,6,6′-tetrachloro-1,1′,3,3′-tetraethylbenzimidazolyl-arbocyanine iodide). After incubation at 37°C for 10 minutes, cells were analyzed by flow cytometry. The number of cells shifting from red to green fluorescence indicates the frequency of cells exhibiting mitochondrial depolarization. Bandpass filters were 525 plus or minus 25 nm for JC-1 greene mission and 610 plus or minus 10 nm for JC-1 red emission.
Cytochrome c analysis
Cells were incubated with ABT-737, L-ASP, or the combination, and cytosolic and mitochondrial fractions were generated using a digitonin-based subcellular fractionation technique.40 For detection of cytochrome c, 30 μg of the cytosolic fraction was supplemented with 5 × SDS-PAGE loading buffer, and then subjected to Western blot analysis. Blots were probed with anti–cytochrome c antibody.
Western blot analyses
Cells were treated, lysed, and sonicated briefly, and centrifuged at 12 000g for 15 minutes. Equal amounts of protein were separated by 4% to 20% Tris-glycine gel (Invitrogen), transferred to a nitrocellulose membrane, and incubated with antibodies. Immunoblots were visualized by enhanced chemiluminescent substrate.
In vivo efficacy against childhood ALL xenografts
The in vivo antileukemic activity of ABT-737 in combination with VXL was assessed using 2 childhood ALL xenografts that we previously established and characterized: ALL-7, biphenotypic ALL established at diagnosis; and ALL-19, common (CD10+) ALL established at relapse.41,42 Groups of 8 female nonobese diabetic/severe combined immunodeficient (NOD/SCID; NOD/LtSz-scid/scid) mice at 7 to 8 weeks of age (Institute of Medical and Veterinary Science, Adelaide, Australia) were inoculated via the tail vein with 5 × 106 ALL-19 or 2.5 × 106 ALL-7 mononuclear cells from the spleens of previously engrafted mice. Leukemia engraftment and response to therapy was monitored by flow cytometric enumeration of the proportion of human CD45+ (huCD45+) cells versus total murine CD45+ plus huCD45+ cells (percentage of huCD45+) in the peripheral blood, as previously described.41,42
When the percentage of huCD45+ cells reached 1% in the peripheral blood, mice were randomized to receive drug or vehicle control treatments. Drugs were administered as described in Figure 6. The proportion of huCD45+ cells in the blood was monitored throughout and following the course of treatment. Mouse event-free survival (EFS) was calculated as the number of days from treatment initiation until the human cells in the blood reached 25%, or when mice were killed due to drug-related toxicity (> 20% weight loss, lethargy, ruffled fur). All animal studies were approved by the Animal Care and Ethics Committee of the University of New South Wales.
Statistical analyses
For in vitro experiments, combination indices (CIs) were calculated using Calcusyn (Biosoft, Cambridge, United Kingdom). Calculation of a CI is a method to numerically quantify drug synergism based on the multiple drug–effect equation of Chou-Talalay derived from enzyme kinetic models.43,44 With this method, a CI lower than 0.9 indicates synergism; a CI of 0.9 to 1.10 indicates additive; and a CI higher than 1.10 indicates antagonism. The CI values were calculated for each fixed-ratio concentration that was used for the cytotoxicity assays. The significance of differences in means was evaluated by SAS version 9.1 (SAS Institute, Cary, NC). Results with a P value less than .05 were regarded as significant.
Mouse EFS was graphically represented by Kaplan-Meier analysis,45 and survival curves were compared by log-rank test where P values less than .05 were considered significant. For comparisons between xenografts and drug treatments, the median EFS for leukemia-bearing, vehicle control mice was subtracted from the median EFS for drug-treated mice to generate a leukemia growth delay (LGD).
Results
ABT-737 synergistically enhanced L-ASP–, vincristine-, or dexamethasone-induced cytotoxicity in ALL cell lines
We determined the cytotoxicity of ABT-737 and VXL, and ABT-737 combined with each of the other drugs at clinically achievable concentrations in 7 ALL cell lines using the DIMSCAN cytotoxicity assay (Table 1; Figure 1). ALL cell lines were used for the in vitro cytotoxicity assays instead of primary patient samples due to their homogeneity, consistent proliferation rate, availability for experimental replication, and to minimize potential artifacts from the stress of a sudden change from in vivo to in vitro conditions. CI values were calculated at the fixed-ratio drug concentrations used for cytotoxicity assays.
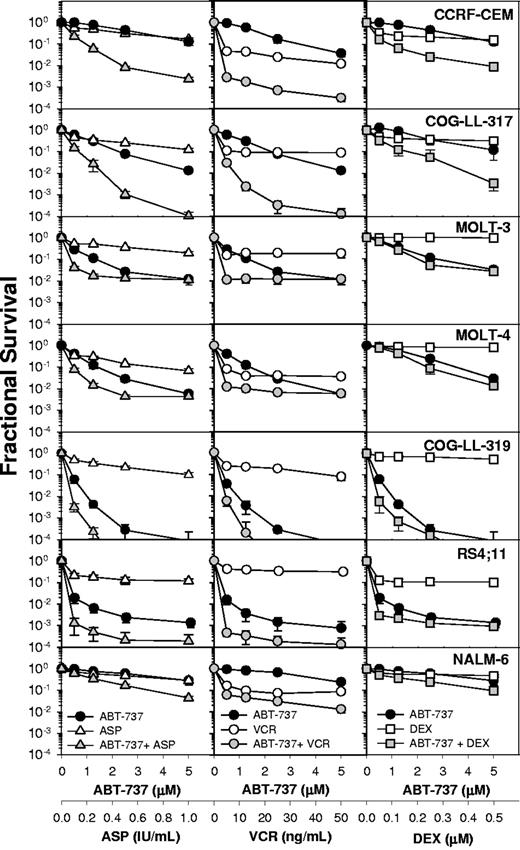
Combination cytotoxicity of ABT-737 and L-ASP, vincristine, or dexamethasone. Dose-response curves of ALL cell lines to ABT-737 (●), L-ASP (△), vincristine (VCR; ○), dexamethasone (DEX; □), and the combinations: ABT-737 + L-ASP (▲), ABT-737 + VCR ( ), and ABT-737 + DEX (gray squares). The concentrations applied for the cell lines were 0.5 to 5 μM for ABT-737; 0.1 to 1 IU/mL for L-ASP; 5 to 50 ng/mL for VCR; and 50 to 500 nM for DEX. Each condition had 12 replicates, and error bars represent standard deviation. The CI values are shown in Table 1.
