Abstract
The anion exchanger 1 (AE1) is encoded by the SLC4A1 gene and catalyzes the electroneutral anion exchange across cell plasma membrane. It is the most abundant transmembrane protein expressed in red cell where it is involved in CO2 transport. Recently, 4 new point mutations of SLC4A1 gene have been described leading to missense mutations in the protein sequence (L687P, D705Y, S731P, or H734R). These point mutations were associated with hemolytic anemia, and it was shown that they confer a cation transport feature to the human AE1. Facing this unexpected property for an electroneutral anion exchanger, we have studied the transport features of mutated hAE1 by expression in xenopus oocytes. Our results show that the point mutations of hAE1 convert the electroneutral anion exchanger to a cation conductance: the exchangers are no longer able to exchange Cl− and HCO3−, whereas they transport Na+ and K+ through a conductive mechanism. These data shed new light on transport mechanisms showing the tiny difference, in terms of primary sequence, between an electroneutral exchange and a conductive pathway.
Introduction
In contrast to immobile cells, erythrocytes are subject to deformations and shears in the blood flow. Therefore, any change in red cell plasma membrane features might have dramatic effect on rheology and lead to hemolytic anemia. It is observed that alteration of proteins involved in red cell cytoskeleton and/or red cell permeability as spectrin, ankyrin, band 4.2, or band 3 are linked to different red cell pathologies such as hereditary spherocytosis, stomatocytosis, elliptocytosis, or Southeast Asian ovalocytosis for instance (for review, see Delaunay1 ). In the present paper, we focused on alterations of band 3, the red cell anion exchanger 1 (AE1) that is one of the oldest studied transmembrane proteins. It is encoded by the SLC4A1 gene and catalyzes the electroneutral anion exchange across cell plasma membrane. Two different polypeptides are synthesized from SLC4A1 gene: one of 911 amino acids that is expressed in red cells and one of 846 amino acids, devoid of the first 65 N-terminal amino acids, that is expressed in kidney α intercalated cells. AE1 is the most abundant transmembrane protein expressed in red cells, where it has different functions such as structuring cell shape, increasing CO2 transport capacity, regulating glycolysis, or signaling senescent erythrocytes. Many different mutations of this transporter have been reported in humans.2 Some of them are asymptomatic but a large group of the different, known mutations of erythroid AE1 is associated with plasma membrane instability leading to red cell pathologies.3 Recently, 4 new point mutations of SLC4A1 gene have been described leading to missense mutations in the protein sequence (L687P, D705Y, S731P, or H734R).4 These mutations were identified in patients with stomatocytosis or spherocytosis, dominantly inherited erythrocyte disorders associated with a Na+ and K+ leak of red cells.1,5,6 In contrast to previously described hAE1 mutations, the pathological effect of L687P, D705Y, S731P, or H734R point mutations is not related to a decreased number of transporters in plasma membrane and hence to plasma membrane instability.7 It was shown that each of these point mutations confers a cation transport feature to the human band 3 in red cells as well as in xenopus oocytes injected with these mutants. These mutations of highly conserved amino acids among anion exchangers are the first described as changing hAE1 transport properties. It has been proposed that they convert hAE1 into a cation transporter, suggesting that anion transport property is abolished and replaced by the cation transport.4 However, the mechanism responsible for the cation leak of hAE1 mutations L687P, D705Y, S731P, or H734R has not been characterized. The 2 cations could be transported by an exchanger or cross the plasma membrane via a nonselective cation conductance.
In the present paper, we investigated by which mechanism the Na+ and K+ leak takes place and the Cl−/bicarbonate transport of the different hAE1 mutants. We characterized hAE1 transport by expression in xenopus oocytes, and our results show that Na+ and K+ diffused independently through the plasma membrane according to their electrochemical gradients, suggesting a “channel-like” transport mechanism. Moreover, these mutations of hAE1 abolish its ability to exchange chloride and bicarbonate, thus the exchanger is converted into a cation conductive pathway.
Materials and methods
hAE1 cDNA with mutations L687P, D705Y, S731P, and H734R
Wild-type and mutated hAE1 were cloned in pSP65. The constructs used were the same as previously described.4
cRNA synthesis
HindIII linearized pSP65hAE1 wt and mutant plasmids were transcribed by SP6 RNA polymerase (Ambion transcription kits; Austin, TX). XhoI linearized hGPA-pcDNA1Neo plasmid was transcribed by T7 RNA polymerase using the Ambion transcription kits. cRNA concentrations were determined on a formamide/formaldehyde agarose gel in MOPS (3-[N-Morpholino]propanesulphonic acid) buffer.
Oocytes
Xenopus laevis were anaesthetized with 0.2% MS222 (3-aminobenzoic acid ethyl ester, methane sulfonate salt) according to the procedure recommended by and with the approval of the ethics committee of CNRS-Université de Nice. The surgery consisted of removing about 5 ovarian lobes containing oocytes.
Oocyte injection
Oocytes were treated as previously published.8 The saline used was MBS (modified Barth saline), with composition in millimolar: NaCl, 85; KCl, 1; NaHCO3, 2.4; MgSO4, 0.82; Ca(NO3)2, 0.33; CaCl2, 0.41; HEPES (N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid), 10; NaOH, 4.5 (pH 7.4), supplemented with penicillin (10 U/mL) and streptomycin (10 μg/mL). Stage V-VI oocytes were then injected with 10 ng hAE1 (wt and mutated) cRNA and with 2.5 ng GPA cRNA when coinjected. Injected oocytes were maintained at 18°C in MBS for 2 or 3 days before running the experiments. For Na+ and K+ content measurements, oocytes were incubated in MBS with 0.5 mM ouabain and 5 μM bumetanide.
