Abstract
Expression of SH2-homology–containing protein-tyrosine phosphatase-1 (SHP-1), a candidate tumor suppressor, is repressed in human T-cell leukemia virus type-1 (HTLV-1)–transformed lymphocyte cell lines, adult T-cell leukemia (ATL) cells, and in other hematologic malignancies. However, the mechanisms underlying regulation and repression of SHP-1 remain unclear. Herein, we cloned the putative full-length, hematopoietic cell–specific SHP-1 P2 promoter and identified the “core” promoter regions. HTLV-1 Tax profoundly represses P2 promoter activity and histone deacetylase-1 (HDAC1) potentiates such inhibition. NF-κB was implicated as both a rate-limiting factor for basal P2 promoter activity and important for Tax-induced promoter silencing (TIPS). Chromatin immunoprecipitation studies demonstrated that NF-κB dissociates from the SHP-1 P2 promoter following the binding of Tax and HDAC1. This is in agreement with coimmunoprecipitation studies where NF-κB competed with HDAC1 for association with Tax protein. We propose that in TIPS, Tax recruits HDAC1 to the SHP-1 P2 promoter and forms an inhibitory complex that results in deacetylation and dissociation of NF-κB from the promoter and attenuation of SHP-1 expression. TIPS provides a possible first step toward HTLV-1 leukemogenesis through its down-modulation of this key immediate early negative regulator of IL-2 signaling.
Introduction
Adult T-cell leukemia (ATL) is an aggressive malignancy of CD4+, CD25+ T cells, and the human T-cell leukemia virus type-1 (HTLV-1) has been identified as the causative agent.1,2 While the understanding of ATL pathogenesis currently remains incomplete, the HTLV-1 virus-encoded Tax protein has been implicated as a major contributor in the development of ATL.3–6 The oncogenic potential of HTLV-1 Tax has been associated with its ability to modulate expression and function of cellular targets involved in cell proliferation and differentiation.7,8 For example, Tax has been shown to induce the activation of NF-κB, CREB, AP-1, and SRF6 as well as to up-regulate IL-2/IL-2 receptor-α,9 IL-15,10 IL-4,11 IL-13,12 and OX40/OX40L6 pathways, resulting in the stimulation of cell growth. Tax can also be a negative regulator of gene expression/function and can down-regulate expression of genes involved in host DNA repair,13 maintaining genetic stability14 and cell cycle progression.15 Of particular importance to this study, Tax has been shown to exert negative effects on at least 3 cellular tumor suppressors—Rb,16–19 hDLG, a human homolog of the Drosophila discs large tumor suppressor protein,6,20–22 and p53.6,23,24 Tax alone is able to immortalize primary human T lymphocytes and transform rodent fibroblast in vitro.25,26 Transgenic mice expressing Tax can also develop tumor in vivo with a wide range of phenotypes.25,27,28
Among the cellular dysfunctions caused by HTLV-1 infection, the loss of IL-2 dependence is remarkable in many HTLV-1–transformed cells.29 In HTLV-1–infected cord blood lymphocytes, the transition from IL-2–dependent to IL-2–independent growth has been shown to correlate with the acquisition of a constitutively activated Jak/STAT pathway, suggesting the involvement of this pathway in HTLV-1–mediated T-cell transformation.30 In addition, proliferation of uncultured leukemic cells from ATL patients has been reported to be associated with constitutive activation of Jak/STAT proteins.31
A number of cellular factors have been demonstrated to negatively regulate Jak/STAT activities, including SHP-1 (SH2-homology–containing protein-tyrosine phosphatase-1), PIAS-3 (protein inhibitors of activated STATs), SOCS-1 (suppressors of cytokine signaling), and CIS (cytokine-inducible SH2-containing protein).32–34 The hematopoietic-specific SHP-1 is expressed exclusively from the P2 promoter located 5′ to the exon 2,35 present constitutively in cells and able to down-regulate signaling immediately upon activation of receptor/kinase complexes.36,37 For example, IL-2 induces association of SHP-1 with the IL-2R complex. Once SHP-1 is recruited to the activated complex, it is able to decrease tyrosine phosphorylation of IL-2Rβ and the associated tyrosine kinases Jak1 and Jak3,36 acting as the earliest negative regulator of IL-2–mediated Jak/STAT signaling.
Previous studies have demonstrated that SHP-1 protein expression is down-regulated or absent in various primary lymphoma and leukemia cells.38–40 This has supported the notion that SHP-1 functions as a tumor suppressor by acting as an antagonist to the growth-promoting and oncogenic potentials of tyrosine kinases. A positive correlation has also been observed between the degree of loss of SHP-1 expression over time in tumor cells and their aggressiveness in vivo.40,41 SHP-1 gene silencing is particularly common in HTLV-1–transformed cells and primary ATL cells.29,36,38–41 However, no mutations in the SHP-1 ORF or promoter have been identified that could contribute to the SHP-1 down-regulation.38,39 Although aberrant promoter methylation may play a role in SHP-1 gene silencing,39,41 no direct relationship between SHP-1 down-regulation and HTLV-1 infection/transformation has been established.
In this study, we sought to clarify the molecular mechanisms by which SHP-1 expression is silenced by HTLV-1 and to investigate if Tax plays a role in this process. A luciferase reporter plasmid was constructed to test the activity of the putative SHP-1 P2 promoter and to map the core promoter elements by deletional analyses. A profound inhibitory effect of Tax on SHP-1 P2 promoter function was observed through cotransfection experiments. In addition, involvement of cellular factors, including NF-κB, CREB, CBP, p300, HDAC1, and PKA on Tax-SHP-1 promoter interaction was investigated. These studies provide the first molecular details of SHP-1 P2 promoter function and its silencing by Tax. A model is proposed for a central role of Tax-induced SHP-1 P2 promoter silencing (TIPS) in the earliest events in HTLV-1 leukemogenesis.
Materials and methods
Cell lines and plasmids
Jurkat large T-antigen cells (Jurkat-LT), Jurkat, HUT78, and SupT1 cell lines were cultured in 10% FBS RPMI 1640 media. 293T and HeLa cell lines were cultured in 10% FBS DMEM media. Human CD4+ T-cell isolation and culture conditions are the same as described previously.38 Plasmid pRSV-RelA and pRSV-p50 were obtained through NIH AIDS Research & Reference Reagent Program from Dr Gary Nabel and Dr Neil Perkins.42,43 PKA-c and 3xκB-Luc (Stratagene, La Jolla, CA), pCREB1 (Open Biosystems Huntsville, AL), pGL3-Control, and pGL3-Enhancer vectors (Promega, Madison, WI) were purchased. The DNA fragments encoding the HTLV-1 wild-type/M22/M47 Tax were subcloned from the original plasmids (gifts of W. Greene44 ) into pcDNA3.1 (+) vector with an N-terminal Flag tag. The following plasmids were generous gifts from S. Grossman (p300)45 ; J. Sui (pCMV-Luc); D. Housman (CBP)46 ; X. Yang and S. J. Marriott (HTLV-1-LTR-Luc)47,48 ; and S. Schreiber (HDAC1).49
Cloning of wild-type SHP-1 P2 promoter
Hematopoietic cell–specific SHP-1 P2 promoter was amplified by polymerase chain reaction (PCR) from fresh human peripheral blood mononuclear cell (PBMC) genomic DNA. Primers were designed according to the published sequence35 with addition of XhoI site and HindIII site at 5′-end of the forward and reverse primer, respectively. The amplified fragments were cloned into the promoterless pGL3-Enhancer vector. The resulting plasmid, pWt-P2-Luc, was sequence confirmed.
Deletional analysis of the SHP-1 P2 promoter
To determine the core sequence responsible for SHP-1 P2 promoter activity, forward primers F10 to F80 and reverse primers R10 to R80 (Figure 1A-C; sequences available upon request) were designed for PCR amplification of serially truncated P2 promoter fragments from pWt-P2-Luc. Each product was truncated by approximately 100-bp. The PCR products were digested, cloned into pGL3-Enhancer as described above in “Cloning of wild-type SHP-1 P2 promoter.” Sequence confirmed, and named after the corresponding primers.
Site-directed mutagenesis
Two NF-κB binding motifs and 2 E-box sequences (CANNTG)50 were predicted in the 277-bp P2 large core promoter using TFSEARCH program (Yutaka Akiyama: “TFSEARCH: Searching Transcription Factor Binding Sites,” http://www.cbrc.jp/research/db/TFSEARCH.html) (Figure 1C). Specific mutants were obtained using the QuickChange Multi Site-directed Mutagenesis Kit (Stratagene) according to the manufacturer's instruction and sequences are shown in Figure 2 legend. The effect of mutations on SHP-1 large core promoter function was evaluated through luciferase reporter assays on lysates from transiently transfected cells. Protein binding to these putative sites was analyzed by electrophoretic mobility shift assay (EMSA) based on a gel shift assay system by Promega and specifically described in Figure 4 legend.