), and ABT-737 + DEX (gray squares). The concentrations applied for the cell lines were 0.5 to 5 μM for ABT-737; 0.1 to 1 IU/mL for L-ASP; 5 to 50 ng/mL for VCR; and 50 to 500 nM for DEX. Each condition had 12 replicates, and error bars represent standard deviation. The CI values are shown in Table 1.
Combination cytotoxicity of ABT-737 and L-ASP, vincristine, or dexamethasone. Dose-response curves of ALL cell lines to ABT-737 (●), L-ASP (△), vincristine (VCR; ○), dexamethasone (DEX; □), and the combinations: ABT-737 + L-ASP (▲), ABT-737 + VCR ( ), and ABT-737 + DEX (gray squares). The concentrations applied for the cell lines were 0.5 to 5 μM for ABT-737; 0.1 to 1 IU/mL for L-ASP; 5 to 50 ng/mL for VCR; and 50 to 500 nM for DEX. Each condition had 12 replicates, and error bars represent standard deviation. The CI values are shown in Table 1.
), and ABT-737 + DEX (gray squares). The concentrations applied for the cell lines were 0.5 to 5 μM for ABT-737; 0.1 to 1 IU/mL for L-ASP; 5 to 50 ng/mL for VCR; and 50 to 500 nM for DEX. Each condition had 12 replicates, and error bars represent standard deviation. The CI values are shown in Table 1.
Dose-response curves to ABT-737 and VXL, and ABT-737 combined with L-ASP, vincristine, or dexamethasone are shown in Figure 1. ABT-737 plus L-ASP showed strong synergy (CI ≤ 0.3) or synergy (CI ≤ 0.7) in all 7 ALL cell lines (Table 1), achieving 2 to 4 logs of cell kill. CEM and NALM-6 cells were not sensitive to ABT-737 or L-ASP as single agents in the range tested, yet the 2 drugs in combination showed more than 1.5 to 2.5 logs of cytotoxicity (Figure 1 left column). Regardless of the sensitivity to single agents, ABT-737 combined with L-ASP showed synergistic cytotoxicity in all ALL cell lines tested. The in vitro cytotoxicity of vincristine as a single agent was not concentration dependent; a 10 times greater concentration resulted in a less than 50% increase in cytotoxicity in all 7 ALL cell lines (Figure 1 middle column). However, in combination with ABT-737, vincristine showed strong to very strong synergy in CEM, COG-LL-317, and RS4-11 cells, and synergy in MOLT-3, MOLT-4, and COG-LL-319 cells (Table 1). Strong synergy was observed with ABT-737 plus dexamethasone in CEM cells, and synergy in COG-LL-317, RS4-11, and NALM-6 cells, but in other cell lines, the combination effect was minimal (Figure 1 right column). Thus, the combination effect of ABT-737 was greatest with L-ASP or vincristine.
The combination of ABT-737 and L-ASP induced proapoptotic proteins and activated caspase-8, caspase-9, and caspase-3
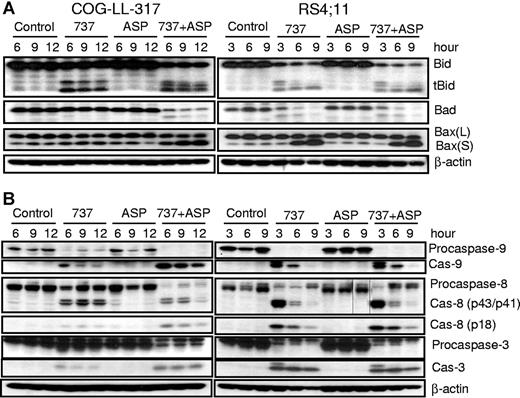
Since ABT-737 synergized most strongly with L-ASP, we determined the effects of ABT-737, L-ASP, and their combination on various Bcl-2 family proteins by Western blot analysis of the COG-LL-317 and RS4-11 cell lines (Figure 2A). We found that Bid was truncated to tBid after treatment with ABT-737 alone, or the combination of ABT-737 and L-ASP.
Activation of various proapoptotic proteins and caspase-8, caspase-9, and caspase-3. Whole-cell extracts from either COG-LL-317 (left panels) or RS4-11 (right panels) cells were incubated with ABT-737 (2.5 μM), L-ASP (2.5 IU/mL), or the combination over time, after which protein extracts were immunoblotted with the specified antibodies for proapoptotic proteins (A) and caspase-8, caspase-9, and caspase-3 (B). The data shown are representative of 3 experiments. Vertical lines have been inserted to indicate where a gel lane was cut. These gels came from 2 different experiments.
Activation of various proapoptotic proteins and caspase-8, caspase-9, and caspase-3. Whole-cell extracts from either COG-LL-317 (left panels) or RS4-11 (right panels) cells were incubated with ABT-737 (2.5 μM), L-ASP (2.5 IU/mL), or the combination over time, after which protein extracts were immunoblotted with the specified antibodies for proapoptotic proteins (A) and caspase-8, caspase-9, and caspase-3 (B). The data shown are representative of 3 experiments. Vertical lines have been inserted to indicate where a gel lane was cut. These gels came from 2 different experiments.