Electrophysiological measurements
Electrophysiological parameters were measured at room temperature as previously described using the 2-electrode voltage clamp technique with a TEV 200 amplifier (Dagan, Minneapolis, MN) monitored by computer through Digidata 1200 A/D converter/PC clamp software (Axon Instruments, Foster City, CA).9 Current and potential electrodes filled with 3 M KCl had a resistance of 1.8 to 2.3 MOhm. A KCl agar-agar bridge (3 M) was used in order to minimize junction potentials. The offset potential between bath and electrodes was zeroed before measuring oocyte resting potential. Resting potential was measured by reading the voltage value on the amplifier display after impaling the oocytes by the 2 electrodes. Following resting potential (Em) determination, oocytes were clamped at holding potentials of −30 mV and clamping potentials from −100 to +80 mV; 20-mV increments were applied for 800 ms and the current values recorded. Im was plotted versus Vm and the mean slope of the I/V curve between −100 mV and −20 mV was taken as an index of the membrane conductance and expressed as microsiemens (μS).
In some experiments, the Na+ in MBS was substituted by NMDG (N-methyl-d-glucamine) or K. For NMDG MBS, the final medium was, in mM: NMDGCl, 85; KCl, 1; KHCO3, 2.4; MgSO4, 0.82; Ca(NO3)2, 0.33; CaCl2, 0.41; HEPES, 10; KOH, 4.5 (pH 7.4). For KCl MBS, the final medium was in mM: KCl, 86; KHCO3, 2.4; MgSO4, 0.82; Ca(NO3)2, 0.33; CaCl2, 0.41; HEPES, 10; KOH, 4.5 (pH 7.4). For experiments where Cl− was substituted by gluconate the, gluconate containing MBS was in mM: NaGluconate, 88; KGluconate, 1; MgSO4, 0.82; Ca(NO3)2, 0.33; CaCl2, 0.41; HEPES, 10; KOH, 4.5 (pH 7.4).
When using inhibitors (La3+, Zn2+, 1 mM), the MBS was devoid of HCO3− and SO42− (NaCl, 87.5 mM KCl, 1 mM; MgCl2, 1 mM; CaCl2, 1.8 mM; HEPES/NaOH, 10 mM [pH 7.4]).
Oocyte Na+ and K+ contents measurements
Na+ and K+ contents were measured as previously described.8 Briefly, one measurement was done on a bunch of 5 oocytes in triplicate for each condition. Results were expressed as micromoles per gram of dry cell solids.
Intracellular pH measurements
Oocyte intracellular pH was measured using selective microelectrodes made as follows. Glass micropipettes were pulled from clean borosilicate capillaries (GC 150F-10) (Harvard Apparatus, Holliston, MA), placed in an oven, and dried at 120°C for 1 hour. The microelectrodes were vapor-silanized with N,N-dimethyltrimethylsilylamine (Fluka 41716; Munich, Germany) and then baked for 2 hours at 150°C. A small drop (0.3 μL) of proton ion exchanger (Hydrogen ionophore I-Cocktail A: ref Fluka 95291) was introduced into the electrode shank. The selective microelectrodes were kept in the dark overnight, tips downward, to allow the exchanger to reach the tip and then were back-filled with phosphate buffer (pH 7.0) and calibrated in solutions of pH 6.8 and 8.0. A standard microelectrode filled with 3 M KCl was used for measurement of membrane potential. The electrodes were connected to a high-impedance probe of a 2 channel FD 223 electrometer (World Precision Instruments, Stevenage, United Kingdom). The bath was grounded via a 3 M KCl agar bridge connected to an Ag-AgCl wire. The slope of the pH microelectrodes was between 54 and 58 mV per pH unit.
The ability of wt and mutant hAE1 to regulate intracellular pH was assessed by measuring intracellular pH of oocytes adapted in MBS without HCO3− then incubated in the following medium: NaCl, 63.4 mM; KCl, 1 mM; HCO3−, 24 mM; MgSO4, 0.82 mM; Ca(NO3)2, 0.33 mM; CaCl2, 0.41 mM; HEPES/NaOH, 5 mM (pH 7.35); CO2, 5% and O2, 95%; and then bathed with MBS without Cl (NaGluconate, 63.4 mM; KGluconate, 1 mM; HCO3−, 24 mM; MgSO4, 0.82 mM; Ca(NO3)2, 0.74 mM; HEPES/NaOH, 5 mM [pH 7.35], CO2, 5% and O2, 95%). Results were given in ΔpHi per minute ± se. ΔpHi was measured when acidified oocytes were exposed to Cl-free medium. It corresponds to the initial slope of the alkalinization.
Statistics
Significance of the results was assessed by Student t test and results were considered significantly different for P < .05. Error bars are standard errors of the mean (SEM).
Chemicals and reagents
Agarose was from GIBCO (Life Technologies, Gaithersburg, MD). Miniprep of DNA was done with a commercial kit from Macherey Nagel (Düren, Germany). Restriction enzymes were from NEBiolabs (Beverly, MA). Unless stated otherwise, all the chemicals used were purchased from Sigma (St Louis, MO).
Plasmid pSP65 hAE1 and hGPA-pcDNA1Neo were a gift from Dr Appelhans.
Results
It was previously shown that the point mutations L687P, D705Y, S731P, and H734R in the putative spanning domain of the erythroid human AE1 were able to induce a cation leak in human red cells.4 Heterologous expression of these mutant AE1 in xenopus oocytes was also shown to induce a Na+ and K+ leak. To further investigate the link between Na+ and K+ movements, the Na+ and K+ contents of control oocytes expressing wild-type hAE1 (wt hAE1) or mutated hAE1 were measured after 48 hours of incubation in either the control medium (MBS) or in a medium where Na+ was substituted by NMDG. To avoid any involvement of the Na+/K+ pump and of the NKCC (Na+-K+-2Cl− cotransporter) in cation movements, the oocytes were incubated in media containing ouabain and bumetanide. As illustrated in Figure 1A, when oocytes expressing mutant hAE1 were incubated in MBS, there was an Na+ uptake and a K+ loss compared with noninjected oocytes or oocytes expressing the wt hAE1. In case of incubation in NMDG MBS (no extracellular Na+; Figure 1B), there is no Na+ uptake. In contrast, we observe a similar Na+ loss in all cases (noninjected and injected oocytes) corresponding to dissipation of Na+ gradient through endogenous Na+ leaks. But in the absence of extracellular Na+ and therefore of Na+ uptake, we do not observe any K+ loss in oocytes expressing mutant hAE1. This blockade can be due to electrical inhibition of K+ efflux in the absence of movement of a counter charge (Na+ influx or Cl− efflux for instance).