Transient transfection and luciferase assay
DNA plasmids were transiently transfected into 1 × 106 Jurkat-LT cells with 6 μL SuperFect Reagent (Qiagen, Valencia, CA). The total amount of DNA was brought to 1.5 μg using pcDNA3.1(+) plasmid. Cells were harvested 60 hours after transfection and lysed in 100 μL 1 × Cell Culture by 513 Reagent (Promega). Upon adjusting protein concentration, 20 μL of each lysate was assayed in replicates for luciferase activity according to the manufacturer's manual using Turner Biosystem TD20/20 luminometer (Turner Designs, Sunnyvale, CA). For siRNA experiments, siRNA against HDAC1 (SI02663472; Qiagen) and control siRNA (AM4611; Ambion, Austin, TX) was transfected into Jurkat-LT cells along with indicated plasmids using Nucleofector kit (VCA-1003; Amaxa, Gaithersburg, MD). Cells were harvested 48 hours later and subjected to luciferase analysis or Western blot.
Chromatin immunoprecipitation (CHIP)
Jurkat cells or freshly isolated human CD4+ T cells were transfected with indicated plasmid DNA using Amaxa Nucleofector kit. CHIP analysis was conducted essentially following the protocol of Upstate CHIP kit (17-295; Lake Placid, NY). Briefly, 5 × 106 cells were cross-linked using 1% formaldehyde, pelleted, resuspended in 200 μL SDS lysis buffer, and sonicated so that the average length of chromosomal DNA became 200 to 1000 bp. After preclearing, chromatin was immunoprecipitated with 6 to 8 μg different antibodies at 4°C overnight. Chromatin solution (20 μL) without immunoprecipitation served as the input DNA control. The core promoter primer sequences F70 and R00 were used for subsequent PCR analysis upon DNA recovery from de–cross-linking and proteinase K digestion.
Immunoprecipitation and Western blot
Following transfection with Amaxa kits, Jurkat cells or human CD4+ T cells were harvested at designated times and lysed in 1 × radioimmunoprecipitation assay (RIPA) buffer containing proteinase inhibitors. Cell lysates were subjected directly to Western blot analysis or examined by combination of immunoprecipitation and Western blot assays.
Results
Cloning of the SHP-1 P2 promoter and identification of its core elements
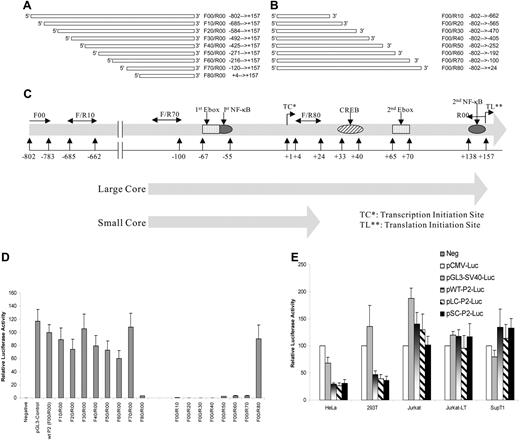
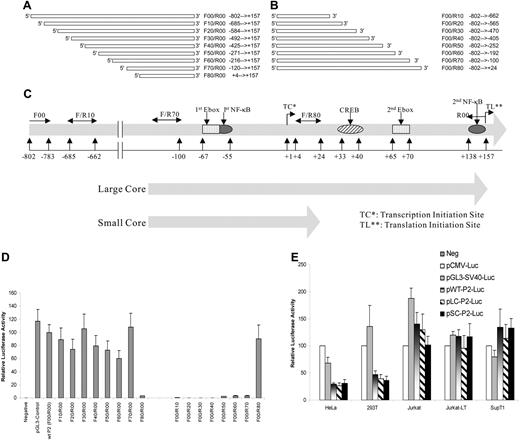
In order to investigate SHP-1 gene expression regulation, a reporter system was established by cloning the putative wild-type SHP-1 P2 promoter including the 5′ untranslated region of exon 2 (− 802 to + 157, with + 1 being the transcription initiation site35 ) in front of luciferase gene in the promoterless pGL3-Enhancer vector. As shown in Figure 1A and 1D, the wild-type SHP-1 P2 promoter (wt-P2-Luc [F00/R00]) was fully functional with comparable activity to the pGL3-Control where luciferase expression is under the control of the SV40 late promoter. The 5′ deletional analysis showed that the promoter activity remains unchanged until the fragment is shortened from − 120 to + 4 bp (F70/R00) (compare F70/R00 with F80/R00; Figure 1A,D). Similarly, the results from 3′ deletional assay indicated that the SHP-1 P2 promoter becomes inactive when the sequences between − 100 bp to + 24 bp are removed (Figure 1B,D). This indicates that the sequence between primer F70 and R80 is essential for SHP-1 P2 promoter function. The fragments defined by the primer set F70/R00 and F70/R80 were named the “large core” (− 120 ∼ + 157 bp) and “small core” (− 120 ∼ + 24 bp) P2 promoters, respectively (Figure 1C). When tested side-by-side, the activity of wild-type full-length and both core P2 promoters demonstrated similar activities (Figure 1E). Of particular note is that the activities of both the full-length and the core SHP-1 P2 promoters were reduced to circa one third of the SV40 and CMV promoters in nonhematopoietic cell lines such as HeLa and 293T, when compared with the hematopoietic cell lines including Jurkat, Jurkat-LT, and SupT1 cells, suggesting the cloned SHP-1 P2 promoter elements are preferentially active in hematopoietic cells.
Cloning and core region identification of SHP-1 P2 promoter. (A) Wild-type, full-length (− 802 bp ∼ + 157 bp, 960 base pairs) hematopoietic cell–specific SHP-1 P2 promoter was PCR amplified using primer pair F00 (forward primer, − 802 bp ∼ − 783 bp) and R00 (reverse primer, + 157 bp ∼ + 138 bp) at 1 × 94°C for 3 minutes, 30 × (94°C 30 seconds, 60°C 40 seconds, and 72°C 60 seconds), followed by 1 × 72°C 5 minutes. The amplified fragment was cloned into the pGL3-Enhancer vector, sequence confirmed, and named pwt-P2-Luc. The forward primers (F10 to F80) were subsequently designed so that a series of circa 100-bp 5′-truncated promoter fragments were achieved using the same reverse primer (R00). Similarly in panel B, a series of 3′-truncations was achieved using the same forward primer (F00) and different reverse primers (R10 to R80). (C) Structure of the full-length wild-type promoter (− 802 bp ∼ + 157 relative to the CAP site), large core (− 120 ∼ + 157), and small core (− 120 ∼ + 24), with putative transcription factor binding motifs labeled. (D) Promoter activity analysis of cloned SHP-1 promoter constructs through luciferase assays of the transient transfected Jurkat-LT cell lysates. (E) A comparison of the SHP-1 promoter activity in hematopoietic cell lines (Jurkat, Jurkat-LT, SupT1) and nonhematopoietic cell lines (HeLa, 293T). pGL3-Control and pCMV-Luc are plasmids that carry luciferase reporter gene driven by an SV40 promoter or a CMV promoter. Wt/LC/SC-P2-Luc: pGL3-based plasmid carrying luciferase reporter driven by the full-length wild-type, large core, or small core SHP-1 P2 promoter. Cell lysates were assayed in triplicates for luciferase activity and values represent the mean (± SD) of 2 experiments.
Cloning and core region identification of SHP-1 P2 promoter. (A) Wild-type, full-length (− 802 bp ∼ + 157 bp, 960 base pairs) hematopoietic cell–specific SHP-1 P2 promoter was PCR amplified using primer pair F00 (forward primer, − 802 bp ∼ − 783 bp) and R00 (reverse primer, + 157 bp ∼ + 138 bp) at 1 × 94°C for 3 minutes, 30 × (94°C 30 seconds, 60°C 40 seconds, and 72°C 60 seconds), followed by 1 × 72°C 5 minutes. The amplified fragment was cloned into the pGL3-Enhancer vector, sequence confirmed, and named pwt-P2-Luc. The forward primers (F10 to F80) were subsequently designed so that a series of circa 100-bp 5′-truncated promoter fragments were achieved using the same reverse primer (R00). Similarly in panel B, a series of 3′-truncations was achieved using the same forward primer (F00) and different reverse primers (R10 to R80). (C) Structure of the full-length wild-type promoter (− 802 bp ∼ + 157 relative to the CAP site), large core (− 120 ∼ + 157), and small core (− 120 ∼ + 24), with putative transcription factor binding motifs labeled. (D) Promoter activity analysis of cloned SHP-1 promoter constructs through luciferase assays of the transient transfected Jurkat-LT cell lysates. (E) A comparison of the SHP-1 promoter activity in hematopoietic cell lines (Jurkat, Jurkat-LT, SupT1) and nonhematopoietic cell lines (HeLa, 293T). pGL3-Control and pCMV-Luc are plasmids that carry luciferase reporter gene driven by an SV40 promoter or a CMV promoter. Wt/LC/SC-P2-Luc: pGL3-based plasmid carrying luciferase reporter driven by the full-length wild-type, large core, or small core SHP-1 P2 promoter. Cell lysates were assayed in triplicates for luciferase activity and values represent the mean (± SD) of 2 experiments.