Hypophosphorylated Bad binds to and inhibits Bcl-2 and Bcl-XL, while hyperphosphorylated Bad is inactive.46,47 ABT-737 alone in RS4-11 cells and the combination of ABT-737 plus L-ASP in both COG-LL-317 and RS4-11 cells increased the relative expression of hypophosphorylated Bad (Figure 2A). We also investigated expression of Bax (Figure 2A), a key mediator of cytochrome c release through mitochondrial membrane permeabilization.48,49 In both cell lines, ABT-737 with and without L-ASP increased the proportion of Bax (S) (Bax in its activated form), in a time-dependent manner. Changes in Bax expression occurred earlier in RS4-11 than in COG-LL-317, perhaps due to differences in the relative sensitivity of the 2 cell lines to ABT-737 as a single agent (Figure 1). These results suggest that ABT-737 alone and together with L-ASP caused the sequestration of Bid from Bcl-2 and Bcl-XL, allowing their activation to tBid, followed by induction of Bax oligomerization. Consistent with the cytotoxicity data (Figure 1), ABT-737 plus L-ASP was more effective in Bax activation than ABT-737 alone in COG-LL-317, while in RS4-11, ABT-737 alone appeared to be the dominant activity.
Since caspase-9 and caspase-3 play key roles in the mitochondrial apoptotic pathway, and caspase-8 has been shown to cleave Bid to tBid,50–52 we examined the effects of ABT-737, L-ASP, and the 2 drugs combined on caspase expression and activation in COG-LL-317 and RS4-11 cell lines (Figure 2B; left and right panels, respectively). Procaspase-8 (57 kDa) was cleaved to the subunits p43/41 and p18 in response to ABT-737 or the combination in both COG-LL-317 and RS4-11. The increase in the p43/p41 subunit and subsequent increase in p18 subunit of caspase-8 was indicative of activation. Caspase-9 and caspase-3 were also cleaved and activated in response to ABT-737 with or without L-ASP. Therefore, the cytotoxic mechanism of ABT-737 with or without L-ASP is associated with caspase activation and involves the mitochondrial pathway. ABT-737 with or without L-ASP also activated caspase-8, which presumably caused subsequent cleavage of Bid to tBid. Since caspase-8 was activated, we investigated Fas, DR4, and DR5 in COG-LL-317 and RS4-11 cell lines, and found that their expression was not changed by ABT-737, with or without L-ASP (data not shown). These data suggest that apoptosis induction by ABT-737 with or without L-ASP occurred primarily through the intrinsic pathway. Again, consistent with the cytotoxicity data (Figure 1), ABT-737 alone appeared to dominate the effect on caspases in RS4-11, while combining ABT-737 with L-ASP enhanced the activity in COG-LL-317.
Cotreatment with ABT-737 and L-ASP induced apoptosis involving mitochondrial membrane depolarization and cytochrome c release
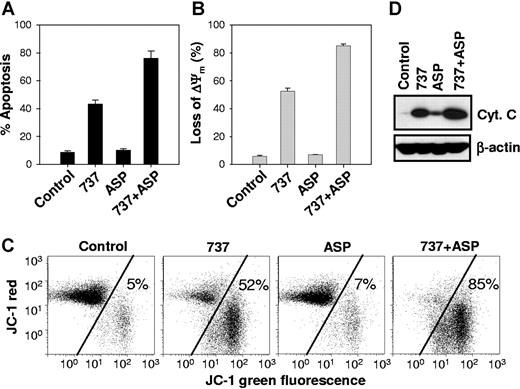
To further understand mechanisms of enhanced cytotoxicity seen with the combination of ABT-737 and its combination with L-ASP, we examined the effects on apoptosis, cytochrome c release, and mitochondrial membrane depolarization. We first investigated whether the observed cytotoxicity was due to apoptosis. COG-LL-317 cells were treated with ABT-737, L-ASP, or the combination and then analyzed for apoptosis by annexin V/FITC and PI staining (Figure 3A). The percentage of apoptotic cells (FITC+/PI−) at 6 hours was 8.6% ± 1.2% in control cells, 8.5% ± 1.3% with L-ASP, 57% ± 1.0% with ABT-737, and 85% ± 4.4% (P =.02 and P =.01 relative to L-ASP and ABT-737 as single agents, respectively) in cells treated with ABT-737 plus L-ASP. Apoptosis at 12 hours of drug exposure was similar to that observed at 6 hours (data not shown). These results suggest that apoptosis is the primary cell death pathway for the combination of ABT-737 and L-ASP.
ABT-737 plus L-ASP caused enhanced apoptosis, mitochondrial membrane depolarization, and release of mitochondrial cytochrome c. Annexin V–FITC and JC-1 assay. COG-LL-317 cells were incubated with ABT-737 (2.5 μM), L-ASP (2.5 IU/mL), or the combination for 6 hours. Then, cells were incubated with annexin V–FITC and PI (A) or JC-1 (B,C) and analyzed by flow cytometry. (A) Bars show the percentage of cells that were annexin V–FITC+/PI−, defined as apoptotic, and error bars represent standard deviation. (B) The loss of Δψm by ABT-737, L-ASP, or ABT-737 + L-ASP was measured by flow cytometry using the JC-1 mitochondrial probe. The bars show the percentage of mitochondrial membrane–depolarized cells. (C) Cytograms showing representative JC-1 assays for mitochondrial membrane potential. The transition of red fluorescence to green indicates mitochondrial membrane depolarization by the drug(s). The values represent the percentage of mitochondrial membrane-depolarized cells. Total number of events analyzed for each condition was 10 000. (D) Cytochrome c release after drug treatment. Cytosolic extracts from COG-LL-317 that had been incubated for 6 hours with ABT-737 (2.5 μM), L-ASP (2.5 IU/mL), or both drugs in combination were prepared and immunoblotted with cytochrome c antibody. The blot shown is representative of 2 separate experiments.