Intracellular Na+ and K+ contents of oocytes expressing wt or mutated hAE1. Na+ and K+ contents were measured in oocytes either noninjected (NI) or injected with the cRNA indicated on x-axis, 2 days after incubation in media containing 0.5 mM ouabain and 5 μM bumetanide. White bars represent Na+ and black bars represent K+ contents of oocytes. (A) Oocytes were incubated in control MBS containing Na+. (B) Oocytes were incubated in MBS where Na+ was totally replaced by impermeant cation NMDG. Data are expressed in micromole of ions per gram of dry weight and are means (± SEM) of 5 experiments (each of them made with 15 oocytes except for mutants D705Y [n = 4] and S731P [n = 3]).
Intracellular Na+ and K+ contents of oocytes expressing wt or mutated hAE1. Na+ and K+ contents were measured in oocytes either noninjected (NI) or injected with the cRNA indicated on x-axis, 2 days after incubation in media containing 0.5 mM ouabain and 5 μM bumetanide. White bars represent Na+ and black bars represent K+ contents of oocytes. (A) Oocytes were incubated in control MBS containing Na+. (B) Oocytes were incubated in MBS where Na+ was totally replaced by impermeant cation NMDG. Data are expressed in micromole of ions per gram of dry weight and are means (± SEM) of 5 experiments (each of them made with 15 oocytes except for mutants D705Y [n = 4] and S731P [n = 3]).
In Table 1, Na+ and K+ contents of control oocytes were subtracted from the Na+ and K+ contents of oocytes expressing the different hAE1 mutations listed in Table 1. Table 1 summarized the net K+ loss and the net Na+ uptake in oocytes expressing the different AE1 mutants, thus giving the stoichiometry of Na+ and K+ movements. It can be observed that Na+ uptake and K+ loss are not significantly different.
It should be noticed that the cation movement induced by expression of mutant hAE1 is also observed in the absence of ouabain and bumetanide. However, it takes longer to observe a similar cation leak without ouabain than with ouabain (Figure S1, available on the Blood website; see the Supplemental Figures link at the top of the online article).
To understand the relationship between Na+ and K+ movements, the conductance of oocytes expressing the different hAE1 mutations was compared with noninjected oocytes or oocytes expressing wt hAE1.
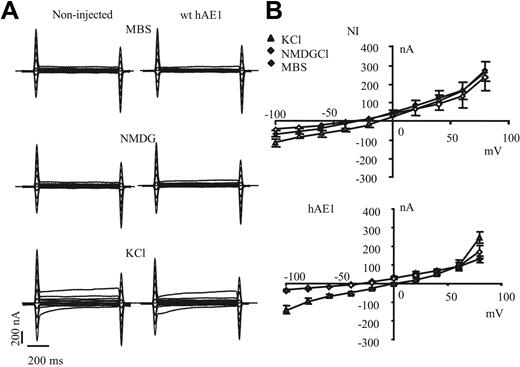
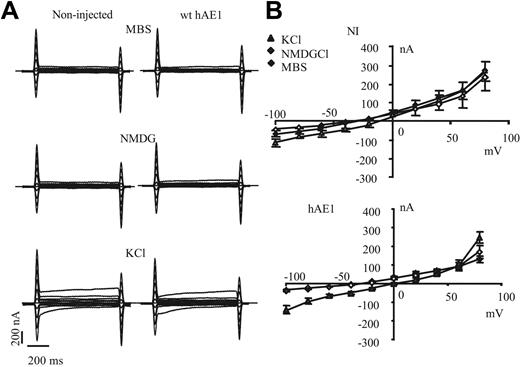
Expression of wt hAE1 in xenopus oocyte does not induce any significant change in the electrical features of oocyte plasma membrane (Figure 2A). The shape of recorded currents is similar between noninjected and wt hAE1–expressing oocytes, and these currents are not significantly sensitive to extracellular substitution of Na+ by NMDG, whereas currents are increased by substitution of extracellular Na+ by K+. In Figure 2B are plotted current/voltage curves of noninjected and wt hAE1–injected oocytes. In KCl medium, the conductance increases and the membrane is depolarized, whereas the absence of permeable extracellular cation (NMDG medium) does not significantly change resting membrane potential or conductance. This is observed for noninjected (NI) oocytes as well as for oocytes expressing wt hAE1. The extracellular Cl− substitution by impermeant anion as gluconate slightly but significantly depolarizes the membrane (by 8 ± 2 mV for wt hAE1 and 6 ± 5 mV for NI) without changing I/V curve shape of NI and wt hAE1–expressing oocytes compared with MBS (curves in gluconate not shown for clarity of the figure). Thus expression of wt hAE1 does not induce any detectable conductance in oocytes, and in our experimental conditions the basal oocyte conductance is mainly a K+ conductance (with also a Cl− component), highly sensitive to the extracellular concentration of K+ as previously published by different groups.10,11
Current recordings of noninjected oocytes or oocytes expressing wt hAE1. (A) The representative electric signal of oocytes (noninjected or injected with wt hAE1 cRNA) measured during voltage clamp protocol either in regular MBS or MBS devoid of Na+. Na+ was substituted by impermeant cation NMDG or by K+. (B) Current-voltage curves obtained with control oocytes (noninjected) or oocytes injected with wild-type hAE1 2 days after injection. The current values were taken at 500 ms. The currents were recorded in control medium (MBS, black diamonds) or in MBS where Na+ was substituted by NMDG (◊), or by K+ (▲). Data presented are means (± SEM) of 20 oocytes coming from different batches.