Inhibition of the SHP-1 P2 promoter activity by HTLV-1 Tax
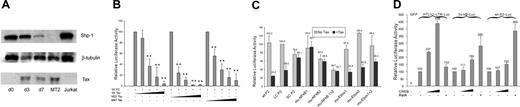
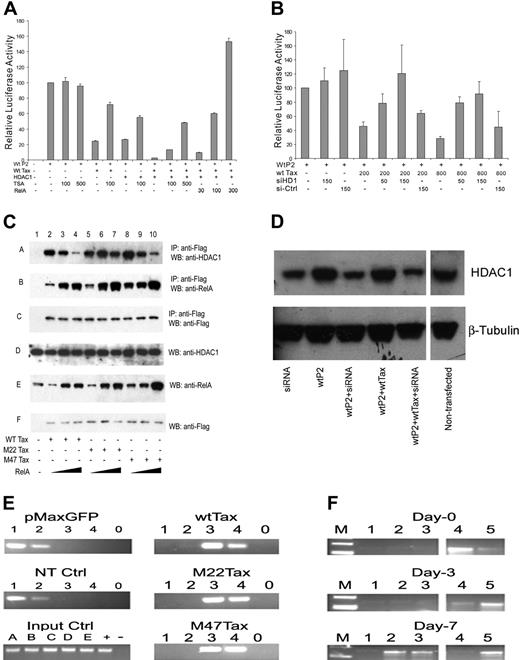
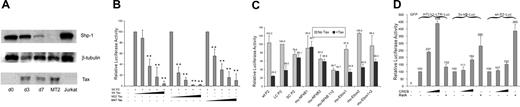
An early indication that Tax may be involved in regulation of SHP-1 gene expression was seen when a gradual loss of SHP-1 was observed with increased Tax expression when fresh CD4+ T cells were transfected with HTLV-1 proviral plasmid (Figure 2A). The effect of wild-type Tax on full-length P2 promoter was next directly examined in a transient transfection luciferase reporter assay. As seen on the left side of Figure 2B, cotransfection of wt Tax significantly inhibited the P2 promoter activity in a dosage-dependent manner. Similar inhibitory effects by Tax were also observed on the large but not the small “core” promoter fragment (Figure 2C). As an initial step to dissect the mechanism of SHP-1 P2 promoter inhibition by Tax, 2 well-characterized Tax mutants, M22 that activates only the CREB/ATF pathway, and M47 that activates only the NF-κB pathway,44 were tested for their effects on the P2 promoter activity. As shown in the middle and right sections of Figure 2A, both M22 and M47 exerted dosage-dependent inhibitory effects on the SHP-1 P2 promoter. The trans-activation characteristics of the N-terminal HA-tagged wild-type M22 and M47 Tax mutants used in this study were confirmed by demonstrating their respective effects on the CREB-dependent expression of HTLV-1 LTR and on an NF-κB–dependent promoter (data not shown).
Repression of wt P2 promoter activity by Tax. (A) Fresh CD4+ cells were transfected with HTLV-1 provirus pACH-wtTax DNA using Amaxa Kit. Cells were then cultured in AIM-V media with 10% fetal bovine serum, IL-2 (100 U/mL), and PHA (1 μg/mL). Cell were collected on day 0 (d0), day 3 (d3), and day 7 (d7) after transfection, and cell lysates were subjected to Western blot analysis. SHP-1 (sc-287) and β-tubulin (sc-9104) antibodies were from Santa Cruz Biotechnology (Santa Cruz, CA). Anti-Tax (Tab172) was from NIH-ARRRP. Jurkat E6-1 and MT2 cell line were used as the positive and negative of SHP-1 expression. (B) Jurkat-LT cells were transfected with 500 ng Wt-P2-Luc plasmid and different amounts of Tax plasmid (0, 10, 50, 200, 1000 ng). The effect of Tax on SHP-1 P2 promoter activity was measured by luciferase assay as described in “Transient transfection and luciferase assay.” Effect of E-box and NF-κB mutations on P2 promoter. Two E-box motifs and 2 NF-κB motifs were predicted in the SHP-1 LC-P2 promoter (Figure 1C). Site-specific mutants were generated to analyze the effects of these sites on the promoter activity. The sequences are shown below: NF-κB1, 5′-CAAGTGA/TGTTCCCCCAAGGG-3′; NF-κB2, 5′-CCTCTCCGGAAGCCCC/TCAGG-3′; Ebox1, AGAAGTAC/TAAGTGAGTTCCC; Ebox2, GGAGCTGCATCT/AGAGGCTTA. The italic sequences are the predicted wild-type motifs, and the bold letters represent the mutated nucleotides. Mu-Ebox1 + 2 and mu-NF-κB1 + 2 represent double mutations in E-boxes or NF-κB motifs, respectively. Asterisks indicate that the differences in values are statistically significant when compared with the values of the corresponding samples without Tax transfection. (C) Luciferase assay was performed to analyze the effect of promoter mutations described in panel B. Wild-type, large or small core SHP-1 P2 promoter plasmids, or the SHP-1 LC-P2 promoter mutant plasmids (0.5 μg) were transfected into Jurkat-LT cells in the presence or absence of 0.2 μg Tax plasmid. (D) Involvement of CREB and RelA in SHP-1 P2 basal promoter regulation was examined by luciferase assay of transiently cotransfected Jurkat-LT cell lysates. CREB-dependent HTLV-1-luc reporter and NF-κB–dependent 3xκb-luc reporter were similarly cotransfected with CREB or RelA encoding plasmids and the cell lysates were used as controls in the luciferase assay. Error bars represent means plus or minus SD.
Repression of wt P2 promoter activity by Tax. (A) Fresh CD4+ cells were transfected with HTLV-1 provirus pACH-wtTax DNA using Amaxa Kit. Cells were then cultured in AIM-V media with 10% fetal bovine serum, IL-2 (100 U/mL), and PHA (1 μg/mL). Cell were collected on day 0 (d0), day 3 (d3), and day 7 (d7) after transfection, and cell lysates were subjected to Western blot analysis. SHP-1 (sc-287) and β-tubulin (sc-9104) antibodies were from Santa Cruz Biotechnology (Santa Cruz, CA). Anti-Tax (Tab172) was from NIH-ARRRP. Jurkat E6-1 and MT2 cell line were used as the positive and negative of SHP-1 expression. (B) Jurkat-LT cells were transfected with 500 ng Wt-P2-Luc plasmid and different amounts of Tax plasmid (0, 10, 50, 200, 1000 ng). The effect of Tax on SHP-1 P2 promoter activity was measured by luciferase assay as described in “Transient transfection and luciferase assay.” Effect of E-box and NF-κB mutations on P2 promoter. Two E-box motifs and 2 NF-κB motifs were predicted in the SHP-1 LC-P2 promoter (Figure 1C). Site-specific mutants were generated to analyze the effects of these sites on the promoter activity. The sequences are shown below: NF-κB1, 5′-CAAGTGA/TGTTCCCCCAAGGG-3′; NF-κB2, 5′-CCTCTCCGGAAGCCCC/TCAGG-3′; Ebox1, AGAAGTAC/TAAGTGAGTTCCC; Ebox2, GGAGCTGCATCT/AGAGGCTTA. The italic sequences are the predicted wild-type motifs, and the bold letters represent the mutated nucleotides. Mu-Ebox1 + 2 and mu-NF-κB1 + 2 represent double mutations in E-boxes or NF-κB motifs, respectively. Asterisks indicate that the differences in values are statistically significant when compared with the values of the corresponding samples without Tax transfection. (C) Luciferase assay was performed to analyze the effect of promoter mutations described in panel B. Wild-type, large or small core SHP-1 P2 promoter plasmids, or the SHP-1 LC-P2 promoter mutant plasmids (0.5 μg) were transfected into Jurkat-LT cells in the presence or absence of 0.2 μg Tax plasmid. (D) Involvement of CREB and RelA in SHP-1 P2 basal promoter regulation was examined by luciferase assay of transiently cotransfected Jurkat-LT cell lysates. CREB-dependent HTLV-1-luc reporter and NF-κB–dependent 3xκb-luc reporter were similarly cotransfected with CREB or RelA encoding plasmids and the cell lysates were used as controls in the luciferase assay. Error bars represent means plus or minus SD.
NF-κB plays an important role in Tax-induced promoter silencing (TIPS)
The above data indicated that Tax might exert its effect through a third transcriptional regulatory pathway that is independent of NF-κB or CREB/ATF. Computational analysis of SHP-1 P2 “core” region revealed several putative binding sites for important transcription factors such as NF-κB, CREB, and E-box binding basic helix-loop-helix (b-HLH) factors (Figure 1C). To test if Tax inhibits SHP-1 gene expression through b-HLH family of transcription factors,51–54 mutations were introduced at the invariant residues of either or both E-box motifs, although no protein-DNA complexes could be detected by EMSA using oligonucleotide probes (20-bp) containing the putative E-box motifs (CANNTG) with nuclear extracts from 4 HTLV-1–positive or 4 HTLV-1–negative cells (data not shown). The resulting mutants were analyzed for their effects on the SHP-1 LC P2 basal promoter activity and TIPS through luciferase assays. As seen in the right section of Figure 2C, the E-box mutations had no significant effects on the basal promoter activity or TIPS, suggesting that Tax unlikely represses SHP-1 P2 promoter through a b-HLH mechanism.