ABT-737 plus L-ASP caused enhanced apoptosis, mitochondrial membrane depolarization, and release of mitochondrial cytochrome c. Annexin V–FITC and JC-1 assay. COG-LL-317 cells were incubated with ABT-737 (2.5 μM), L-ASP (2.5 IU/mL), or the combination for 6 hours. Then, cells were incubated with annexin V–FITC and PI (A) or JC-1 (B,C) and analyzed by flow cytometry. (A) Bars show the percentage of cells that were annexin V–FITC+/PI−, defined as apoptotic, and error bars represent standard deviation. (B) The loss of Δψm by ABT-737, L-ASP, or ABT-737 + L-ASP was measured by flow cytometry using the JC-1 mitochondrial probe. The bars show the percentage of mitochondrial membrane–depolarized cells. (C) Cytograms showing representative JC-1 assays for mitochondrial membrane potential. The transition of red fluorescence to green indicates mitochondrial membrane depolarization by the drug(s). The values represent the percentage of mitochondrial membrane-depolarized cells. Total number of events analyzed for each condition was 10 000. (D) Cytochrome c release after drug treatment. Cytosolic extracts from COG-LL-317 that had been incubated for 6 hours with ABT-737 (2.5 μM), L-ASP (2.5 IU/mL), or both drugs in combination were prepared and immunoblotted with cytochrome c antibody. The blot shown is representative of 2 separate experiments.
We next investigated the effects of treatment with the single agents or the combination on mitochondrial membrane potential and cytochrome c release in the COG-LL-317 cell line. Mitochondrial membrane depolarization, as determined by the JC-1 mitochondrial probe, was induced by ABT-737 as a single agent 6 hours after treatment, and the combination with L-ASP resulted in a greater than additive effect (P =.02 and P =.01 relative to L-ASP and ABT-737 as single agents, respectively) compared with the single agents (Figure 3B,C). Mitochondrial membrane depolarization was delayed to 12 hours for L-ASP as a single agent, but ABT-737 plus L-ASP resulted in a greater effect (P =.003) at 12 hours than either single agent (data not shown). We also examined cytochrome c release from mitochondria into the cytosol by Western blot analysis after treatment with ABT-737, L-ASP, or the combination after 3 or 6 hours in COG-LL-317. Cytochrome c release was increased by ABT-737 and to a lesser degree by L-ASP, but was greatest for ABT-737 plus L-ASP (Figure 3D). These results suggest that ABT-737 and ABT-737 plus L-ASP synergized to induce apoptosis via mitochondrial membrane depolarization, leading to cytochrome c release.
Effect of ABT-737 on vincristine, L-ASP, or dexamethasone cytotoxicity in normal hematopoietic cells
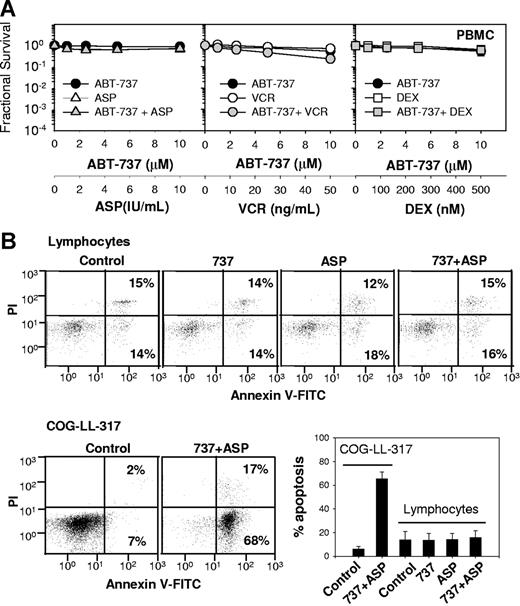
To address whether the cytotoxicity of ABT-737 combined with vincristine, L-ASP, or dexamethasone is preferential for leukemia cells relative to nonmalignant cells we studied peripheral blood mononuclear cells (PBMCs) (Figure 4). Apoptosis was evaluated by assessing annexin V staining by flow cytometry (Figure 4B,C). In COG-LL-317 treated with ABT-737 plus L-ASP for 6 hours, 68% plus or minus 3% cells were positive for apoptosis (annexin V–FITC+; PI−), while 6% plus or minus 2% were positive in unstimulated control cells (P < .001). For normal lymphocytes, which were gated from PBMCs using a standard forward- and side-scatter lymphocyte gate, mean apoptosis was 12.6% plus or minus 5.1% in unstimulated controls, 13.3% plus or minus 2.0% with ABT-737, 13.3% plus or minus 4.4% with L-ASP, and 15.8% plus or minus 4.1% with ABT-737 plus L-ASP–treated cells. Apoptosis in treated lymphocytes was not statistically different from untreated controls (P > .5).