Current recordings of noninjected oocytes or oocytes expressing wt hAE1. (A) The representative electric signal of oocytes (noninjected or injected with wt hAE1 cRNA) measured during voltage clamp protocol either in regular MBS or MBS devoid of Na+. Na+ was substituted by impermeant cation NMDG or by K+. (B) Current-voltage curves obtained with control oocytes (noninjected) or oocytes injected with wild-type hAE1 2 days after injection. The current values were taken at 500 ms. The currents were recorded in control medium (MBS, black diamonds) or in MBS where Na+ was substituted by NMDG (◊), or by K+ (▲). Data presented are means (± SEM) of 20 oocytes coming from different batches.
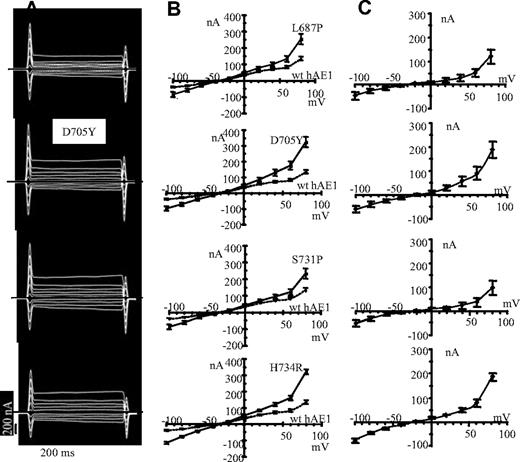
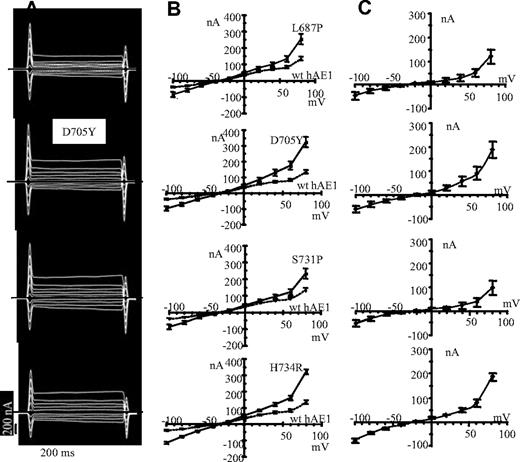
In contrast to wt hAE1, expression of hAE1 mutations H734R, S731P, D705Y, and L687P stimulates the oocyte membrane currents (Figure 3A). In Figure 3B are plotted the I/V curves of oocytes expressing the different mutations or the wt hAE1. To analyze the conductance due to hAE1 mutations, the currents obtained in wt hAE1–injected oocytes were subtracted from the currents induced by mutant hAE1 expression. The calculated I/V curves are presented in Figure 3C. These curves represent the conductance only due to point mutations in hAE1. A similar shape is observed for the 4 mutants with a tendency to inward and outward rectification.
Current recordings in oocytes expressing mutated hAE1. (A) The representative electric signal of oocytes injected with the different hAE1 mutants following voltage clamp protocol. Oocytes were incubated in MBS. (B) The current-voltage curves of oocytes injected with mutant hAE1 (━) compared with wt hAE1 expressing oocytes (----) are plotted. Presented data are means (± SEM) of n oocytes coming from different batches: n = 10 for L687P; n = 11 for D705Y; n = 15 for S731P; n = 23 for H734R; and n = 21 for wt hAE1. (C) Calculated I/V curves giving the features of the current due to hAE1 point mutations only. These calculated I/V curves were obtained by subtracting the mean basal current of wild-type hAE1-injected oocytes (corresponding to dotted line in panel B) to the mean current induced by expression of hAE1 mutations (corresponding to plain line in panel B).
Current recordings in oocytes expressing mutated hAE1. (A) The representative electric signal of oocytes injected with the different hAE1 mutants following voltage clamp protocol. Oocytes were incubated in MBS. (B) The current-voltage curves of oocytes injected with mutant hAE1 (━) compared with wt hAE1 expressing oocytes (----) are plotted. Presented data are means (± SEM) of n oocytes coming from different batches: n = 10 for L687P; n = 11 for D705Y; n = 15 for S731P; n = 23 for H734R; and n = 21 for wt hAE1. (C) Calculated I/V curves giving the features of the current due to hAE1 point mutations only. These calculated I/V curves were obtained by subtracting the mean basal current of wild-type hAE1-injected oocytes (corresponding to dotted line in panel B) to the mean current induced by expression of hAE1 mutations (corresponding to plain line in panel B).
To characterize which ions are responsible for the observed currents, the extracellular ion composition was modified. Currents were recorded in media containing NMDGCl or KCl instead of NaCl. The extracellular Cl− was also removed and substituted by gluconate. Table 2 resumes the resting potential of plasma membrane for oocytes expressing each mutation of hAE1 in different media. For all the mutations, there is a hyperpolarization in a medium where Na+ was substituted by an impermeant cation: NMDG and a depolarization in a medium where Na+ was substituted by K+. Removal of extracellular Cl− does not significantly change the resting potential. The modifications of resting potential are accompanied by changes in oocyte conductance as illustrated in Figure 4. The current-voltage curves illustrate the conductance of oocytes expressing the different hAE1 point mutations, bathed in NMDG-containing (no Na+ and 1 mM KCl) or KCl-containing (no Na+ and 86 mM KCl) medium or gluconate-containing medium compared with control conditions (MBS with 85 mM NaCl and 1 mM KCl). For the 4 different mutants, inward currents are slightly reduced, whereas outward currents increase in NMDG-containing medium. This is expected if Na+ movements are involved in the observed currents. The increased positive currents observed in NMDG medium could be Na+ and K+ leaving the oocyte. In a KCl-containing medium, without extracellular Na+, the increased conductance suggests involvement of K+ movements in the observed currents, whereas Cl− substitution by gluconate does not significantly modify the control I/V curves measured in MBS.