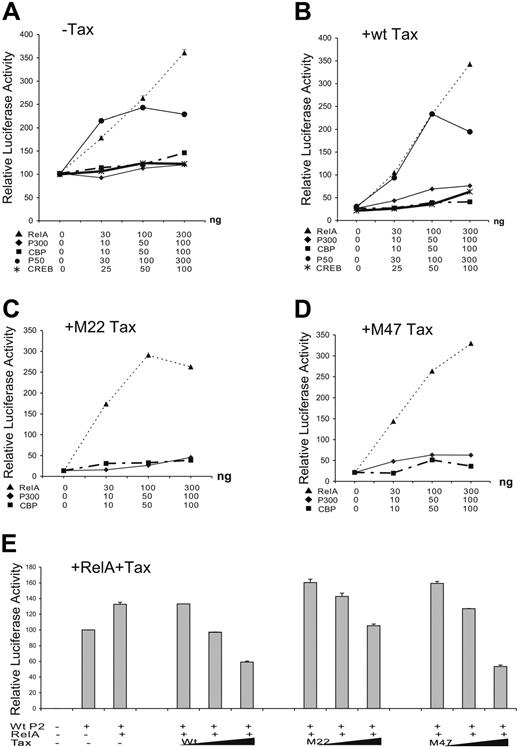
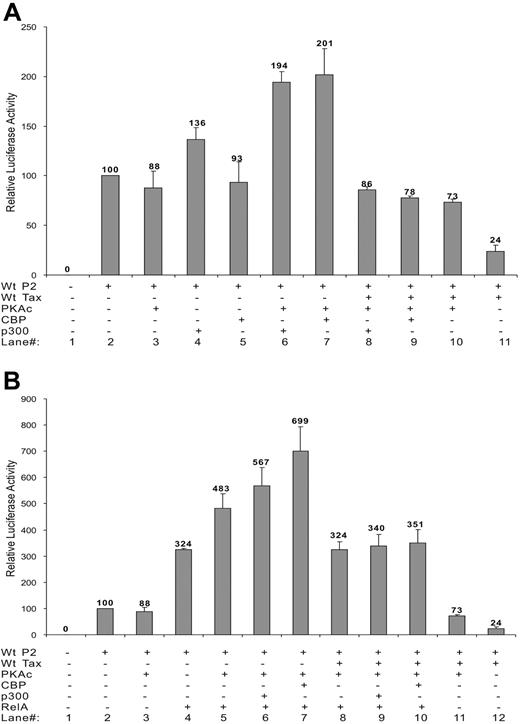
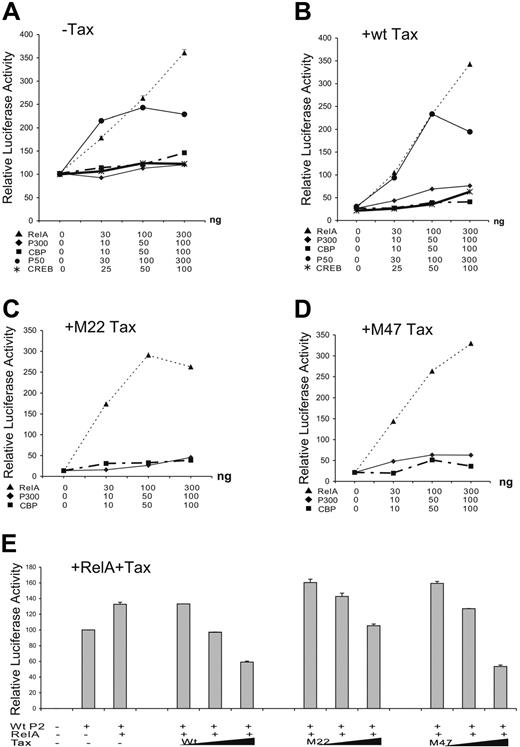
The effects of CREB of the CREB/ATF pathway and RelA component of the NF-κB pathway on SHP-1 P2 basal promoter were next investigated by transient transfection. Data in Figure 2D indicated that only RelA strongly activated the P2 promoter. This observation was further examined through dosage titration that included CREB, p300, CBP, RelA, and p50. When transfected individually with the wt-P2-Luc luciferase reporter plasmid, RelA had a significant stimulatory effect on the SHP-1 P2 promoter activity, while the effects of CREB, p300, and CBP were minimal (Figure 3A), indicating the RelA could be the rate-limiting factor in SHP-1 transcription regulation. Consequently, RelA is shown to be the most potent blocker of TIPS in Figure 3B-D. In the presence of 200 ng wild-type or mutant Tax plasmids, the P2 promoter activity could be fully restored by cotransfecting 30 ng RelA plasmid compared with approximately 50% restoration by CREB, CBP, or p300 at 100 ng. It is worth noting that p50 component of NF-κB achieved a similar effect compared with RelA on basal and Tax-inhibited promoter activity (Figure 3A,B), albeit it reached plateau at higher DNA concentrations. These results suggest that the NF-κB plays an active role in TIPS.
Effect of NF-κB, CREB, CBP, and p300 on TIPS. Jurkat-LT cells were plated in 12-well tissue culture dishes at 1 × 106cells/well and 1.5 μg plasmids was transiently transfected using SuperFect transfection Reagent (Qiagen). Wild-type SHP-1 P2 promoter (wt-P2-Luc, 500 ng) was cotransfected with increasing amounts of p65 (RelA) or p50 of NF-κB family (0, 30, 100, and 300 ng), CREB (0, 25, 50, 100 ng), CBP (0, 10, 50, 100 ng), or p300 (0, 10, 50, 100 ng) in the absence (A) or presence of 200 ng wt Tax (B), M22 Tax (C), or M47 Tax (D). (E) Wild-type SHP-1 P2 promoter (wt-P2-Luc, 500 ng) was cotransfected without or with 30 ng RelA plasmid and 100, 200, or 400 ng wt, M22, or M47 Tax. Cell lysates were assayed in triplicates for luciferase activity and values represent the mean ± SD of 2 experiments. The relative luciferase activity was normalized against basal SHP-1 P2 promoter luciferase reporter alone (set as 100).
Effect of NF-κB, CREB, CBP, and p300 on TIPS. Jurkat-LT cells were plated in 12-well tissue culture dishes at 1 × 106cells/well and 1.5 μg plasmids was transiently transfected using SuperFect transfection Reagent (Qiagen). Wild-type SHP-1 P2 promoter (wt-P2-Luc, 500 ng) was cotransfected with increasing amounts of p65 (RelA) or p50 of NF-κB family (0, 30, 100, and 300 ng), CREB (0, 25, 50, 100 ng), CBP (0, 10, 50, 100 ng), or p300 (0, 10, 50, 100 ng) in the absence (A) or presence of 200 ng wt Tax (B), M22 Tax (C), or M47 Tax (D). (E) Wild-type SHP-1 P2 promoter (wt-P2-Luc, 500 ng) was cotransfected without or with 30 ng RelA plasmid and 100, 200, or 400 ng wt, M22, or M47 Tax. Cell lysates were assayed in triplicates for luciferase activity and values represent the mean ± SD of 2 experiments. The relative luciferase activity was normalized against basal SHP-1 P2 promoter luciferase reporter alone (set as 100).
To further evaluate the role of RelA in TIPS, a reverse titration experiment was performed to determine if addition of Tax could counteract the stimulatory effect of RelA on the SHP-1 P2 promoter. As expected, cotransfection of wt and M47 Tax, and to a lesser extent M22, inhibited RelA-stimulated P2 activity in a dosage-dependent manner (Figure 3E), further implicating RelA as one of the main molecular targets involved in TIPS.
Mutational analysis of RelA's role in TIPS
Computer analysis also predicted 2 putative NF-κB binding motifs (− 65 ∼ − 55 and + 145 ∼ + 154) in the large core promoter region with threshold scores around 80% (Figure 1C). To confirm the role of RelA in the basal promoter activity as well as TIPS, site-specific mutants were generated and the effects were evaluated. As seen in Figure 2C, mutations in either one (mu-NF-κB1 or mu-NF-κB2) or both (mu-NF-κB1 + 2) NF-κB binding sites led to the reduction of the P2 promoter activity. While mutation within the first NF-κB motif (mu-NF-κB1) had minimal effect, the second NF-κB motif mutant and the double mutant reduced the wild-type large core promoter activity to 65% and 39%, respectively. Most importantly, single or double NF-κB motif mutants cannot be inhibited by Tax, indicating both motifs are indispensable for TIPS. Consistent with this result, Tax could not repress SHP-1 small core (SC) promoter where the second NF-κB motif is absent (Figures 1C and 2C).