Cytotoxicity of ABT-737 plus vincristine, dexamethasone, or L-ASP in PMBCs. (A) Dose-response curves of PBMCs to ABT-737 (●), L-ASP (△), vincristine (VCR; ○), dexamethasone (DEX; □), and the combinations (gray symbols). The concentrations of ASP used for PBMCs were increased to 1 to 10 IU/mL to assure examination of cytotoxicity in clinically achievable concentrations. Each condition had 12 replicates, and error bars represent standard deviation. (B) Cells (COG-LL-317 and PBMCs) were incubated with ABT-737 (2.5 μM), ASP (1 IU/mL), or the combination for 6 hours. Then, apoptosis assessed with annexin V and PI staining measured by flow cytometry. COG-LL-317 cells were used as positive controls. For PBMCs, apoptosis was assessed only in lymphocytes that fell within a standard forward- and side-scatter lymphocyte gate. Cells that were annexin V–FITC+, PI− were defined as apoptotic. The bar graph shows the percentage of cells in apoptosis, and the values represent means (± SD) of 3 samples, and the experiments were repeated twice. Apoptosis in COG-LL-317 for ABT-737 + L-ASP was significantly higher than control (P < .001), while neither single agents nor ABT-737 + L-ASP induced significant apoptosis in lymphocytes.
Cytotoxicity of ABT-737 plus vincristine, dexamethasone, or L-ASP in PMBCs. (A) Dose-response curves of PBMCs to ABT-737 (●), L-ASP (△), vincristine (VCR; ○), dexamethasone (DEX; □), and the combinations (gray symbols). The concentrations of ASP used for PBMCs were increased to 1 to 10 IU/mL to assure examination of cytotoxicity in clinically achievable concentrations. Each condition had 12 replicates, and error bars represent standard deviation. (B) Cells (COG-LL-317 and PBMCs) were incubated with ABT-737 (2.5 μM), ASP (1 IU/mL), or the combination for 6 hours. Then, apoptosis assessed with annexin V and PI staining measured by flow cytometry. COG-LL-317 cells were used as positive controls. For PBMCs, apoptosis was assessed only in lymphocytes that fell within a standard forward- and side-scatter lymphocyte gate. Cells that were annexin V–FITC+, PI− were defined as apoptotic. The bar graph shows the percentage of cells in apoptosis, and the values represent means (± SD) of 3 samples, and the experiments were repeated twice. Apoptosis in COG-LL-317 for ABT-737 + L-ASP was significantly higher than control (P < .001), while neither single agents nor ABT-737 + L-ASP induced significant apoptosis in lymphocytes.
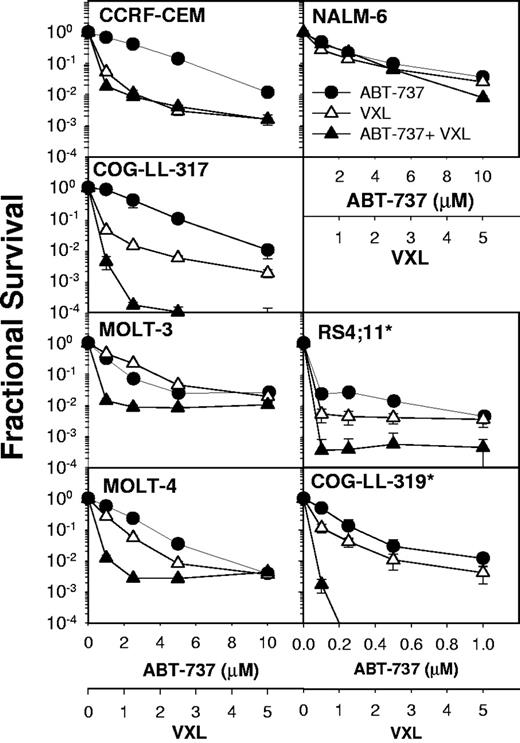
ABT-737 synergistically enhanced the combined cytotoxicty of VXL in leukemia cell lines
As a prelude to in vivo combination efficacy experiments, the in vitro cytotoxicity of the 4-drug combination (VXL plus ABT-737) was evaluated in 7 leukemia cell lines using the DIMSCAN cytotoxicity assay (Figure 5). Initially, we tested the combination cytotoxicity of VXL in our panel of 7 ALL cell lines. Then, using the same concentrations and conditions for VXL cytotoxicity, in vitro 4-drug combination cytotoxicity was evaluated for VXL plus ABT-737. Due to the high single-agent activity of ABT-737 in the RS4-11 and COG-LL-319 cell lines, for these later lines, ABT-737 concentrations of 0.1 to 1 μM were used, while in other cell lines, ABT-737 was tested at 1 to 10 μM. Synergistic cytotoxicity (defined as a CI < 1) was observed in 5 of 7 ALL cell lines. The mean CIs that were calculated from concentrations applied for cytotoxicity were 0.04 (COG-LL-319), 0.19 (COG-LL-317), 0.63 (MOLT-3), 0.97 (MOLT-4), and 0.013 (RS4-11). In CEM and NALM-6 cells, ABT-737 did not synergistically enhance the cytotoxicity of VXL (CI=1.08 and 1.8, respectively).
Four-drug combination cytotoxicity of ABT-737 in combination with VXL in ALL cell lines. Dose-response curves of ALL cell lines to ABT-737 (●), VXL (△), and the combinations of all 4 drugs (▲). The concentrations for VCR, DEX, and L-ASP applied were 0.5 to 5 ng/mL, 50 to 500 nM, and 0.1 to 1 IU/mL, respectively, where 1 U on the VXL axis corresponds to 1 ng/mL VCR, 100 nM DEX, and 0.2 IU/mL L-ASP. The concentrations for ABT-737 applied were 1 to 10 μM for CEM, COG-LL-317, MOLT-3, MOLT-4, and NALM-6; concentrations of 0.1 to 1 μM were used for RS4-11* and COG-LL-319*. Each condition had 12 replicates, and error bars represent SD. *Cell lines especially sensitive to ABT-737 as a single agent (Figure 1) that required lower dosing for combination drug testing.