Current sensitivity to changes in extracellular ion composition. Oocytes expressing the different hAE1 point mutations (A: L687P, B: D705Y, C: S731P, and D: H734R) were bathed in different media: MBS (Na+, K+, and Cl−-containing medium, ♦), NMDG medium (Na+ was substituted by NMDG, ◊), KCl medium (Na+ was substituted by K+, ▲), and gluconate medium (NaCl and KCl were substituted by NaGluconate and KGluconate, ○). For each mutation, the same batch of 5 oocytes was voltage-clamped in the different media. The data plotted are from one representative experiment; data are means (± SEM) of 5 oocyte measurements. When not seen, the sem was smaller than the symbols.
Current sensitivity to changes in extracellular ion composition. Oocytes expressing the different hAE1 point mutations (A: L687P, B: D705Y, C: S731P, and D: H734R) were bathed in different media: MBS (Na+, K+, and Cl−-containing medium, ♦), NMDG medium (Na+ was substituted by NMDG, ◊), KCl medium (Na+ was substituted by K+, ▲), and gluconate medium (NaCl and KCl were substituted by NaGluconate and KGluconate, ○). For each mutation, the same batch of 5 oocytes was voltage-clamped in the different media. The data plotted are from one representative experiment; data are means (± SEM) of 5 oocyte measurements. When not seen, the sem was smaller than the symbols.
All the currents were recorded in absence of ouabain and bumetanide. It has been controlled that the presence of these compounds did not change the shape of I/V curves, whereas addition of ouabain could be responsible for a depolarization of about 10 mV.
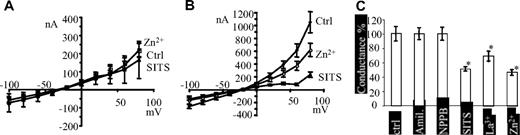
To further characterize the cation conductance induced by expression of hAE1 mutants, the effects of some drugs on currents were assessed. The conductance of oocytes was measured in MBS medium without bicarbonate and divalent anions (to avoid precipitation with divalent cations) or in this medium with either SITS (4-acetamido-4′isothiocyanato-stilbene-2,2′-disulfonic acid), Zn2+, La3+, glybenclamide, amiloride, TEA (tetra-ethyl ammonium), or NPPB (5-nitro-2-(3-phenylpropylamino)benzoic acid). Amiloride, TEA, La3+, and Zn2+ have been used to block different endogenous cation conductances in xenopus oocyte.12,13 SITS has been shown to block the cation leak of red cell from patients with H734R, S731P, D705Y, or L687P mutation of band 3.4
It is observed that none of the inhibitors significantly modified the I/V curves of control oocytes NI or expressing wt hAE1. In Figure 5A are plotted the I/V curves of oocytes expressing wt hAE1 in presence of Zn2+ or SITS (similar results with NI oocytes, not shown). In contrast, the current of oocytes expressing H734R mutation of hAE1 is inhibited by Zn2+ and SITS as shown in Figure 5B. Other drugs, TEA (10 mM), La3+ (1 mM), amiloride (0.5 mM), NPPB (0.5 mM) and glybenclamide (0.5 mM), were tested on conductance of oocytes expressing wt hAE1 or H734R mutation. None of these compounds has any effect on I/V curves of wt hAE1–expressing oocytes, and, except for La3+, they do not inhibit the current induced by H734R mutation (not shown). The graph bar in Figure 5C summarized the inhibitory data on conductance of oocytes expressing H734R mutation of hAE1. SITS was also assessed on the conductance induced by the other hAE1 point mutations. The current induced by S731P and L687P mutations is partially blocked and the conductance measured between −100 and −20 mV was decreased by 30% and 40%, respectively, with 0.5 mM SITS.
Inhibitor sensitivity of currents induced by H734R mutation of hAE1. In these experiments, oocytes were bathed in MBS medium without bicarbonate and divalent anions to avoid precipitation with divalent cations. (A) Current/voltage curves of control oocytes expressing wt hAE1 in the absence of inhibitors (●, mean of 10 oocytes) or in the presence of 0.5 mM SITS (▲, mean of 5 oocytes) or 1 mM Zn2+ (□, mean (± SEM) of 8 oocytes). (B) Current/voltage curves of oocytes expressing H734R mutation in the absence of inhibitor (●) or in the presence of 0.5 mM SITS (▲) or 1 mM Zn2+ (○). Data are mean (± SEM) of 10 oocytes. (C) The graph bar summarizes the effect of some inhibitors on the conductance of H734R-expressing oocytes. The conductance was calculated from I/V curves between − 80 and − 20 mV in the absence of inhibitors and expressed as 100%. The conductance calculated in the presence of amiloride (0.1 mM), NPPB (0.5 mM), Zn2+ (1 mM), La3+ (1 mM), or SITS (0.5 mM) was expressed in percentage of the corresponding control oocytes and compared with the control by Student t test. (*P < .05).
Inhibitor sensitivity of currents induced by H734R mutation of hAE1. In these experiments, oocytes were bathed in MBS medium without bicarbonate and divalent anions to avoid precipitation with divalent cations. (A) Current/voltage curves of control oocytes expressing wt hAE1 in the absence of inhibitors (●, mean of 10 oocytes) or in the presence of 0.5 mM SITS (▲, mean of 5 oocytes) or 1 mM Zn2+ (□, mean (± SEM) of 8 oocytes). (B) Current/voltage curves of oocytes expressing H734R mutation in the absence of inhibitor (●) or in the presence of 0.5 mM SITS (▲) or 1 mM Zn2+ (○). Data are mean (± SEM) of 10 oocytes. (C) The graph bar summarizes the effect of some inhibitors on the conductance of H734R-expressing oocytes. The conductance was calculated from I/V curves between − 80 and − 20 mV in the absence of inhibitors and expressed as 100%. The conductance calculated in the presence of amiloride (0.1 mM), NPPB (0.5 mM), Zn2+ (1 mM), La3+ (1 mM), or SITS (0.5 mM) was expressed in percentage of the corresponding control oocytes and compared with the control by Student t test. (*P < .05).