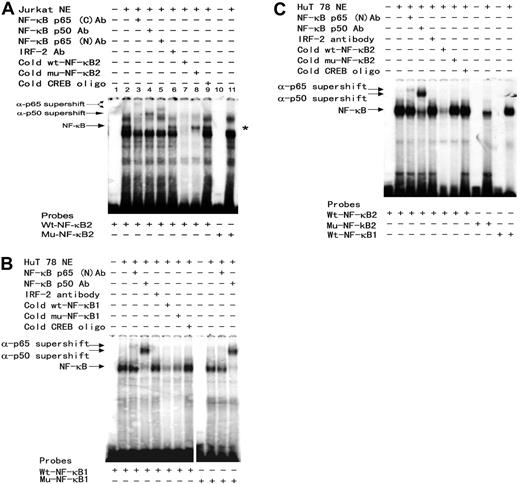
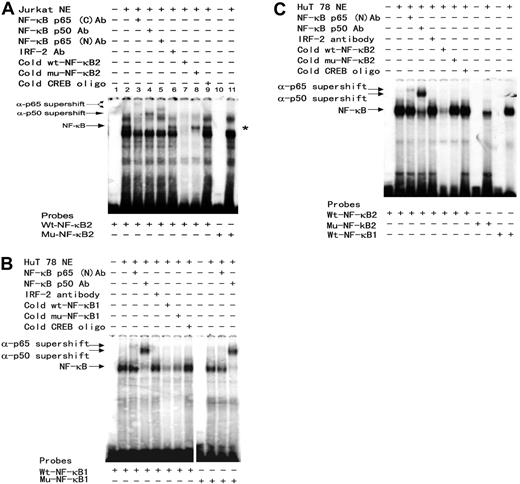
Binding of the NF-κB p50/p65 to these putative NF-κB motifs and their mutants was verified by EMSA. DNA-protein complexes were detected upon incubation of a 20-bp oligonucleotide probe containing the wild-type second NF-κB motif with the Jurkat (Figure 4A) or HUT78 (Figure 4C) nuclear extracts. The NF-κB-DNA complex was confirmed by supershift analysis with NF-κB–specific and irrelevant antibodies. The specificity of this NF-κB-DNA complex was further confirmed by competition with 100 × molar excess of unlabeled wt-NF-κB2, mu-NF-κB2, and irrelevant CREB oligonucleotide probes as well as by its absence (Figure 4A) among the protein-DNA complexes formed with site-specific NF-κB2 mutant probe. These results indicate that p50 and p65 indeed bind to the second NF-κB motif and when combined with the results of luciferase reporter assay in Figure 2C, support the notion that NF-κB is actively involved in SHP-1 P2 promoter activity and TIPS.
Evaluation of protein-DNA complex formation on the putative NF-κB motifs by EMSA. The sense (as listed in Figure 2 legend) and antisense synthetic oligonucleotides representing either wild-type or single mutant NF-κB sequences were annealed and 32P-labeled to form the double-stranded DNA probes. The 32P-labeled double-stranded 20-bp oligonucleotide probes were incubated with 7.5 μg PMA-stimulated Jurkat (A) or HUT78 (B,C) for 30 minutes at room temperature (RT). For antibody supershift analysis, nuclear extracts were incubated for 10 minutes at RT with 1 μL antibody (Santa Cruz Biotechnology) specific for NF-κB p65 (sc-372x, C-term; and sc-109x, N-term), NF-κB p50 (sc-7178x), or IRF-2 (sc-498x) prior to addition of the 32P-labeled wild-type, mutant NF-κB1, or NF-κB2 probes as indicated at the bottom of each panel. For oligonucleotide competition experiments, 100-fold molar excess of indicated unlabeled probes was added to each binding reaction. The DNA-protein complexes were resolved on 4% native PAGE and exposed to KODAK X-Omat LS films (Rochester, NY). A vertical line has been inserted into panel B to indicate where gel lanes were cut. Note that all images in panel B came from the same experiment. *indicates absence of NF-κB protein-DNA complex in lane 11 when a mutant probe is used.
Evaluation of protein-DNA complex formation on the putative NF-κB motifs by EMSA. The sense (as listed in Figure 2 legend) and antisense synthetic oligonucleotides representing either wild-type or single mutant NF-κB sequences were annealed and 32P-labeled to form the double-stranded DNA probes. The 32P-labeled double-stranded 20-bp oligonucleotide probes were incubated with 7.5 μg PMA-stimulated Jurkat (A) or HUT78 (B,C) for 30 minutes at room temperature (RT). For antibody supershift analysis, nuclear extracts were incubated for 10 minutes at RT with 1 μL antibody (Santa Cruz Biotechnology) specific for NF-κB p65 (sc-372x, C-term; and sc-109x, N-term), NF-κB p50 (sc-7178x), or IRF-2 (sc-498x) prior to addition of the 32P-labeled wild-type, mutant NF-κB1, or NF-κB2 probes as indicated at the bottom of each panel. For oligonucleotide competition experiments, 100-fold molar excess of indicated unlabeled probes was added to each binding reaction. The DNA-protein complexes were resolved on 4% native PAGE and exposed to KODAK X-Omat LS films (Rochester, NY). A vertical line has been inserted into panel B to indicate where gel lanes were cut. Note that all images in panel B came from the same experiment. *indicates absence of NF-κB protein-DNA complex in lane 11 when a mutant probe is used.
Specific binding to the first NF-κB site could be detected and verified by antibody supershift (Figure 4B and data not shown). PhosphorImager (Storm 820, ImageQuant; GE Healthcare, Piscataway, NJ) analysis shows that the NF-κB binding to the NF-κB second site is twice as intense as to the first site, reflecting a likely difference in the NF-κB's binding affinity of these 2 motifs. In addition, the single mutation made in the NF-κB first site reduced but did not completely eliminate NF-κB binding (Figure 4B), which may count for the limited reduction in basal promoter activity of this mutant (Figure 2C). However, identical mutation totally abolished TIPS, indicating either NF-κB or other factors binding to this location is essential for Tax's inhibitory effect on SHP-1 P2 promoter.
Involvement of HDAC1 in the TIPS
Since Tax55,56 and NF-κB57,58 interact with mammalian histone deacetylase 1 (HDAC1) and HDAC1 has been previously associated with transcriptional silencing,55,57,59 possible involvement of HDAC1 in TIPS was tested. As shown in Figure 5A, HDAC1 can inhibit the P2 promoter to a similar level as wt Tax, about 25% of basal activity. While addition of HDAC1 inhibitor TSA alone had no significant effect on promoter activity, 100 ng/mL TSA could restore the HDAC1-inhibited P2 promoter activity from 27% to 54%, roughly 2-fold, and the wt Tax–inhibited P2 activity from 24% to 72%, roughly 3-fold. Coexpression of HDAC1 and wt Tax further inhibited the SHP-1 P2 promoter to 3% of its basal activity, which required an additional amount of TSA (500 ng/mL) to reverse. To confirm the role of HDAC1 in TIPS, siRNA against HDAC1 was transfected with SHP-1 P2 luc construct with or without wt Tax. The presence of 150 pmol HDAC1-specific siRNA not only reduced the steady-state level of endogenous HDAC1 protein (Figure 5D) but also significantly reversed TIPS (circa 3-fold) in luciferase reporter assay (Figure 5B). These results clearly indicate the HDAC1's involvement in TIPS.
Effect of HDAC1 and NF-κB on TIPS. (A) Jurkat-LT cell transfection and luciferase assay were performed as previously described. Plasmids used were as follows: wild-type SHP-1 P2 promoter (wt-P2-Luc), 500 ng; wt Tax, 200 ng; HDAC1, 500 ng; and RelA, 30 ng, 100 ng, or 300 ng. TSA (100 ng/mL or 500 ng/mL) was added 24 hours before harvesting the cells. Cell lysates were assayed in triplicates for luciferase activity and values represent the mean ± SD of 2 experiments. The relative luciferase activity was normalized against basal SHP-1 P2 promoter luciferase reporter alone (set as 100). (B,D) Jurkat-LT cells were cotransfected with 500 ng wtP2-Luc, different amounts of wtTax plasmid, and siRNA against HDAC1 as indicated on X-axis. Cells were harvested 48 hours after transfection, and cell lysates were analyzed by luciferase assay (B) and by Western blot (D, anti-HDAC1, upper; and anti–β-tubulin, lower, from Santa Cruz Biotechnology catalog no. sc-7872 and sc-9104, respectively). A nonspecific siRNA was used as a control. (C) Effect of NF-κB on association of HDAC1 with Tax. Jurkat-LT cells (5 × 106) were transfected with 3 μg Flag-tagged Tax constructs (wt or M22 or M47) and 100 ng or 800 ng RelA (p65 of NF-κB) plasmid. Sixty hours later, cells were harvested and lysed in 1 × RIPA lysis buffer with protease inhibitors (catalog no. 1169-7498; Roche Diagnostics, Indianapolis, IN). The proteins in association with Tax were precipitated with anti-Flag M2 agarose beads (A2220; Sigma) and identified by Western blots using HDAC1 antibody (lane A, sc-7872; Santa Cruz Biotechnology), anti-RelA antibody (lane B, sc-372; Santa Cruz Biotechnology), or anti-Flag antibody (lane C for Tax detection, A8592; Sigma). As controls, Western blots were also performed using cell lysates without immunoprecipitation against HDAC1, RelA, and Flag (lanes D, E, and F, respectively). IP indicates immunoprecipitation; WB, Western blot. (E) CHIP analysis. Jurkat cells were transfected, using Amaxa kit VCA-1003, with 3 μg of WtTax, M22, or M47 Tax-expressing plasmids and a control pMaxGFP plasmid. Sixty hours after transfection, cells were harvested and CHIP assay was performed using Upstate kit (17-295). Primers for PCR analysis were as follows: F70, 5′AGTGCCACCCTGCTCTGCTTC3′; R00, 5′CCTGGGGGCTTCCGGAGAGG3′. Antibodies used here were as follows: (lane 1) anti-p65; (lane 2) anti-p50; (lane 3) anti-Tax; (lane 4) anti-HDAC1; (lane 0) rabbit isotype IgG control. (Left images; from top to bottom) GFP control, no transfection control, and input control. (A-E) Input DNA control from wtTax, M22, M47, GFP, and no transfection, respectively. + indicates positive PCR control with the pGL3-LC-P2 plasmid; −, no DNA template control. (Right images; from top to bottom) Jurkat cells transfected with wtTax, M22, and M47. (F) CHIP assay of histone H3-K9 deacetylation at P2 promoter in freshly isolated CD4+ T cells transfected with HTLV-1 proviral DNA (pACH-wtTax). (Lane 1) no antibody; (lane 2) anti-Tax; (lane 3) anti-HDAC1; (lane 4) anti–acetyl-H3-K9; (lane 5) anti-H3. Antibodies used for CHIP assays in panels E,F: from NIH-ARRRP: anti-Tax (Tab172); from Santa Cruz Biotechnology: anti-HDAC1 (sc-7872); anti–NF-κB (p65) (sc-372X); anti–NF-κB (p50) (sc-7178X); and rabbit control IgG (sc-2027); from Cell Signaling Technology: anti–acetyl-H3-K9 (catalog no. 9671) and anti-H3 (catalog no. 9715). Spaces were inserted into both panels D and F to indicate where the gel lanes were cut. Note that the gel images in each panel came from the same experiment.