Four-drug combination cytotoxicity of ABT-737 in combination with VXL in ALL cell lines. Dose-response curves of ALL cell lines to ABT-737 (●), VXL (△), and the combinations of all 4 drugs (▲). The concentrations for VCR, DEX, and L-ASP applied were 0.5 to 5 ng/mL, 50 to 500 nM, and 0.1 to 1 IU/mL, respectively, where 1 U on the VXL axis corresponds to 1 ng/mL VCR, 100 nM DEX, and 0.2 IU/mL L-ASP. The concentrations for ABT-737 applied were 1 to 10 μM for CEM, COG-LL-317, MOLT-3, MOLT-4, and NALM-6; concentrations of 0.1 to 1 μM were used for RS4-11* and COG-LL-319*. Each condition had 12 replicates, and error bars represent SD. *Cell lines especially sensitive to ABT-737 as a single agent (Figure 1) that required lower dosing for combination drug testing.
In vivo sensitivity of ALL xenografts to VXL and ABT-737 combination therapy
The in vivo efficacy of ABT-737 was assessed in combination with VXL, which was designed to mimic induction and reinduction therapy protocols administered to pediatric patients with ALL. Pediatric ALL samples directly established as xenografts in NOD/SCID mice provide a validated, clinically relevant experimental model of the disease.41,42 These combinations were tested against ALL-7 and ALL-19, xenografts derived from patients who relapsed and died within 13 months of diagnosis, and exhibited intrinsic resistance to chemotherapy.41,42
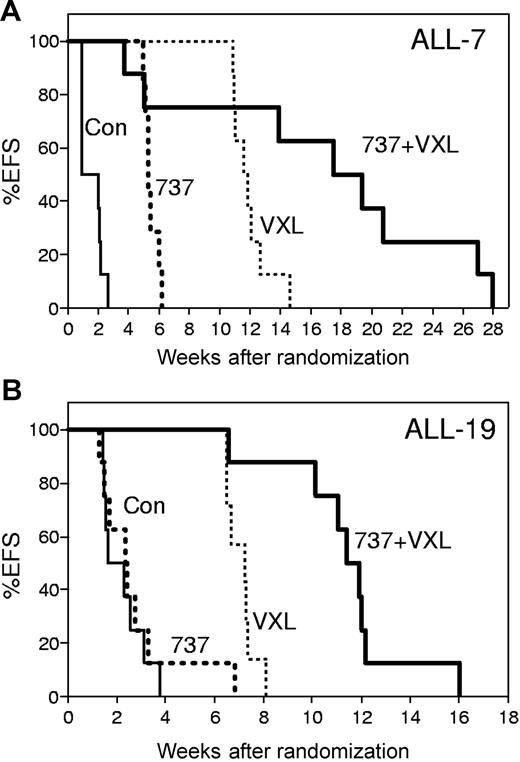
The median EFS of leukemia-bearing, vehicle-treated control mice was 10.4 days (range, 6.6-18.3 days) and 14.0 days (range, 10.2-22.0 days) after treatment initiation for ALL-7 and ALL-19, respectively (Figure 6; Table 2). The VXL combination significantly delayed the progression of ALL-7 and ALL-19 by 72 and 37 days, respectively. ABT-737 used as a single agent caused a significant delay in the progression of ALL-7 (LGD of 27 days), but was ineffective against ALL-19 (LGD of 3 days). However, the addition of ABT-737 to the VXL regimen delayed the progression of both xenografts to a greater extent than would be expected if the effects of the individual components were additive. The median EFS of mice engrafted with ALL-7 and treated with VXL plus ABT-737 was 129 days (range, 26-191 days), with 2 early events (< 6 weeks) due to drug-related toxicity. The LGD for ALL-7 was 119 days, which was 20 days greater than the sum of the LGDs induced by VXL or ABT-737 alone.
In vivo efficacy of ABT-737 combined with a VXL treatment regimen. NOD/SCID mice were inoculated with ALL-7 (A) or ALL-19 (B), monitored for engraftment, and treated with vehicle control (Con; thin lines), ABT-737 (737; bold dotted lines), a combination of vincristine, dexamethasone, and L-ASP (VXL; thin dotted lines), or VXL plus ABT-737 (VXL + 737; bold lines). Drugs were administered by intraperitoneal injection: vincristine (Sigma-Aldrich, Castle Hill, Australia), 0.15 mg/kg in saline every 7 days for 4 weeks; dexamethasone (Sigma-Aldrich), 5 mg/kg in saline Monday to Friday for 4 weeks; L-ASP (Aventis, Lane Cove, Australia), 1000 IU/kg in saline Monday to Friday for 4 weeks; and ABT-737, 25 mg/kg in DMSO (final concentration < 1%), 30% propylene glycol, 5% Tween 80, and 65% dextrose (pH 4-5), Monday to Friday for 4 weeks. The EFS of mice was quantified as the time taken from the initiation of treatment until leukemia cells reached 25% in the peripheral blood, or for mice to be killed due to treatment-related toxicity. Each line represents the proportion of mice remaining event-free over time.
In vivo efficacy of ABT-737 combined with a VXL treatment regimen. NOD/SCID mice were inoculated with ALL-7 (A) or ALL-19 (B), monitored for engraftment, and treated with vehicle control (Con; thin lines), ABT-737 (737; bold dotted lines), a combination of vincristine, dexamethasone, and L-ASP (VXL; thin dotted lines), or VXL plus ABT-737 (VXL + 737; bold lines). Drugs were administered by intraperitoneal injection: vincristine (Sigma-Aldrich, Castle Hill, Australia), 0.15 mg/kg in saline every 7 days for 4 weeks; dexamethasone (Sigma-Aldrich), 5 mg/kg in saline Monday to Friday for 4 weeks; L-ASP (Aventis, Lane Cove, Australia), 1000 IU/kg in saline Monday to Friday for 4 weeks; and ABT-737, 25 mg/kg in DMSO (final concentration < 1%), 30% propylene glycol, 5% Tween 80, and 65% dextrose (pH 4-5), Monday to Friday for 4 weeks. The EFS of mice was quantified as the time taken from the initiation of treatment until leukemia cells reached 25% in the peripheral blood, or for mice to be killed due to treatment-related toxicity. Each line represents the proportion of mice remaining event-free over time.