It is noticeable that the complete absence of extracellular Ca2+ did not modify the I/V curves of oocytes expressing mutant hAE1 (not shown).
Human glycophorin A (hGPA) is a chaperone protein associated with band 3 in human red cell membrane. It has been shown that coexpression of hGPA and hAE1 in xenopus oocytes enhanced DIDS-sensitive Cl− transport by increasing the accumulation rate of band 3 in plasma membrane.14,15 Therefore, the effect of coexpression of hGPA and mutant hAE1 was investigated on oocyte conductance.
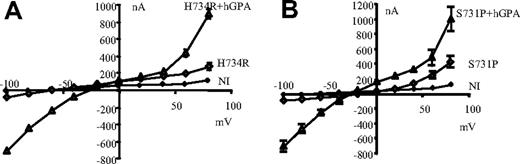
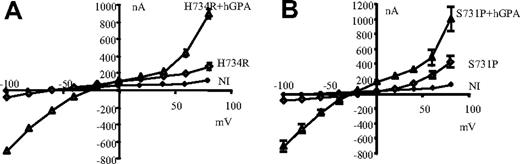
As shown in Figure 6, coinjection of hGPA and mutant hAE1 could increase conductances induced by mutations H734R and S731P of hAE1. It should be noticed that hGPA expression alone does not change the electrical features of oocytes (not shown for clarity of the figure). The GPA coexpression induces an inward and outward rectification as well as a reversal potential shift toward zero for mutant H734R. This is due to changes in Na+ and K+ contents of these oocytes compared with oocytes expressing only mutated AE1. (Na+ and K+ contents of oocytes coexpressing H734R and GPA were 148 ± 2 and 14 ± 2 μmol/g dry weight, respectively; measurements were done on the same oocytes as those used to record presented I/V curves. Intracellular Na+ and K+ concentrations tend to equilibrate with extracellular concentrations, thus pulling membrane potential toward zero.) These large currents are also sensitive to SITS, Zn2+, or La3+ and to extracellular Na+ as well as K+ concentrations (Figures S3,S4).
Effect of GPA on currents induced by hAE1 mutations. Current-voltage curves measured in control oocytes (NI) or injected with mutant hAE1 (H734R or S731P: ◊) or coinjected with mutant hAE1 and human glycophorin A (▲). Oocytes were bathed in MBS. Data presented are mean (± SEM) of 5 oocytes. In some cases, the SEM is smaller than the symbols and thus not visible in the graph. The conductance of oocytes injected with hGPA alone was the same as for NI oocytes.
Effect of GPA on currents induced by hAE1 mutations. Current-voltage curves measured in control oocytes (NI) or injected with mutant hAE1 (H734R or S731P: ◊) or coinjected with mutant hAE1 and human glycophorin A (▲). Oocytes were bathed in MBS. Data presented are mean (± SEM) of 5 oocytes. In some cases, the SEM is smaller than the symbols and thus not visible in the graph. The conductance of oocytes injected with hGPA alone was the same as for NI oocytes.
Four different point mutations of hAE1 unmask a Na+ and K+ conductance. It was therefore of interest to see whether these mutations convert the anion exchanger to a nonselective cation “channel” or whether the 2 functions, channel and exchanger, coexist in the same protein. As shown previously, the Cl− uptake in oocytes expressing these hAE1 mutants is very small compared with wild-type hAE1.4 To assess the Cl−/HCO3− exchange activity of mutant hAE1, we measured the ability of the different hAE1 (wild type and mutated) to alkalinize the oocyte after an acid load.
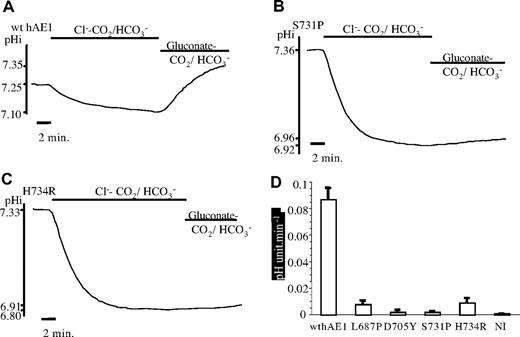
Oocytes were coinjected with hGPA and wild-type or mutated hAE1 to ensure an optimal expression of the exchanger. In Figure 7, the curves are representative of the time course of oocyte pH changes, and the bar graph illustrates the velocity of pH recovery after the acid load of oocytes expressing wt or mutated hAE1. Oocytes expressing wt hAE1 are acidified in MBS containing CO2/HCO3− buffer and then alkalinized by removal of extracellular Cl−. In contrast, none of the studied mutants is able to alkalinize the oocyte after the acid load. Moreover, the slope of the acid load is faster than for wt hAE1, indicating a less efficient Cl−/HCO3− exchange. Indeed, the rate of the initial acidification depends also on the ability of the anion exchanger to equilibrate intracellular and extracellular bicarbonate. If wild-type hAE1–injected oocytes are taken as a 100% of pH recovery rate, all the mutants strongly decrease this pH recovery rate by more than 90%.
Intracellular pH measurements. Traces are intracellular pH measurements of representative oocytes expressing GPA and wt (A) or mutated hAE1 (B: S731P: C: H734R) as a function of time and extracellular medium composition. The graph bars (D) illustrate the initial speed of pH recovery after intracellular acidification for control oocytes (noninjected) or oocytes expressing GPA and wt hAE1 or GPA and the 4 different point mutations of hAE1. Data are mean (± SEM) of 5 (NI), 10 (L687P), 7 (D705Y), 10 (S731P), 8 (H734R), or 16 (wt hAE1) oocytes coming from different xenopus.