Effect of HDAC1 and NF-κB on TIPS. (A) Jurkat-LT cell transfection and luciferase assay were performed as previously described. Plasmids used were as follows: wild-type SHP-1 P2 promoter (wt-P2-Luc), 500 ng; wt Tax, 200 ng; HDAC1, 500 ng; and RelA, 30 ng, 100 ng, or 300 ng. TSA (100 ng/mL or 500 ng/mL) was added 24 hours before harvesting the cells. Cell lysates were assayed in triplicates for luciferase activity and values represent the mean ± SD of 2 experiments. The relative luciferase activity was normalized against basal SHP-1 P2 promoter luciferase reporter alone (set as 100). (B,D) Jurkat-LT cells were cotransfected with 500 ng wtP2-Luc, different amounts of wtTax plasmid, and siRNA against HDAC1 as indicated on X-axis. Cells were harvested 48 hours after transfection, and cell lysates were analyzed by luciferase assay (B) and by Western blot (D, anti-HDAC1, upper; and anti–β-tubulin, lower, from Santa Cruz Biotechnology catalog no. sc-7872 and sc-9104, respectively). A nonspecific siRNA was used as a control. (C) Effect of NF-κB on association of HDAC1 with Tax. Jurkat-LT cells (5 × 106) were transfected with 3 μg Flag-tagged Tax constructs (wt or M22 or M47) and 100 ng or 800 ng RelA (p65 of NF-κB) plasmid. Sixty hours later, cells were harvested and lysed in 1 × RIPA lysis buffer with protease inhibitors (catalog no. 1169-7498; Roche Diagnostics, Indianapolis, IN). The proteins in association with Tax were precipitated with anti-Flag M2 agarose beads (A2220; Sigma) and identified by Western blots using HDAC1 antibody (lane A, sc-7872; Santa Cruz Biotechnology), anti-RelA antibody (lane B, sc-372; Santa Cruz Biotechnology), or anti-Flag antibody (lane C for Tax detection, A8592; Sigma). As controls, Western blots were also performed using cell lysates without immunoprecipitation against HDAC1, RelA, and Flag (lanes D, E, and F, respectively). IP indicates immunoprecipitation; WB, Western blot. (E) CHIP analysis. Jurkat cells were transfected, using Amaxa kit VCA-1003, with 3 μg of WtTax, M22, or M47 Tax-expressing plasmids and a control pMaxGFP plasmid. Sixty hours after transfection, cells were harvested and CHIP assay was performed using Upstate kit (17-295). Primers for PCR analysis were as follows: F70, 5′AGTGCCACCCTGCTCTGCTTC3′; R00, 5′CCTGGGGGCTTCCGGAGAGG3′. Antibodies used here were as follows: (lane 1) anti-p65; (lane 2) anti-p50; (lane 3) anti-Tax; (lane 4) anti-HDAC1; (lane 0) rabbit isotype IgG control. (Left images; from top to bottom) GFP control, no transfection control, and input control. (A-E) Input DNA control from wtTax, M22, M47, GFP, and no transfection, respectively. + indicates positive PCR control with the pGL3-LC-P2 plasmid; −, no DNA template control. (Right images; from top to bottom) Jurkat cells transfected with wtTax, M22, and M47. (F) CHIP assay of histone H3-K9 deacetylation at P2 promoter in freshly isolated CD4+ T cells transfected with HTLV-1 proviral DNA (pACH-wtTax). (Lane 1) no antibody; (lane 2) anti-Tax; (lane 3) anti-HDAC1; (lane 4) anti–acetyl-H3-K9; (lane 5) anti-H3. Antibodies used for CHIP assays in panels E,F: from NIH-ARRRP: anti-Tax (Tab172); from Santa Cruz Biotechnology: anti-HDAC1 (sc-7872); anti–NF-κB (p65) (sc-372X); anti–NF-κB (p50) (sc-7178X); and rabbit control IgG (sc-2027); from Cell Signaling Technology: anti–acetyl-H3-K9 (catalog no. 9671) and anti-H3 (catalog no. 9715). Spaces were inserted into both panels D and F to indicate where the gel lanes were cut. Note that the gel images in each panel came from the same experiment.
Competitive association of HDAC1 and NF-κB to HTLV-1 Tax protein and endogenous SHP-1 promoter
Based on the previous finding that HDAC1 can interact directly with RelA to negatively regulate gene expression57 and that both HDAC1 and RelA can bind to Tax,55,56,60–62 we hypothesized that Tax may exert its inhibitory effect by recruiting HDAC1 to the SHP-1 P2 promoter site and competitively displacing NF-κB from the promoter possibly through deacetylation. Indeed, like TSA, RelA could also counteract the combined inhibitory effect of wt Tax and HDAC1 on the SHP-1 P2 promoter, as shown in the last 3 columns of Figure 5A. To further address this possibility, 3 μg Tax (wt, M22, or M47) was cotransfected into Jurkat cells in the presence of increasing amounts of RelA plasmid. Interaction of RelA and HDAC1 with the Flag-tagged Tax proteins was determined by coprecipitation using an anti-Flag antibody followed by Western blot analysis with RelA- or HDAC1-specific antibodies. As shown in Figure 5C, endogenous HDAC1 was coimmunoprecipitated with all 3 forms of Tax (lanes 2, 5, and 8). In addition, binding of HDAC1 to wt and M47 Tax were greatly reduced with increased expression of exogenous RelA (Figure 5C lanes 2-4 and 8-10), thus providing evidence that a competition exists between HDAC1 and NF-κB for their association with Tax. Interestingly, the binding of HDAC1 to M22 Tax was not significantly affected by increased RelA expression (comparing Figure 5C lanes 5-7 to 2-4 and 8-10), suggesting association of HDAC1 and NF-κB with M22 is qualitatively different compared with their association with wt or M47 Tax (eg, higher affinity binding between M22 and HDAC1).
The effect of HTLV-1 Tax on association of NF-κB and HDAC1 with the endogenous SHP-1 P2 promoter was further evaluated using direct chromatin immunoprecipitation (CHIP) assays. As shown in Figure 5E, transfection of HTLV-1 wild-type or mutant Tax plasmids into Jurkat cells led to an association of both Tax (lane 3) and HDAC1 (lane 4) with the large core P2 promoter while simultaneously eliminating both NF-κB p65 (lane 1) and p50 (lane 2) binding to the promoter (compare the Tax plasmid–transfected samples versus nontransfected and GFP-transfected controls). CHIP assay also revealed that the loss of histone H3-K9 acetylation, a known target for HDAC1-associated gene repression,63 at the SHP-1 P2 promoter also correlated with detection of both Tax and HDAC1 during the 7-day period that was examined (Figure 5F). This supports the hypothesis that Tax recruits HDAC1 onto the promoter and causes not only dissociation of NF-κB but also deacetylation of H3K9.