Similarly, the median EFS of mice engrafted with ALL-19 and treated with VXL plus ABT-737 was 82 days (range, 46-85 days), although no mice in this group experienced drug-related toxicity. The LGD resulting from the addition of ABT-737 to the VXL regimen was 68 days, which was 28 days greater than would be expected if the effects of the drug combination were additive.
For ALL-7, log-rank analysis showed that ABT-737, VXL, and ABT-737 plus VXL increased EFS (P < .001) relative to controls, and ABT-737 plus VXL showed increased EFS relative to either VXL or ABT-737 (P < .02). One mouse engrafted with ALL-7 and treated with ABT-737 plus VXL died at 186 days with no evidence of leukemia infiltration of major organs. For ALL-19, log-rank analysis showed that ABT-737 alone did not increase EFS relative to controls (P = .51), while VXL and ABT-737 plus VXL showed increased EFS (P < .001) relative to controls, and ABT-737 plus VXL demonstrated increased EFS relative to either VXL or ABT-737 (P < .01).
Discussion
Despite significant improvements in primary therapy, relapse remains a major problem in pediatric ALL, outcomes after relapse remain poor, and relapsed ALL is more common than new diagnoses of many pediatric malignancies.53 The 3-year survival after marrow, central nervous system (CNS), and testes relapse among patients enrolled on the Childrens Cancer Group-1900 series of studies was 28%, 60%, and 60%, respectively.6 Patients who have a second bone marrow relapse have a 3-year survival of only 8%, although third remissions are attainable.5 Thus, there is a critical need for new drugs with novel mechanisms of action that might improve outcome for recurring ALL. At the same time, several multiagent regimens, including the combination of vincristine, prednisone, and L-ASP, are reported to provide complete response rates of approximately 40% in multiple-relapse patients,6,54–56 and the likelihood in recurrent ALL of a complete response to familiar drug combinations often deters patient entry onto single-agent trials of new drugs.
One strategy to improve accrual onto phase 1 trials in pediatric ALL is to identify new drugs likely to contribute to enhanced leukemia killing by standard multidrug reinduction regimens, but with minimal added systemic toxicity. With the latter philosophy in mind, we have examined the potential for ABT-737, a small-molecule inhibitor of the antiapoptotic proteins Bcl-XL, Bcl-2, and Bcl-w to enhance the antileukemia activity of VXL.
In the first part of this study, we demonstrated that ABT-737 in combination with either vincristine, dexamethasone, or L-ASP (at clinically achievable concentrations for the latter drugs) synergistically induced apoptotic cell death in leukemia cell lines tested in bone marrow–level oxygen tension. ABT-737 as a single agent and in combination with L-ASP (with which the combination effect was greatest), induced cell death through the mitochondria-dependent apoptotic pathway, mediated by mitochondrial membrane depolarization, cytochrome c release, and caspase activation. L-ASP is an important component of primary57,58 and relapse59 ALL therapy. Effective asparagine depletion is associated with successful remission reinduction,11 and in the context of multiagent therapy, L-ASP contributes substantially to reduction of minimal residual disease.60,61 Resistance to L-ASP has been associated with poor long-term outcome in leukemia.14–16 Thus, the ability to sensitize leukemia cells to this critical agent may have important therapeutic implications, particularly in the relapse setting.
Up-regulation of Bcl-2 correlates with chemoresistance in a variety of cancers, and Bcl-XL overexpression has been reported as an independent predictor of EFS in pediatric ALL.23 In addition, the ratio of Bcl-2 to Bax mRNA expression, which may determine the fate of cells following an apoptotic stimulus, is often increased in patients with ALL and AML,62,63 and study found an increase in the Bcl-2/Bax ratio in recurring ALL bone marrow compared with diagnostic samples.63 The number of cell lines used in the current study was insufficient to reveal a clear relationship between Bcl-2 expression and ABT-737 activity. However, by binding antiapoptotic proteins Bcl-2 and Bcl-XL with subnanomolar affinity,18 ABT-737 competitively displaces and liberates sequestered proapoptotic proteins, lowering the threshold for apoptosis.18,64 This may be 1 mechanism for the synergy observed with ABT-737 and other chemotherapeutic agents, and a more detailed assessment of expression levels of all Bcl-2 family proteins in ALL cell lines will be necessary to determine the underlying mechanistic basis for this synergy.
Antiapoptotic Bcl-2 members exert inhibitory effects on glucocorticoid-induced cell death in lympoid malignancies,65,66 and therefore, ABT-737 might be expected to synergize dexamethasone cytotoxicity. In our experiments, ABT-737 enhanced dexamethasone cytotoxicity to a different extent depending on the cell lines used. We speculate that combining ABT-737 with dexamethasone could be less effective in some cell lines based on the report that ABT-737 inhibits Bcl-2, Bcl-XL, and Bcl-w, but not Mcl-1.18 Furthermore, recent data indicate that Mcl-1 may play a role in glucocorticoid resistance.67 Thus, the combination effect could vary depending on which mechanism is involved with glucocorticoid resistance among cell lines.