Intracellular pH measurements. Traces are intracellular pH measurements of representative oocytes expressing GPA and wt (A) or mutated hAE1 (B: S731P: C: H734R) as a function of time and extracellular medium composition. The graph bars (D) illustrate the initial speed of pH recovery after intracellular acidification for control oocytes (noninjected) or oocytes expressing GPA and wt hAE1 or GPA and the 4 different point mutations of hAE1. Data are mean (± SEM) of 5 (NI), 10 (L687P), 7 (D705Y), 10 (S731P), 8 (H734R), or 16 (wt hAE1) oocytes coming from different xenopus.
Discussion
The 4 hAE1 mutants studied here are able to induce a cation leak when expressed in xenopus oocytes. Our results show that this leak is associated with a net Na+ and K+ transport with a stoichiometry of 1 K+ for 1 Na+. It is observed that in the absence of extracellular Na+ there is no K+ loss. This result rules out the existence of 2 independent Na-Cl and K-Cl cotransporters functioning simultaneously. Indeed, in this case, in the absence of extracellular Na+ there can still be a KCl loss. The increased conductance of oocytes expressing mutant hAE1, associated with a 1 for 1 stoichiometry of Na+ and K+ net fluxes, also rules out the involvement of an electrogenic Na+/K+ exchange. Moreover, there is no increase of the Cl− conductance of oocytes expressing mutant compared with wt hAE1 and resting potential of these oocytes is sensitive to Na+ and K+ extracellular concentrations. Thus, this conductance has the features of a nonspecific cation conductance allowing diffusion of Na+ and K+ according to their electrochemical gradients. It is observed that the 4 different mutations induced a similar shape of currents that differ only by their intensity. Thus, mutations L687P, D705Y, S731P, and H734R turn on a similar Na+ and K+ conductance in xenopus oocytes. This conductance is specifically observed in oocytes expressing mutated hAE1 and it is not observed in oocytes expressing wt hAE1. This cation conductance is small and this is expected when the net cation leak is considered. Indeed in vivo, the cation leak is compensated in erythrocytes by Na/K ATPase activity, and in oocytes the net cation leak is around 10 nmol/day/oocyte expressing mutant hAE1 (in presence of ouabain, see Table 1; the dry weight of one oocyte is about 0.5 mg). With the equation converting into current net ion movements through the plasma membrane (1 μ equivalent of ion transported per hour corresponds to 26.8 μA of current through the membrane, knowing that 1 A corresponds to 1 Coulomb/s and the charge of one monovalent ion is 1.6 × 10−19 Coulomb), the cation current associated with the 10 nmol Na+ or K+ transported per day per oocyte should be around 12 nA, which is approximately the order of magnitude of the presented currents in oocytes (Figure 3C).
The pharmacology of this cation conductance is difficult to study in oocytes with traditional band 3 inhibitors such as DIDS or niflumic acid since it was observed that these drugs are potent activators of oocyte endogenous cation conductances. Only SITS could be successfully used in our electrophysiological study and it was shown that, at relatively high concentration (0.5 mM), this compound decreased the cation conductance of oocytes expressing point mutations of hAE1. Compared with anion exchange through band 3, the cation conductance is not as sensitive to SITS; only a partial inhibition (30% to 50%, Figure 5 and “Results”) is observed with 0.5 mM SITS. Moreover, in contrast to the anion exchange, the cation conductance is not NPPB sensitive. However, the SITS sensitivity of the cation conductance induced by hAE1 point mutation expression in oocytes is also observed for the bumetanide- and ouabain-resistant K influx in erythrocytes from people carrying the mutated AE1.4 None of the compounds used in our pharmacological study was a potent inhibitor of the cation conductance. Only a partial inhibition could be observed with 0.5 mM SITS or 1 mM Zn2+ or La3+ (like the red cell voltage-dependent cation channel16 ). Thus the cation conductance induced by hAE1 point mutations exhibit unusual pharmacological features compared with anion exchanger and also to known oocyte cation channels.
Nonselective cation channels have been described in xenopus oocyte membrane. These endogenous cation conductances are observed in different conditions such as stimulation by bisphosphonate, DIDS, maitotoxin, hyperpolarization, or acidification for instance.17–20 Therefore, it could be envisioned that overexpression of mutated hAE1 could result in the activation of a nonselective cation channel in oocyte membrane.21 In the past, expression studies of different channels or membrane proteins in xenopus oocytes have demonstrated the relevance of interactions between studied proteins and oocyte endogenous transporters.22–26 However, the present data disagree with this interpretation. Only specific point mutations are able to confer to hAE1 the possibility to enhance a putative endogenous cation conductance; the wild-type hAE1 is not able to stimulate any cation conductance in oocyte (Figure 2). Moreover, it was possible to increase the cation conductance induced by H734R or S731P point mutations by coexpressing human glycophorin A, an AE1 chaperone in red cell. The feature of this cation conductance is different from what has been characterized in oocyte membrane up to now. In contrast to some endogenous cation conductances, the mutated hAE1-induced cation conductance is insensitive to amiloride (unlike the nonselective cation channel9,17–19 ) to TEA and to extracellular Ca2+ concentration.20,27,28 Moreover, in human red cells carrying these mutated hAE1, a similar cation leak is observed and red cell cation channels might be different from oocyte cation channels. The cation leak observed in red cells from patient, with AE1 point mutations is sensitive to SITS as is the conductance of oocytes expressing these mutated AE1.4 Thus, the simplest interpretation of our data is that point mutations are able to unmask a cation “channel” activity in the otherwise well-known electroneutral anion exchanger 1. The association of a cation transport to the anion exchanger has already been documented for the anion exchanger of trout erythrocytes.8 Indeed, hypo-osmotic swelling of trout red cell stimulates a Na+ and K+ leak through the anion exchanger.29
It should be noticed that appearance of the cation leak in mutated hAE1 is correlated to the disappearance of Cl−/HCO3− exchange activity. It was previously shown that these mutant AE1 in erythrocytes are not able to transport anions.4 These data suggest that the anion exchanger is indeed converted to a cation conductive pathway when amino acids L687, D705, S731, or H734 are mutated as described.