The effect of PKA on the basal SHP-1 P2 promoter activity and its involvement in TIPS
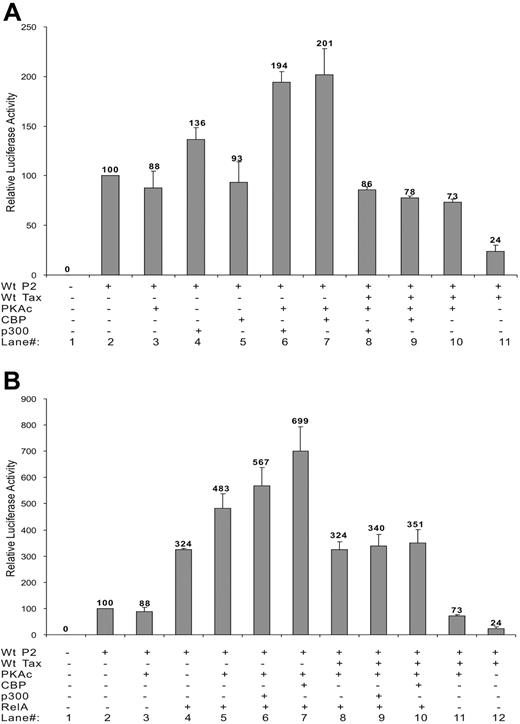
Azran et al64 have shown that PKA-c phosphorylates RelA and increases its binding to CBP/p300 and P/CAF coactivators, resulting in an increased nuclear translocation of Tax-p65(RelA)-CBP ternary complex and increased transcriptional activity. The effect of PKA-c on the SHP-1 P2 promoter and its involvement in TIPS were investigated. As shown in Figure 6A, individually expressing PKA-c had no effect on basal SHP-1 P2 activity (lanes 2,3). However, expression of exogenous CBP or p300 together with PKA-c led to an approximately 2-fold increase in the basal promoter activity (Figure 6A lanes 6,7). Expression of PKA-c could also further stimulate the SHP-1 P2 promoter in the presence of ectopically expressed RelA (Figure 6B lanes 4,5). Such stimulatory effect could be potentiated by addition of CBP or p300 (Figure 6B lanes 6,7).
Effect of PKA-c on basal SHP-1 P2 promoter activity and TIPS. Jurkat-LT cells were transfected with different combination of plasmids. Dosages of plasmids used were as follows: wt-P2-Luc, 500 ng; wt Tax, 200 ng; RelA, 300 ng; CBP, 100 ng; p300, 100 ng; PKA-c, 400 ng. Luciferase activity was measured as previous described. (A) Effect of PKA-c on CBP, p300 activation of SHP-1 P2 promoter in the absence or presence of Tax. (B) Effect of PKA-c on RelA activation of SHP-1 P2 promoter in the presence or absence of CBP, p300, and Tax. Cell lysates were assayed in triplicates for luciferase activity and values represent the mean ± SD of 2 experiments. The relative luciferase activity was normalized against basal SHP-1 P2 promoter luciferase reporter alone (set as 100). Data presented in panels A-B were collected simultaneously, separated only for clearer illustration, and thus can be compared directly against each other.
Effect of PKA-c on basal SHP-1 P2 promoter activity and TIPS. Jurkat-LT cells were transfected with different combination of plasmids. Dosages of plasmids used were as follows: wt-P2-Luc, 500 ng; wt Tax, 200 ng; RelA, 300 ng; CBP, 100 ng; p300, 100 ng; PKA-c, 400 ng. Luciferase activity was measured as previous described. (A) Effect of PKA-c on CBP, p300 activation of SHP-1 P2 promoter in the absence or presence of Tax. (B) Effect of PKA-c on RelA activation of SHP-1 P2 promoter in the presence or absence of CBP, p300, and Tax. Cell lysates were assayed in triplicates for luciferase activity and values represent the mean ± SD of 2 experiments. The relative luciferase activity was normalized against basal SHP-1 P2 promoter luciferase reporter alone (set as 100). Data presented in panels A-B were collected simultaneously, separated only for clearer illustration, and thus can be compared directly against each other.
The involvement of PKA-c in TIPS was demonstrated by a roughly 3-fold restoration of the Tax-repressed SHP-1 P2 promoter activity in the presence of PKA-c (24% to 73%, Figure 6A, compare lanes 10 and 11). Additional data show that Tax effectively abolished PKA-c's stimulatory effects on the SHP-1 P2 promoter when ectopically expressed CBP and p300 (Figure 6A, compare lane 7 with 9 and lane 6 with 8) or RelA (Figure 6B, compare lane 5 with 8) were examined alone. Tax also inhibits PKA-c's stimulatory effect (Figure 6B) on RelA ectopically coexpressed with p300 (lanes 6 and 9) or CBP (lanes 7 and 10) to a level seen with RelA alone (lanes 4, 5, and 8), further suggesting that PKA-c is another molecule targeted by Tax in the TIPS process.
Discussion
Lack of SHP-1 tyrosine phosphatase expression, a key negative regulator in several intracellular signal transduction pathways, in HTLV-1–positive T-cell lines and primary ATL cells has previously been reported.29,36,38 However, the mechanism(s) of SHP-1 P2 promoter basal transcription, SHP-1 gene silencing in HTLV-1–transformed lymphocytes, and its role in the development of ATL remains unclear. In particular, it is not known whether anyHTLV-1–encoded viral proteins are involved in the process of SHP-1 silencing. The focus of this study was to evaluate if Tax played a role in mediating SHP-1 gene expression. In order to assess its activity, the full-length SHP-1 P2 promoter35 was cloned and demonstrated to be fully functional in mediating luciferase reporter expression in a hematopoietic cell–specific manner (Figure 1). Two “core” promoter regions within − 120 to + 157 bp and − 120 to + 24 bp relative to the CAP site35 were defined through serial deletions (Figure 1). Additional studies showed that, compared with CREB, CBP, and p300, NF-κB (RelA) was the rate-limiting factor for basal SHP-1 P2 promoter activity (Figure 3A), similar to that shown for other viral and cellular genes, such as SV40,65,66 HIV-LTR,44 IL-6,67 as well as E-selectin and VCAM-1.68 The involvement of RelA in TIPS was most convincingly demonstrated through mutations within both NF-κB motifs that led to complete loss of TIPS.
The fact that the SHP-1 P2 promoter activity could be inhibited by wild-type Tax and M22/M47 mutants (Figure 2B) suggests neither the NF-κB nor the CREB/ATF pathway is the sole determinant in TIPS. The involvement of M22 in TIPS appears qualitatively different from that of wt and M47 Tax. Compared with the wild-type and M47 Tax, the M22 mutant exhibited the most potent inhibitory effect on the SHP-1 P2 promoter (Figure 2B) but was the least effective in repressing RelA-stimulated promoter activity (Figure 3E). One possible explanation for this observation is that M22 may contribute to TIPS through multiple actions. On one hand, it is possible M22 exerts its inhibitory effect through sequestration of NF-κB presumably in the cytoplasm. This is supported by the observation of Geleziunas et al that M22 fails to activate either IKKα or IKKβ69 and thus cannot induce NF-κB nuclear translocation. The fact that M22 Tax was the least effective in repressing RelA-stimulated promoter activity (Figure 3E) indicates that it may not be able to completely sequester NF-κB in the cytoplasm when RelA is in excess. On the other hand, M22 could also mediate its inhibitory effect through increased affinity with HDAC1 (Figure 5C), leading to inactivation of both NF-κB and CREB.70–72 Thus Tax, through recruitment of HDAC1, may be able to use both the NF-κB and CREB/ATF pathways to achieve its silencing effect. Interestingly, Miyazato et al recently also reported that both NF-κB and CREB/ATF defective Tax mutants were capable of inactivating p53's transcriptional activity.24 It should be pointed out that in the case of SHP-1 P2 promoter, NF-κB is the major target of Tax-mediated promoter silencing and CREB has a minimal effect in its basal activity and TIPS (Figures 1D and 3A,B).
HDAC1 was initially implicated as one of the mediators involved in TIPS based on the observation that the HDAC1 inhibitor TSA relieves TIPS as effectively as it does on the HDAC1-mediated repression of basal SHP-1 P2 promoter activity (Figure 5A). Reversal of TIPS by the HDAC1-specific siRNA further confirmed the role of HDAC1 in SHP-1 P2 promoter silencing. The ability of RelA to effectively counteract the combinatorial inhibitory effect of Tax and HDAC1 (Figure 5A) led us to explore the possible interaction among these 3 proteins. Physical interactions among HDAC1, RelA, and Tax were demonstrated through coprecipitation and Western blot analysis (Figure 5C). The association of HDAC1 and RelA to wt Tax and M47 Tax appeared to be mutually exclusive. This is further confirmed by CHIP assay where binding of HDAC1 and dissociation of NF-κB could be detected at the same time that Tax was expressed and became associated with the endogenous SHP-1 P2 promoter in Tax-transfected Jurkat cells (Figure 5C) or HTLV-1 provirus–transfected CD4+ T cells (J.C. and W.A.M., unpublished data, May 2006).