Bcl-2 family proteins are key regulators of the mitochondrial apoptotic pathway.64 They are grouped based on regions of Bcl-2 homology (BH domains): multidomain antiapoptotic (Bcl-2, Bcl-XL, Bcl-w, Mcl-1, Bfl-1/A1), multidomain proapoptotic (Bax, Bak), and BH3-only proapoptotic (Bid, Bim, Bad, Bik, Noxa, PUMA, Bmf, Hrk).48,68 The “activating” BH3-only proteins (Bid, Bim) directly induce Bax and/or Bak oligomerization, while the “sensitizing” proteins (Bad, Bik) competitively bind antiapoptotic proteins, liberating the “activating” proteins which can then activate Bax/Bak.48,51,52,68–70 The function of ABT-737 is analogous to that of the “sensitizing” BH3-only proteins.18,48,68
We observed activation of Bad in COG-LL-317 cells treated with ABT-737 plus L-ASP and in RS4-11 cells treated with ABT-737 or the combination with L-ASP (Figure 2A), indicating that activated Bad (and/or ABT-737 itself) was available to inhibit Bcl-XL function. Bax (S) was increased by ABT-737 and by the combination in an additive fashion in both cell lines. Bak was increased by ABT-737 and by ABT-737 plus L-ASP in RS4-11 (data not shown), consistent with disruption of Bcl-2 and Bcl-XL sequestering Bax/Bak via competitive displacement by ABT-737.18,71,72 Similar to activated Bad, tBid can also induce oligomerization and insertion of Bax into the mitochondrial membrane, initiating apoptosis.50–52,73 Moreover, ABT-737 and ABT-737 plus L-ASP also led to truncation of Bid to tBid, probably through displacement of Bid from Bcl-2 or Bcl-XL, leading to activation by caspase-8. Caspase-dependent apoptosis is a reported mechanism for both vincristine and glucocorticoid cytotoxicity in ALL,74 although the route of their cytotoxicity is not fully defined. The synergistic cytotoxicty of ABT-737 plus vincristine or dexamethasone suggests that ABT-737 may share a common mechanism of cytotoxicity with vincristine or dexamethasone, which needs to be further defined.
To explore whether the combination of ABT-737 plus VXL is preferential for leukemia relative to normal cells, we compared the cytotoxicity of ABT-737 plus VXL in leukemia cell lines to PBMCs. In ALL cell lines, ABT-737 as a single agent or in combination with other leukemia drugs showed multilog cell killing, but PBMCs were minimally affected at the same or higher concentrations, consistent with the minimal hematopoietic toxicity reported in animal studies.18 These results were confirmed by examining apoptosis in PBMCs (gating on lymphocytes) using flow cytometry. These data suggest that the combination of ABT-737 with vincristine, dexamethasone, or L-ASP kills ALL cells efficiently but minimally affects normal hematopoietic cells. Our study agrees with a previously reported lack of significant effects of ABT-737 on nonmalignant hematopoietic cells.75
To provide preclinical data in support of a clinical trial testing a novel multiagent combination regimen for recurrent ALL, we determined if the cytotoxicity for ALL cell lines of VXL was enhanced by ABT-737, and we observed synergy between VXL and ABT-737 in most of the ALL cell lines tested. Considering that synergism was not observed in CEM and NALM-6 cell lines among the 7 ALL cell lines tested, we would expect that the combination may not be effective against all ALL. Future studies should focus on establishing models of leukemia subgroups that are responsive and resistant to BH3-mimetics to facilitate identifying molecular markers associated with resistance.
We tested VXL, ABT-737, or VXL plsu ABT-737 in 2 systemic ALL xenograft models and demonstrated enhanced mouse EFS for both models. Interestingly, while ABT-737 alone showed activity in the ALL-7 xenograft model, ABT-737 did not have single-agent activity in the ALL-19 xenografts, yet ABT-737 still enhanced the activity of VXL in both the ALL-7 and ALL-19 xenograft models. It has been previously reported that responses of mouse xenografts to vincristine and dexamethasone correlated significantly with patient outcome, and that the in vitro sensitivity of xenografts to dexamethasone correlated with in vivo responses.42 Therefore, the enhanced activity of antileukemia drug combinations by ABT-737 observed in the xenografts may predict similar activity in patients.
In conclusion, these data demonstrate that: (1) In ALL cell lines cultured in bone marrow–level hypoxia, ABT-737 synergized with vincristine-, dexamethasone-, or L-ASP–induced cytotoxicity in vitro, with the strongest synergy observed with the combination of ABT-737 and L-ASP; (2) ABT-737 in combination with L-ASP induced apoptosis, and ABT-737 enhanced the antileukemia activity of L-ASP, mainly through the mitochondrial pathway; (3) the combination of ABT-737 and L-ASP was cytotoxic against leukemia cells but not normal hematopoietic cells; and (4) ABT-737 significantly enhanced the activity of a 3-drug induction-type regimen (VXL) against pediatric ALL cell lines and also against pediatric ALL xenografts in immunodeficient mice. We conclude that a combination chemotherapy regimen incorporating a small-molecule BH3-mimetic into VXL warrants clinical investigation in recurring ALL, and potentially as front-line therapy for relatively chemotherapy-resistant ALL subgroups.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
Children's Cancer Institute Australia for Medical Research is associated with the University of New South Wales and Sydney Children's Hospital. We thank Dr Richard Sposto for the support in statistical analyses of data, and Nancy Yen and Daniel Cabral for their technical support.
Authorship
Contribution: M.H.K., C.P.R., and R.B.L. designed research; M.H.K., Y.H.K., B.S., M.A.S., T.H., and U.W.K. performed research and analyzed data; and M.H.K. wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Min H. Kang, Division of Hematology-Oncology, Childrens Hospital Los Angeles, 4650 Sunset Blvd MS no. 57, Los Angeles, CA 90027; e-mail: mkang@chla.usc.edu.