How these different point mutations are able to convert the electroneutral anion exchanger to a Na+ and K+ conductive pathway is an open question. All the mutations are located around putative helices 8 (L687P) and 9 (D705Y) and the loop connecting spanning domains 9 and 10 (S731P and H734R) (according to the topology of AE1 by Zhu et al30 ). It can be observed that, first, these mutations change highly conserved residues among all the different known anion exchangers, AE1, AE2, and AE3. It can be assumed that they play an important part in the anion exchange function of these proteins. Second, the nature of these mutations suggests that they could induce noticeable changes in hAE1 ternary structure either by introducing prolines (L687P or S731P) or by removing a negative charge (D705Y) in a highly conserved sequence (KGSG(F/L)HLD) among all the anion exchangers known so far or by adding a positive charge (H734R). Another point is that these different mutations induced a similar effect, converting the anion exchanger to a Na+ and K+ transporter. Thus, they are probably responsible for a similar structural modification of the protein. This structural change is accompanied by changes in inhibitor sensitivity. In human red cells, the mutated AE1 are no more sensitive to covalent binding of H2-DIDS in contrast to wt AE1, suggesting a conformational change of mutated proteins.4 In contrast to anion exchange, the cation transport is sensitive to Zn2+ and La3+: new sites appeared in the “cation conductive” AE1. The sensitivity to a classical band 3 inhibitor SITS, although maintained, is decreased and other inhibitors of the anion exchanger such as NPPB do not block the cation conductance. Further work will be needed to understand the role of these amino acids in AE1 spatial organization.
Our data showing the conversion of the electroneutral anion exchanger AE1 to a nonselective cation conductance as a result of a point mutation led us to wonder about the role of the AE1 in other red cell pathologies where a cation leak has been described such as malaria or sickle cell disease. Could the new permeability pathway (NPP)31 or P-sickle32 be linked to AE1 structural changes?
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgment
The authors would like to thank their colleague Dr Olivier Soriani for fruitful discussions about electrophysiology.
Authorship
Contribution: S.M. and N.G. performed experiments; F.B. analyzed and interpreted data; and H.G. designed and performed research, interpreted data, and wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Hélène Guizouarn, Laboratoire de physiologie cellulaire et moléculaire, UMR6548 CNRS-Université de Nice, Bâtiment de sciences naturelles, 28 av. Valrose, 06108 Nice cedex 2, France; e-mail: helene.guizouarn@unice.fr.

![Figure 1. Intracellular Na+ and K+ contents of oocytes expressing wt or mutated hAE1. Na+ and K+ contents were measured in oocytes either noninjected (NI) or injected with the cRNA indicated on x-axis, 2 days after incubation in media containing 0.5 mM ouabain and 5 μM bumetanide. White bars represent Na+ and black bars represent K+ contents of oocytes. (A) Oocytes were incubated in control MBS containing Na+. (B) Oocytes were incubated in MBS where Na+ was totally replaced by impermeant cation NMDG. Data are expressed in micromole of ions per gram of dry weight and are means (± SEM) of 5 experiments (each of them made with 15 oocytes except for mutants D705Y [n = 4] and S731P [n = 3]).](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/110/6/10.1182_blood-2006-12-063420/7/m_zh80180707330001.jpeg?Expires=1765289695&Signature=NVk~kBTcArX1HbspLbDb4GhapuM5N3rY5wkiktwdpKOcn5ZP3iWNIneNB18ek8I~jYgj6sHIMfbZpyAFUQZkDzkwSrlA4tsPAIf0quwJRVIPe09vCDBNW9XrEz9pTDXPX68SPtsieZ7vGQo~8GFm-QVUZ1D-mYxiymwR0NPXOxEUAJxWebt1iMP16SMOLDMtvY0MxElrLVk6NEIcHtzmKWiazG2qYdTuqvu6bEZl~xkXi58QTRYmwU1cFjg-sHhi5ZU1i3J~lJf26oHk7xQi5oa3-GBNOZlJu9ysXPMiYi1km1szJocWsqnU8SgtHfePAGYivhJNs-K1yHnNTwDFLQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)




![Figure 1. Intracellular Na+ and K+ contents of oocytes expressing wt or mutated hAE1. Na+ and K+ contents were measured in oocytes either noninjected (NI) or injected with the cRNA indicated on x-axis, 2 days after incubation in media containing 0.5 mM ouabain and 5 μM bumetanide. White bars represent Na+ and black bars represent K+ contents of oocytes. (A) Oocytes were incubated in control MBS containing Na+. (B) Oocytes were incubated in MBS where Na+ was totally replaced by impermeant cation NMDG. Data are expressed in micromole of ions per gram of dry weight and are means (± SEM) of 5 experiments (each of them made with 15 oocytes except for mutants D705Y [n = 4] and S731P [n = 3]).](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/110/6/10.1182_blood-2006-12-063420/7/m_zh80180707330001.jpeg?Expires=1765346093&Signature=p7gYEkuuG65aVnMi7DwhjplLeShU4PWdrUWMZ5i3i~oRdKZE-vvKrtfw-OmQ~-8EEpDpAwSyC8Cnt460Mb3EP5XBUGddPfumvPQw4tsvWdORDgUsynP2BZ5RAn5-UPhSVug~Iv93yxz1SBc9hY1sIuedexnyvbB0QRrIUaz3ujcVO6QK2kkCOwBH9tg2x50Zo3ptNXS2HBXs8iZZVI4s4wn9tgPgmR9w9vg3TSGjBeskyvdpIcoWlHT-1jSTUSW1AzOWjrnsmB9QsK50sz5BSLOSBfPtKTH9P-b8-K3nRY7omuGCNd70BErkWs~ykjFVzy2kCElpIO72quqmitErsA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)