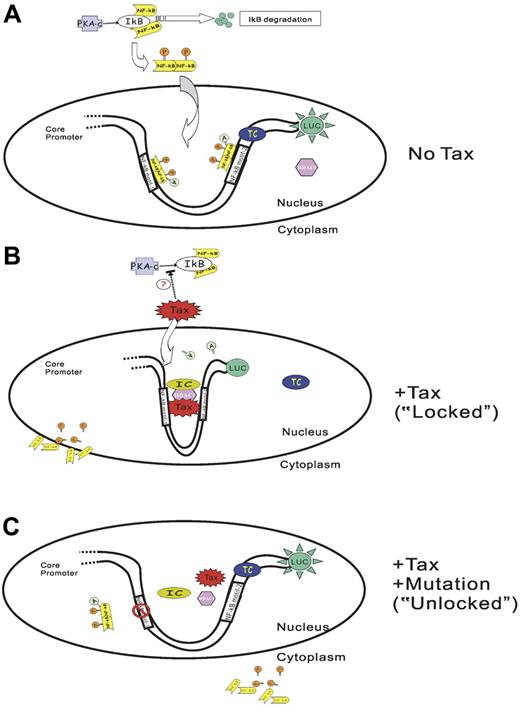
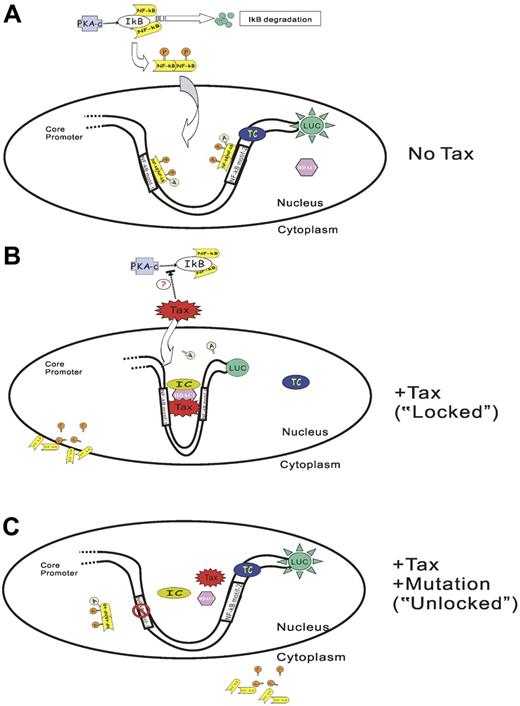
Direct association of Tax with HDAC1 was previously implicated in the regulation of the HTLV-1 promoter.55,56 It was proposed that HDAC1 inhibited the transactivation function of Tax on HTLV-1 promoter by competing with CBP in binding with Tax.55 Tax is also known to interact directly with RelA62 and other NF-κB subunits.61 In light of our data and the previous report that HDAC1 could negatively regulate gene expression by deacetylating NF-κB (RelA) through direct interaction,57 we propose a likely mechanism of TIPS as Tax recruiting HDAC1 to the promoter while displacing NF-κB (RelA) at the same time leading to the repression of the P2 promoter activity (Figure 7). The dissociation of NF-κB (RelA) from Tax protein may be a result of deacetylation by HDAC1, which will lead to both the displacement of NF-κB from the SHP-1 P2 promoter and its subsequent exportation to the cytoplasm. Our mutagenesis data suggest that DNA sequences within the 2 NF-κB motifs are absolutely required for TIPS. Since no Tax-responsive element-1 is present in the full-length SHP-1 P2 promoter sequence,35,73 it is probable that Tax exerts its effect through protein-protein interactions. As illustrated in Figure 7B, one likely model is that Tax together with HDAC1 interact with yet-undetermined cellular factor(s) to form an inhibitory complex that bridges both NF-κB binding motifs. Transcription from SHP-1 P2 promoter is inhibited in such a “locked” position.
Model of Tax-induced SHP-1 P2 promoter silencing (TIPS). In the absence of Tax (A), PKA (protein kinase A) phosphorylates IκB and leads to IκB degradation. NF-κB is released and subsequently phosphorylated and dimerized. The dimerized NF-κB translocates into the nucleus, binds to the NF-κB motifs on the promoter, and is further acetylated by CBP/p300. RNA polymerase II (RNAP II) is recruited to the promoter to form a transcriptional complex (TC). The SHP-1 P2 promoter is thus activated and generates luciferase activity. The presence of Tax (B) serves to recruit HDAC1, and probably other inhibitory molecules onto the SHP-1 P2 promoter to form an inhibitory complex (IC) at the sequence within or surrounding the 2 NF-κB motifs. Tax-associated HDAC1 also deacetylates NF-κB, and reduces the binding of NF-κB on Tax and its association with P2 promoter. The promoter is converted into a “locked” pattern and is therefore silenced. Moreover, inhibition of PKA-induced IκB phosphorylation and degradation may also occur in the presence of Tax, which prevents NF-κB from being activated. On the other hand, mutation of either NF-κB motif (only one is illustrated) will prevent Tax-IC complex formation (C), and the promoter is opened in an “unlocked” position thereby leading to a loss of TIPS effect.
Model of Tax-induced SHP-1 P2 promoter silencing (TIPS). In the absence of Tax (A), PKA (protein kinase A) phosphorylates IκB and leads to IκB degradation. NF-κB is released and subsequently phosphorylated and dimerized. The dimerized NF-κB translocates into the nucleus, binds to the NF-κB motifs on the promoter, and is further acetylated by CBP/p300. RNA polymerase II (RNAP II) is recruited to the promoter to form a transcriptional complex (TC). The SHP-1 P2 promoter is thus activated and generates luciferase activity. The presence of Tax (B) serves to recruit HDAC1, and probably other inhibitory molecules onto the SHP-1 P2 promoter to form an inhibitory complex (IC) at the sequence within or surrounding the 2 NF-κB motifs. Tax-associated HDAC1 also deacetylates NF-κB, and reduces the binding of NF-κB on Tax and its association with P2 promoter. The promoter is converted into a “locked” pattern and is therefore silenced. Moreover, inhibition of PKA-induced IκB phosphorylation and degradation may also occur in the presence of Tax, which prevents NF-κB from being activated. On the other hand, mutation of either NF-κB motif (only one is illustrated) will prevent Tax-IC complex formation (C), and the promoter is opened in an “unlocked” position thereby leading to a loss of TIPS effect.
Our findings also support the involvement of other factors and/or posttranslational modifications in TIPS and reveal the complex nature of SHP-1 gene down-regulation during HTLV-1 transformation. For example, Zhang et al showed that SHP-1 promoter was methylated in HTLV-1–positive cell lines.39,74 Results from Figure 5F of this study revealed histone deacetylation occurred in the presence of Tax and HDAC1. A comprehensive study is currently under way to evaluate the effects of Tax on histone code changes at the SHP-1 P2 promoter. Our data further demonstrate that the SHP-1 P2 promoter is up-regulated by an elevated level of activated PKA in the presence of ectopically expressed RelA, CBP, or p300 (Figure 6). Our results are in agreement with previous report that PKA-c, the catalytic subunit of cAMP-activated PKA, can phosphorylate RelA or CREB75,76 at specific serine residues resulting in increased binding of CBP to RelA and/or CREB64,77 and transcription activation. As shown in Figure 6, PKA-c could reverse Tax inhibitory effect by circa 3-fold. One possible explanation is that PKA is able to reverse TIPS by supplying additional NF-κB in its phosphorylated form. Alternatively, TIPS, in part, could also be a result of Tax directly interfering with the phosphorylation/dephosphorylation state of NF-κB through PKA, which in turn could affect formation of transcriptional competent complexes on the SHP-1 P2 promoter.
In this study, Tax's involvement in negative regulation of SHP-1 expression was established for the first time. SHP-1 is known to have a central role in regulating intracellular levels of phosphotyrosine and to act as an immediate early negative regulator of Jak/STAT signaling. The importance of SHP-1 in the very early stages of T-cell development and TCR signaling has been demonstrated in vivo through studies with moth-eaten and viable moth-eaten mice where homozygosity for these allelic mutations is associated with early onset of severe autoimmune and immunodeficiency disease that leads to death by ages 3 and 9 weeks, respectively.78–80 The early death of these mice has prevented the role of SHP-1 to be assessed more fully; however, the hypothesis that SHP-1 functions as a tumor suppressor is supported by the correlation between the induction of SHP-1 expression and controlled cancer cell growth (reviewed in81 ). It is likely that SHP-1 gene silencing serves as one of the earliest targets in Tax-mediated leukemogenesis. As a candidate tumor suppressor gene and an early negative regulator of cell signaling and proliferation, loss of SHP-1 gene expression may be a first step leading to uncontrolled cell growth. This, together with Tax mediated up-regulation of other genes involved in cell growth and its pleiotropic effects on DNA repair, genetic instability, and apoptosis, could usher a path toward leukemogenesis and ultimately full ATL transformation.
Preliminary reports of this work were presented at the 11th Conference on Retroviruses and Opportunistic Infections (CROI) at San Francisco, 2004; 12th CROI at Boston, 2005; the 12th International Conference on Human Retrovirology at Montego Bay (Jamaica), 2005; and the International Meeting of the Institute of Human Virology at Baltimore, 2006.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Note added in proof.
Wlodarski et al recently reported similar mapping of the SHP-1 P2 core promoter.82
Acknowledgments
This work was supported by the National Foundation for Cancer Research (W.A.M), by NIH CA104936 (W.A.M.), and by NIH AI058804 (Q.Z.)
We thank Drs W. Greene, S. Grossman, D. Housman, S. Marriott, S. Schreiber, J. Sui, and X. Yang for gifts of reagents.
National Institutes of Health
Authorship
Contribution: J.C., Q.Z., and W.A.M. designed research; J.C., A.R.K., K.N., A.M., K.M.N., H.T., M.D., and C.X. performed research; J.C., A.R.K., K.N., K.M.N., H.T., Q.Z., and W.A.M. analyzed data; and J.C., Q.Z., and W.A.M. wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Wayne A. Marasco, Department of Cancer Immunology and AIDS, Dana-Farber Cancer Institute, Harvard Medical School, 44 Binney St, Boston, MA 02115; e-mail: wayne_marasco@dfci.harvard.edu.